Improvement of Ethylene Removal Performance by Adsorption/Oxidation in a Pin-Type Corona Discharge Coupled with Pd/ZSM-5 Catalyst
Abstract
:1. Introduction
2. Results and Discussion
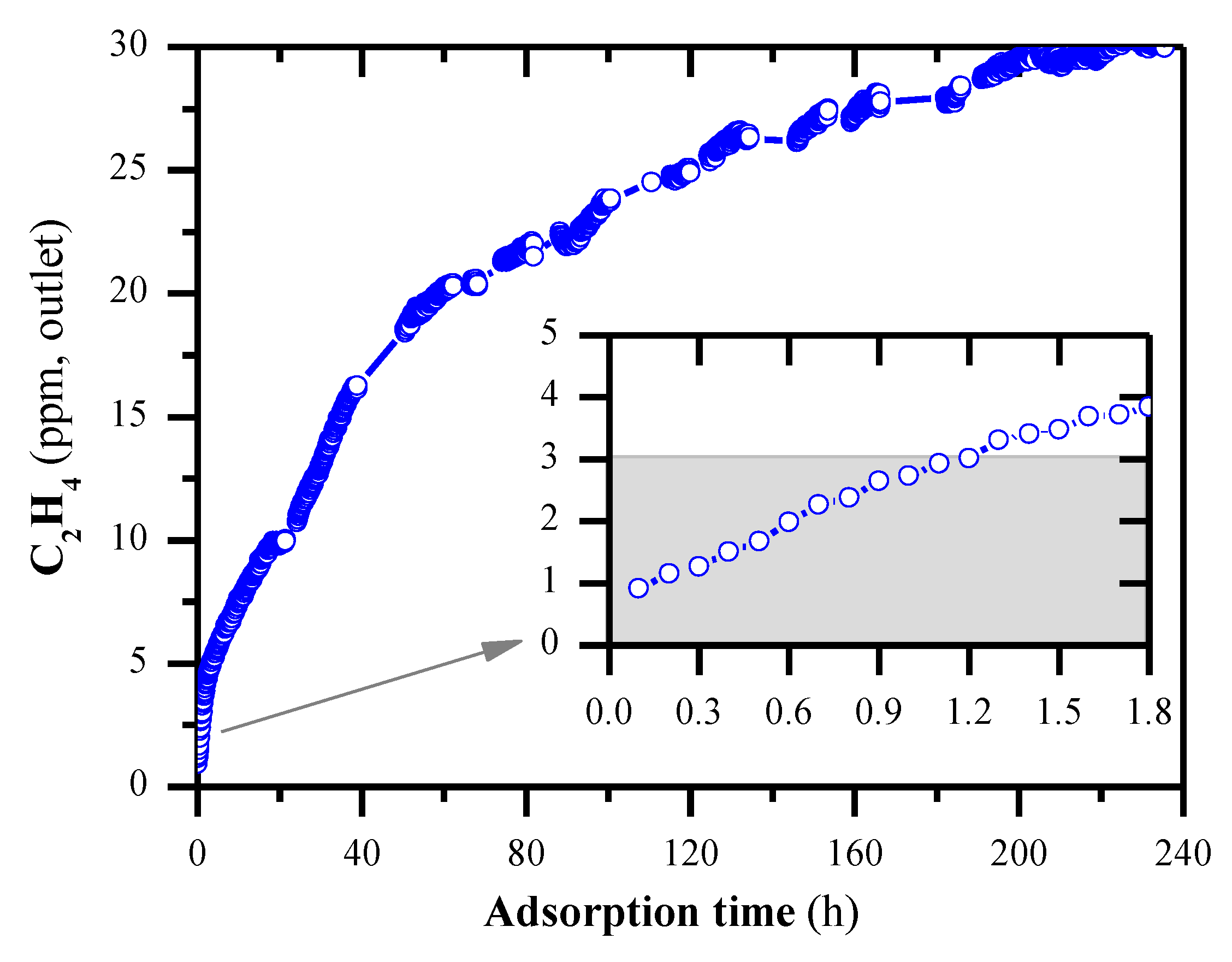
2.1. Adsorption Capacity
2.2. Thermal-Programed Oxidization (TPO) of Ethylene
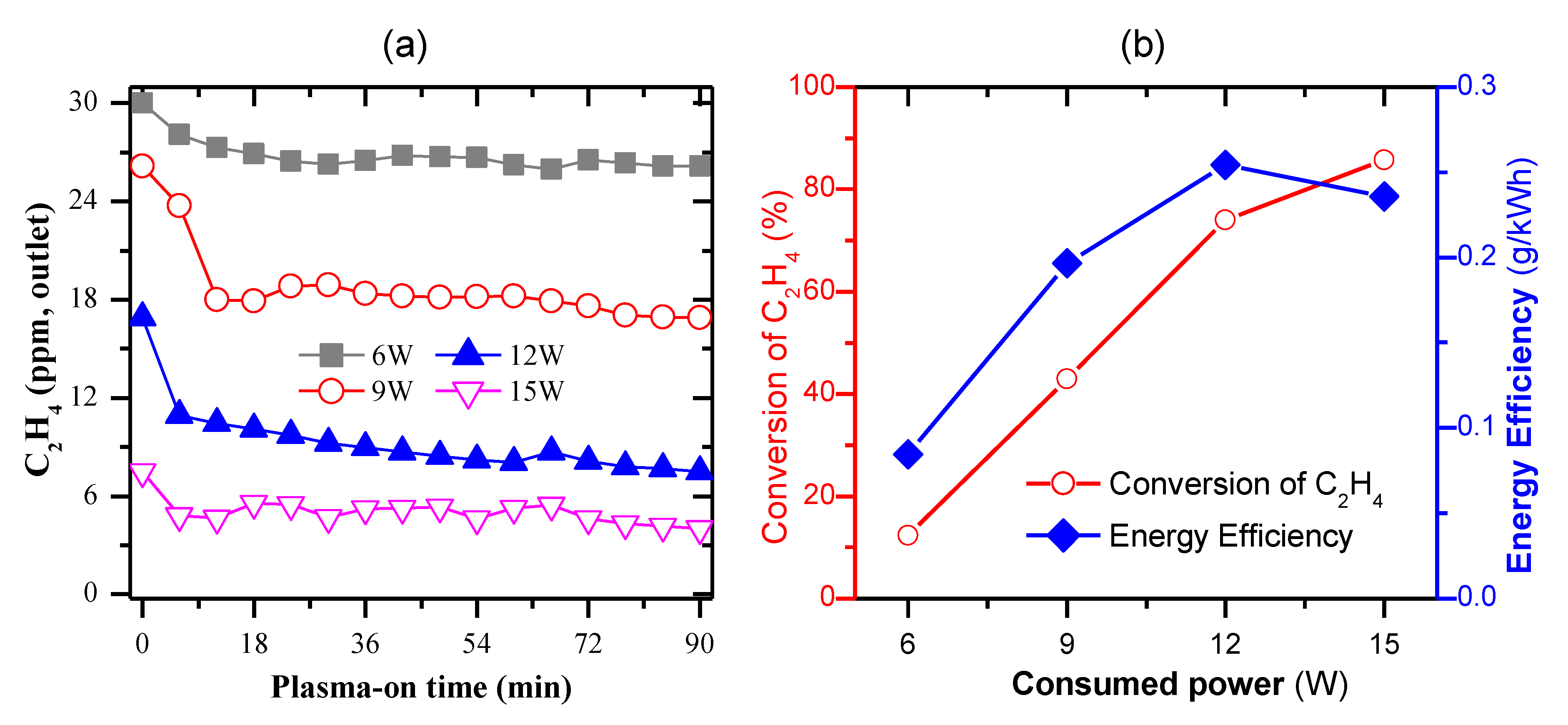
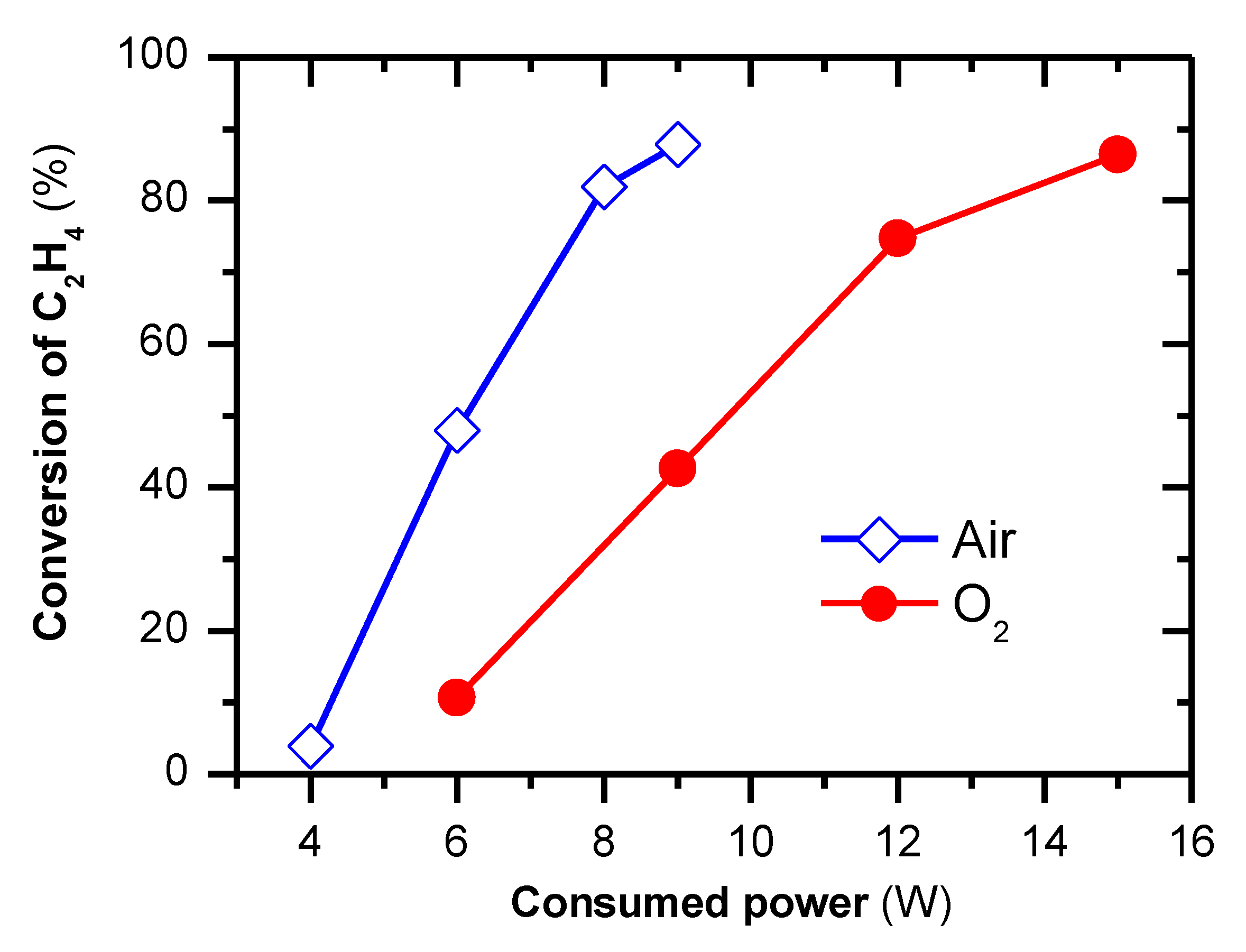
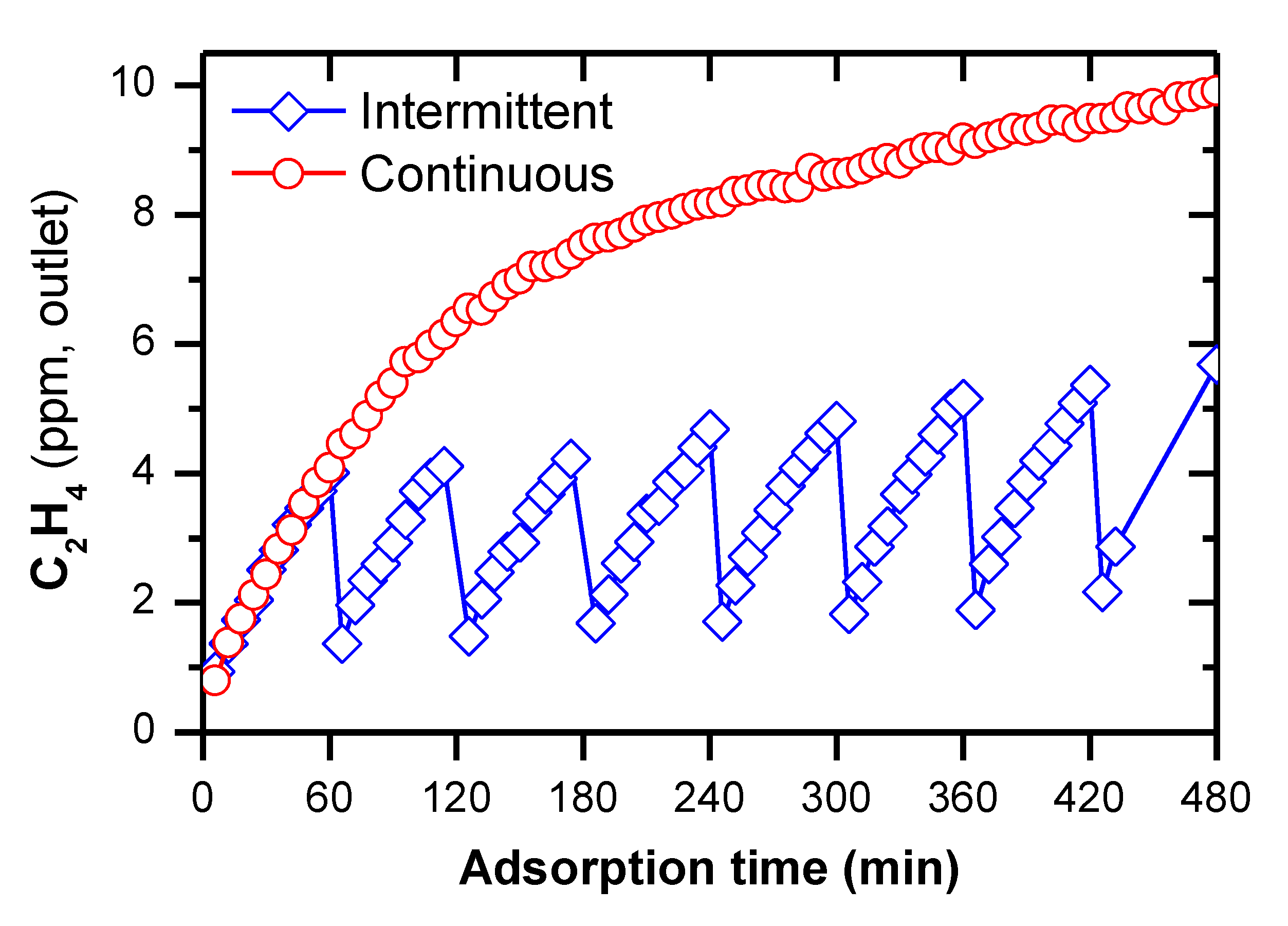
2.3. Removal of C2H4 under the Continuous Operation of Plasma-Catalytic Reaction
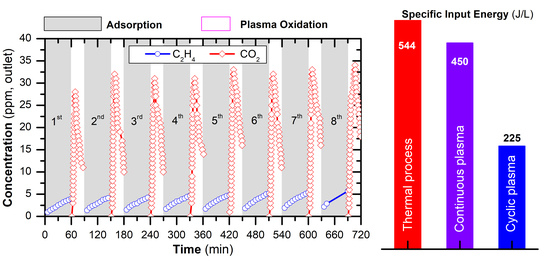
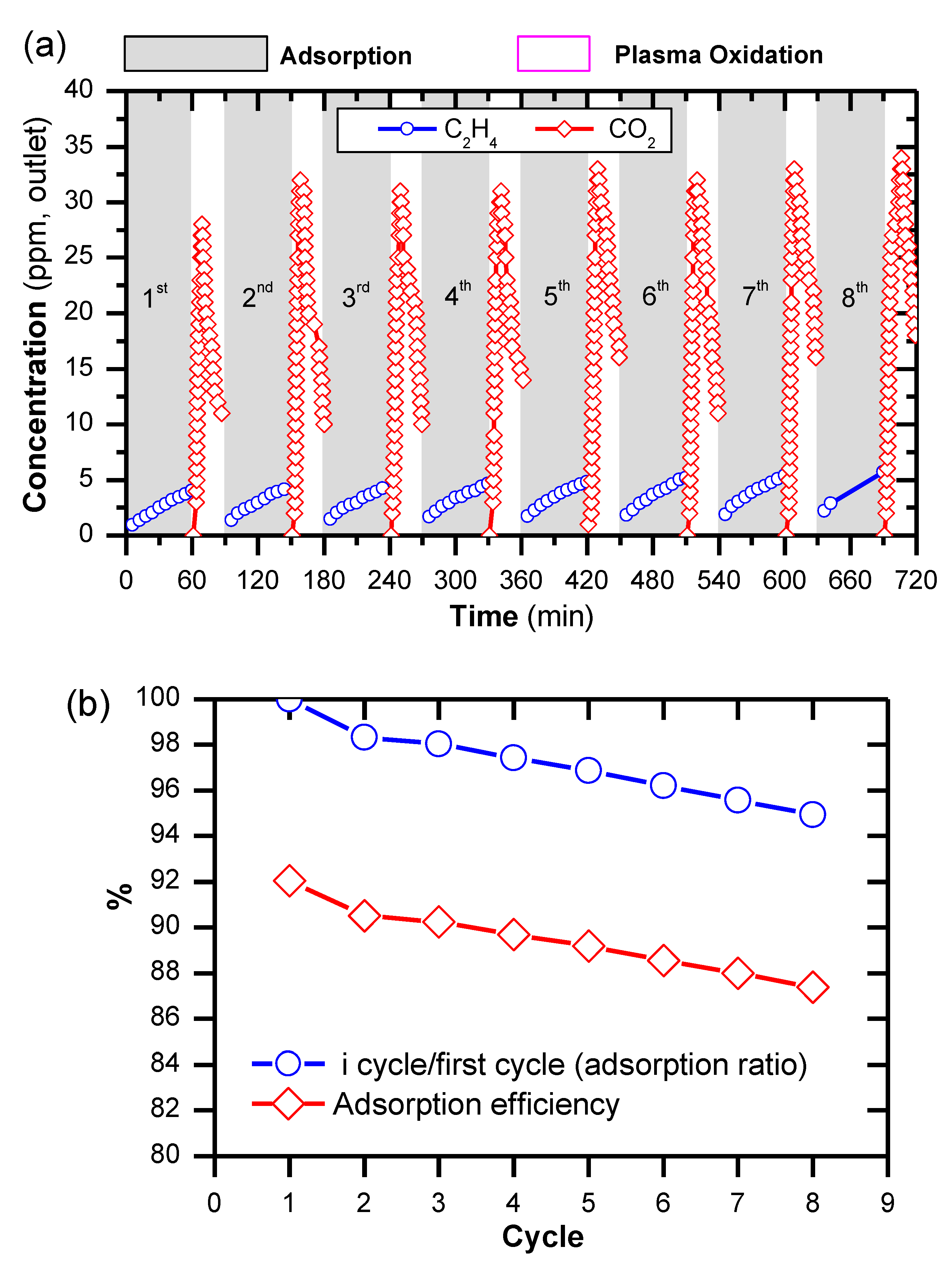
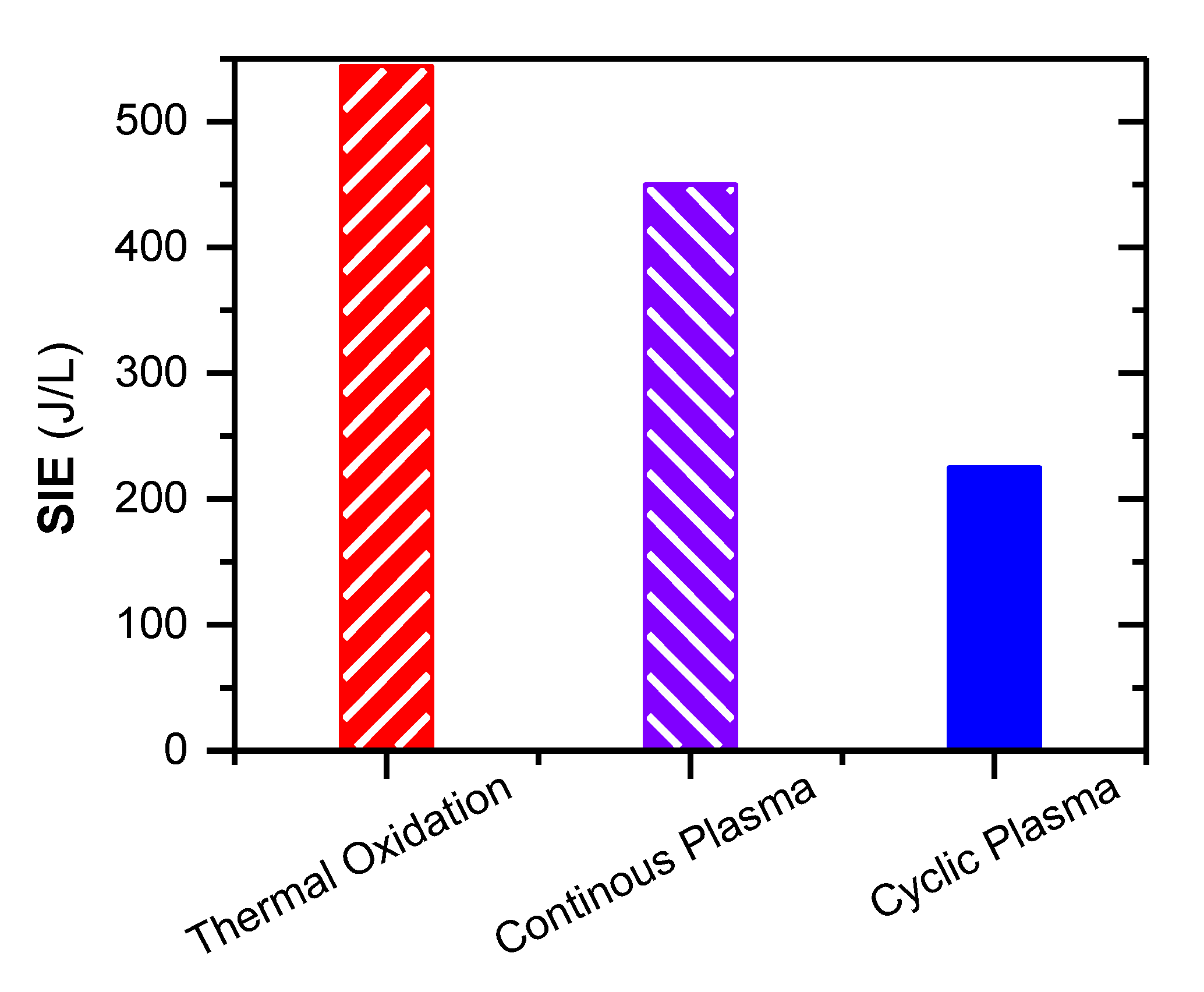
2.4. Removal of C2H4 by a Cyclic Adsorption-Plasma Oxidation Process
3. Materials and Methods
3.1. Synthesis of Pd/ZSM-5
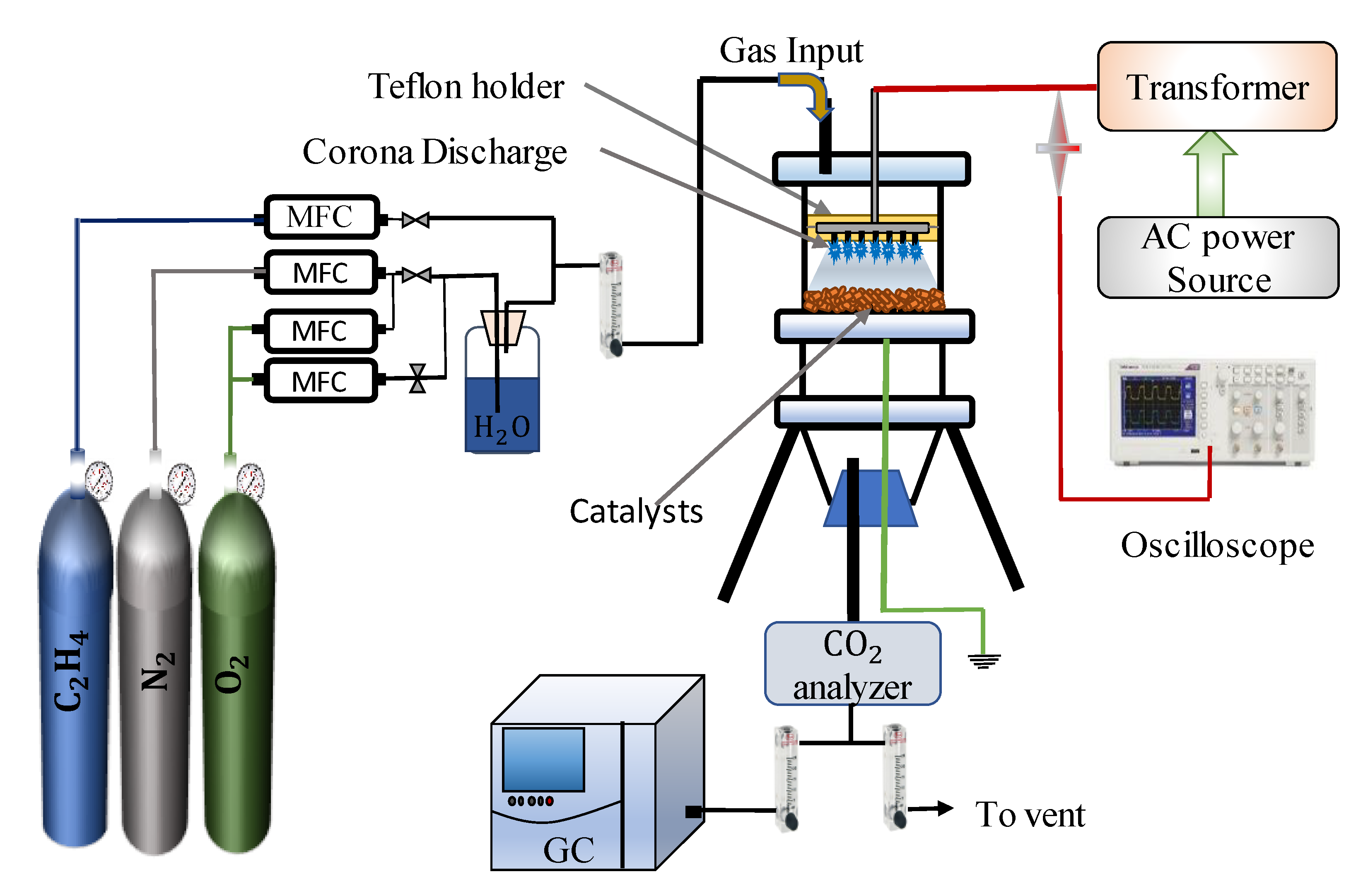
3.2. Experimental Setup
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gandhi, M.S.; Ananth, A.; Mok, Y.S.; Song, J.I.; Park, K.H. Time dependence of ethylene decomposition and byproducts formation in a continuous flow dielectric-packed plasma reactor. Chemosphere 2013, 91, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Keller, N.; Ducamp, M.N.; Robert, D.; Keller, V. Ethylene removal and fresh product storage: A challenge at the frontiers of chemistry. Toward an approach by photocatalytic oxidation. Chem. Rev. 2013, 113, 5029–5070. [Google Scholar] [CrossRef] [PubMed]
- El Blidi, A.; Rigal, L.; Malmary, G.; Molinier, J.; Torres, L. Ethylene removal for long term conservation of fruits and vegetables. Food Qual. Prefer. 1993, 4, 119–126. [Google Scholar] [CrossRef]
- Oka, A.; Motodate, T.; Takahashi, K.; Takaki, K.; Koide, S. Influence of oxygen concentration on ethylene removal using dielectric barrier discharge. Jpn. J. Appl. Phys. 2015, 4, 5882. [Google Scholar]
- Trinh, Q.H.; Gandhi, M.S.; Mok, Y.S. Adsorption and plasma-catalytic oxidation of acetone over zeolite-supported silver catalyst. Jpn. J. Appl. Phys. 2014, 54, 01AG04. [Google Scholar] [CrossRef]
- Trinh, Q.H.; Mok, Y.S. Effect of the adsorbent/catalyst preparation method and plasma reactor configuration on the removal of dilute ethylene from air stream. Catal. Today 2015, 256, 170–177. [Google Scholar] [CrossRef]
- Trinh, Q.H.; Lee, S.B.; Mok, Y.S. Removal of ethylene from air stream by adsorption and plasma-catalytic oxidation using silver-based bimetallic catalysts supported on zeolite. J. Hazard. Mater. 2015, 285, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Liu, H.; He, C.; Shen, Z.; Wang, T. Synergistic effects and mechanism of a non-thermal plasma catalysis system in volatile organic compound removal: A review. Catal. Sci. Technol. 2018, 8, 936–954. [Google Scholar] [CrossRef]
- Cools, P.; De Geyter, N.; Morent, R. Plasma-Catalytic Removal of VOCs. In Plasma Catalysis: Fundamentals and Applications; Tu, X., Whitehead, J.C., Nozaki, T., Eds.; Springer: Cham, Switzerland, 2019; pp. 145–180. ISBN 978-3-030-05189-1. [Google Scholar]
- Vandenbroucke, A.M.; Morent, R.; De Geyter, N.; Leys, C. Non-thermal plasmas for non-catalytic and catalytic VOC abatement. J. Hazard. Mater. 2011, 195, 30–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Jo, J.O.; Huynh, D.L.; Mongre, R.K.; Ghosh, M.; Singh, A.K.; Lee, S.B.; Mok, Y.S.; Hyuk, P.; Jeong, D.K. Growth-inducing effects of argon plasma on soybean sprouts via the regulation of demethylation levels of energy metabolism-related genes. Sci. Rep. 2017, 7, 41917. [Google Scholar] [CrossRef] [PubMed]
- Fylladitakis, E.D.; Theodoridis, M.P.; Moronis, A.X. Review on the history, research, and applications of electrohydrodynamics. IEEE Trans. Plasma Sci. 2014, 42, 358–375. [Google Scholar] [CrossRef]
- Trinh, Q.H.; Kim, S.H.; Mok, Y.S. Removal of dilute nitrous oxide from gas streams using a cyclic zeolite adsorption-plasma decomposition process. Chem. Eng. J. 2016, 302, 12–22. [Google Scholar] [CrossRef]
- Nguyen, D.B.; Nguyen, V.T.; Heo, I.J.; Mok, Y.S. Removal of NOx by selective catalytic reduction coupled with plasma under temperature fluctuation condition. J. Ind. Eng. Chem. 2019, 72, 400–407. [Google Scholar] [CrossRef]
- Mizuno, A.; Craven, M. Plasma Catalysis Systems. In Plasma Catalysis: Fundamentals and Applications; Tu, X., Whitehead, J.C., Nozaki, T., Eds.; Springer: Cham, Switzerland, 2019; pp. 21–46. ISBN 978-3-030-05189-1. [Google Scholar]
- Tendero, C.; Tixier, C.; Tristant, P.; Desmaison, J.; Leprince, P. Atmospheric pressure plasmas: A review. Spectrochim. Acta Part B At. Spectrosc. 2006, 61, 2–30. [Google Scholar] [CrossRef]
- Kim, K.N.; Lee, S.M.; Mishra, A.; Yeom, G.Y. Atmospheric pressure plasmas for surface modification of flexible and printed electronic devices: A review. Thin Solid Films 2016, 598, 315–334. [Google Scholar] [CrossRef]
- Mok, Y.S.; Kim, S.G.; Ba Nguyen, D.; Trinh, Q.H.; Lee, H.W.; Kim, S.B. Plasma-catalytic oxidation of ethylene over zeolite-supported catalysts to improve the storage stability of agricultural products. Catal. Today 2019, 337, 208–215. [Google Scholar] [CrossRef]
- Mok, Y.S.; Kim, S.G.; Nguyen, D.B.; Trinh, Q.H.; Lee, H.W.; Kim, S.B. Removal of dilute ethylene using repetitive cycles of adsorption and plasma-catalytic oxidation over Pd/ZSM-5 catalyst. J. Phys. D Appl. Phys. 2020. Submitted Manuscript. [Google Scholar]
- Nguyen, D.B.; Lee, W.G. Effects of ambient gas on cold atmospheric plasma discharge in the decomposition of trifluoromethane. RSC Adv. 2016, 6, 26505–26513. [Google Scholar] [CrossRef]
- Poling, B.E.; Thomson, G.H.; Friend, D.G.; Rowley, R.L.; Wilding, W.V. Section 2: Physical and Chemical Data. In Perry’s Chemical Engineers’ Handbook; The McGraw-Hill Companies, Inc.: New York, NY, USA, 2007; ISBN 0071542094. [Google Scholar]
- Kucharczyk, B.; Szczygieł, B.; Chȩcmanowski, J. The effect of catalyst precursors and conditions of preparing Pt and Pd-Pt catalysts on their activity in the oxidation of hexane. Open Chem. 2017, 15, 182–188. [Google Scholar] [CrossRef] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saud, S.; Nguyen, D.B.; Kim, S.-G.; Lee, H.W.; Kim, S.B.; Mok, Y.S. Improvement of Ethylene Removal Performance by Adsorption/Oxidation in a Pin-Type Corona Discharge Coupled with Pd/ZSM-5 Catalyst. Catalysts 2020, 10, 133. https://doi.org/10.3390/catal10010133
Saud S, Nguyen DB, Kim S-G, Lee HW, Kim SB, Mok YS. Improvement of Ethylene Removal Performance by Adsorption/Oxidation in a Pin-Type Corona Discharge Coupled with Pd/ZSM-5 Catalyst. Catalysts. 2020; 10(1):133. https://doi.org/10.3390/catal10010133
Chicago/Turabian StyleSaud, Shirjana, Duc Ba Nguyen, Seung-Geon Kim, Ho Won Lee, Seong Bong Kim, and Young Sun Mok. 2020. "Improvement of Ethylene Removal Performance by Adsorption/Oxidation in a Pin-Type Corona Discharge Coupled with Pd/ZSM-5 Catalyst" Catalysts 10, no. 1: 133. https://doi.org/10.3390/catal10010133