MicroRNA Polymorphisms in Cancer: A Literature Analysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. List of Cancer Associated Polymorphisms within miRNA Encoding Genes

| miRNA Name | rs Number | Substitution | Location | Genomic Context (Host Genes) | |
|---|---|---|---|---|---|
| Protein-Coding | Non-Coding Transcriptional Unit | ||||
| hsa-mir-27a | rs11671784 | G>A | pre-mature | ||
| rs895819 | T>A, T>C, T>G | pre-mature | |||
| hsa-mir-146a | rs2910164 | C>G | seed | CTC-231O11.1 (exon, S) | |
| hsa-mir-149 | rs71428439 | A>G | pre-mature | GPC1 (intron, S) | |
| rs2292832 | T>C | pre-mature | GPC1 (intron, S) | ||
| hsa-mir-196a-2 | rs11614913 | C>T | mature | HOXC6 (intron, S) | |
| hsa-mir-202 | rs12355840 | C>A, C>T, C>G | pre-mature | MIR202HG (intron/exon, As) | |
| hsa-mir-423 | rs6505162 | A>C | pre-mature | NSRP1 (intron, S) | MIR3184 (intron, S) |
| hsa-mir-499a | rs3746444 | A>G | seed | MYH7B (intron, S) | |
| hsa-mir-603 | rs11014002 | C>T | pre-mature | KIAA1217 (intron, S) | |
| hsa-mir-605 | rs2043556 | T>C | pre-mature | PRKG1 (intron, S) | |
| hsa-mir-608 | rs4919510 | C>G | mature | SEMA4G (intron, S), MRPL43 (intron, As) | |
| hsa-mir-612 | rs12803915 | G>A | pre-mature | NEAT1 (exon, S) | |
| hsa-mir-618 | rs2682818 | A>C | pre-mature | LIN7A (intron, As) | |
| hsa-mir-646 | rs6513497 | T>G | mature | MIR646HG (intron, S) | |
| hsa-mir-933 | rs79402775 | G>A | mature | ATF2 (intron, As) | |
| hsa-mir-1206 | rs2114358 | G>A | pre-mature | PVT1 (intron, S) | |
| hsa-mir-1307 | rs7911488 | A>G | pre-mature | USMG5 (intron, As) | |
| hsa-mir-3144 | rs67106263 | G>A | mature | ||
| hsa-mir-5197 | rs2042253 | T>C | pre-mature | ||
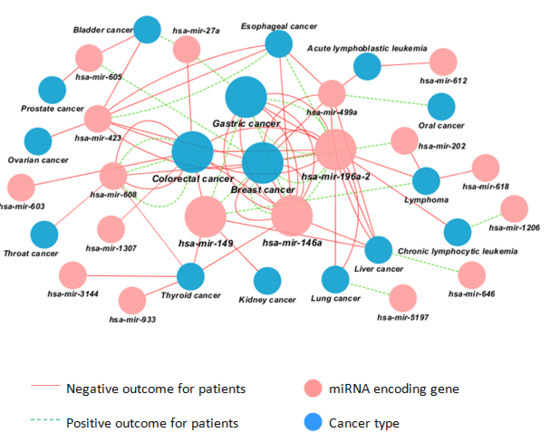
2.2. Visualization of 75 Associations between miR-SNPs and Cancer

2.3. Future Directions
3. Experimental Section
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kunej, T.; Godnic, I.; Horvat, S.; Zorc, M.; Calin, G.A. Cross talk between microRNA and coding cancer genes. Cancer J. 2012, 18, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Obsteter, J.; Dovc, P.; Kunej, T. Genetic variability of microRNA regulome in human. Mol. Genet. Genomic Med. 2015, 3, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Yan, J.; Noltner, K.; Feng, J.; Li, H.; Sarkis, D.A.; Sommer, S.S.; Rossi, J.J. SNPs in human miRNA genes affect biogenesis and function. RNA 2009, 15, 1640–1651. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.U.; Miyazaki, H.; Ochiya, T. The roles of microRNAs in breast cancer. Cancers (Basel) 2015, 7, 598–616. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yu, C.Y.; Wang, J.L.; Guan, J.; Chen, H.Y.; Fang, J.Y. MicroRNA sequence polymorphisms and the risk of different types of cancer. Sci. Rep. 2014, 4, 3648. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Li, Y.; Wu, J. A risk of digestive tract neoplasms susceptibility in miR-146a and miR-196a2. Fam. Cancer 2015, 14, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Ren, Y.; Fang, X.; Yin, Z.; Li, X.; Wu, W.; Guan, P.; Zhou, B. Prognostic role of common microRNA polymorphisms in cancers: Evidence from a meta-analysis. PLoS ONE 2014, 9, e106799. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Gu, J.; Eng, C.; Ellis, L.M.; Hildebrandt, M.A.; Lin, J.; Huang, M.; Calin, G.A.; Wang, D.; Dubois, R.N.; et al. Genetic polymorphisms in microRNA-related genes as predictors of clinical outcomes in colorectal adenocarcinoma patients. Clin. Cancer Res. 2012, 18, 3982–3991. [Google Scholar] [CrossRef] [PubMed]
- Ryan, B.M.; McClary, A.C.; Valeri, N.; Robinson, D.; Paone, A.; Bowman, E.D.; Robles, A.I.; Croce, C.; Harris, C.C. Rs4919510 in hsa-mir-608 is associated with outcome but not risk of colorectal cancer. PLoS ONE 2012, 7, e36306. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Wan, S.; Zhou, F.; Qu, F.; Li, B.; Myers, R.E.; Fu, X.; Palazzo, J.P.; He, X.; Chen, Z.; et al. Genetic polymorphisms in pre-microRNA genes as prognostic markers of colorectal cancer. Cancer Epidemiol. Biomark. Prev. 2012, 21, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Pardini, B.; Rosa, F.; Naccarati, A.; Vymetalkova, V.; Ye, Y.; Wu, X.; di Gaetano, C.; Buchler, T.; Novotny, J.; Matullo, G.; et al. Polymorphisms in microRNA genes as predictors of clinical outcomes in colorectal cancer patients. Carcinogenesis 2015, 36, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Bai, H.; Hu, H. Rs11671784 g/a variation in miR-27a decreases chemo-sensitivity of bladder cancer by decreasing miR-27a and increasing the target RUNX-1 expression. Biochem. Biophys. Res. Commun. 2015, 458, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tian, X.; Zhang, B.; Chen, J. Polymorphisms in microRNA-related genes are associated with survival of patients with T-cell lymphoma. Oncologist 2014, 19, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Rawlings-Goss, R.A.; Campbell, M.C.; Tishkoff, S.A. Global population-specific variation in miRNA associated with cancer risk and clinical biomarkers. BMC Med. Genomics 2014, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Bansal, C.; Sharma, K.L.; Misra, S.; Srivastava, A.N.; Mittal, B.; Singh, U.S. Common genetic variants in pre-micrornas and risk of breast cancer in the north indian population. Ecancermedicalscience 2014, 8, 473. [Google Scholar] [PubMed]
- Qi, P.; Wang, L.; Zhou, B.; Yao, W.J.; Xu, S.; Zhou, Y.; Xie, Z.B. Associations of miRNA polymorphisms and expression levels with breast cancer risk in the Chinese population. Genet. Mol. Res. 2015, 14, 6289–6296. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Pan, Y.; Xu, Y.; Deng, Q.; Sun, H.; Gao, T.; Wang, S. Associations of polymorphisms in microRNAs with female breast cancer risk in Chinese population. Tumour Biol. 2015, 36, 4575–4582. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hao, X.; Feng, Z.; Liu, Y. Genetic polymorphisms in miRNAs and susceptibility to colorectal cancer. Cell Biochem. Biophys. 2015, 71, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Tang, W. Associations of polymorphisms in mir-196a2, mir-146a and mir-149 with colorectal cancer risk: A meta-analysis. Pathol. Oncol. Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Ma, X.L.; Zhao, C.; Liu, T.; Du, Y.L.; Kong, W.Q.; Wei, B.L.; Yu, J.Y.; Li, Y.Y.; Huang, J.W.; et al. Associations of single nucleotide polymorphisms in miR-146a, miR-196a, miR-149 and miR-499 with colorectal cancer susceptibility. Asian Pac. J. Cancer Prev. 2014, 15, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yang, S.; Chaugai, S.; Wang, Y.; Wang, D.W. Meta-analysis of Hsa-mir-499 polymorphism (rs3746444) for cancer risk: Evidence from 31 case-control studies. BMC Med. Genet. 2014, 15, 126. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.J.; Ding, Y.; Mao, Y.Y.; Jing, F.Y.; Zhang, Z.Y.; Jiang, L.F.; Guo, J.F.; Sun, X.J.; Jin, M.J.; Chen, K. Associations between hsa-mir-603 polymorphism, lifestyle-related factors and colorectal cancer risk. Cancer Biomark. 2014, 14, 225–231. [Google Scholar] [PubMed]
- Tang, R.; Qi, Q.; Wu, R.; Zhou, X.; Wu, D.; Zhou, H.; Mao, Y.; Li, R.; Liu, C.; Wang, L.; et al. The polymorphic terminal-loop of pre-mir-1307 binding with mbnl1 contributes to colorectal carcinogenesis via interference with dicer1 recruitment. Carcinogenesis 2015, 36, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Zorc, M.; Omejec, S.; Tercic, D.; Holcman, A.; Dovc, P.; Kunej, T. Catalog of genetic variants within mature microRNA seed regions in chicken. Poult. Sci. 2015, 94, 2037–2040. [Google Scholar] [CrossRef] [PubMed]
- MiRNA SNiPer. Available online: http://www.integromics-time.com/miRNA-SNiPer/ (accessed on 29 June 2015).
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Cancers: Medlineplus. Available online: https://www.nlm.nih.gov/medlineplus/cancers.html (acccessed on 29 June 2015).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pipan, V.; Zorc, M.; Kunej, T. MicroRNA Polymorphisms in Cancer: A Literature Analysis. Cancers 2015, 7, 1806-1814. https://doi.org/10.3390/cancers7030863
Pipan V, Zorc M, Kunej T. MicroRNA Polymorphisms in Cancer: A Literature Analysis. Cancers. 2015; 7(3):1806-1814. https://doi.org/10.3390/cancers7030863
Chicago/Turabian StylePipan, Veronika, Minja Zorc, and Tanja Kunej. 2015. "MicroRNA Polymorphisms in Cancer: A Literature Analysis" Cancers 7, no. 3: 1806-1814. https://doi.org/10.3390/cancers7030863
APA StylePipan, V., Zorc, M., & Kunej, T. (2015). MicroRNA Polymorphisms in Cancer: A Literature Analysis. Cancers, 7(3), 1806-1814. https://doi.org/10.3390/cancers7030863