Sucrose-Assisted Solution Combustion Synthesis of Doped Strontium Ferrate Perovskite-Type Electrocatalysts: Primary Role of the Secondary Fuel
Abstract
:1. Introduction
2. Results and Discussion
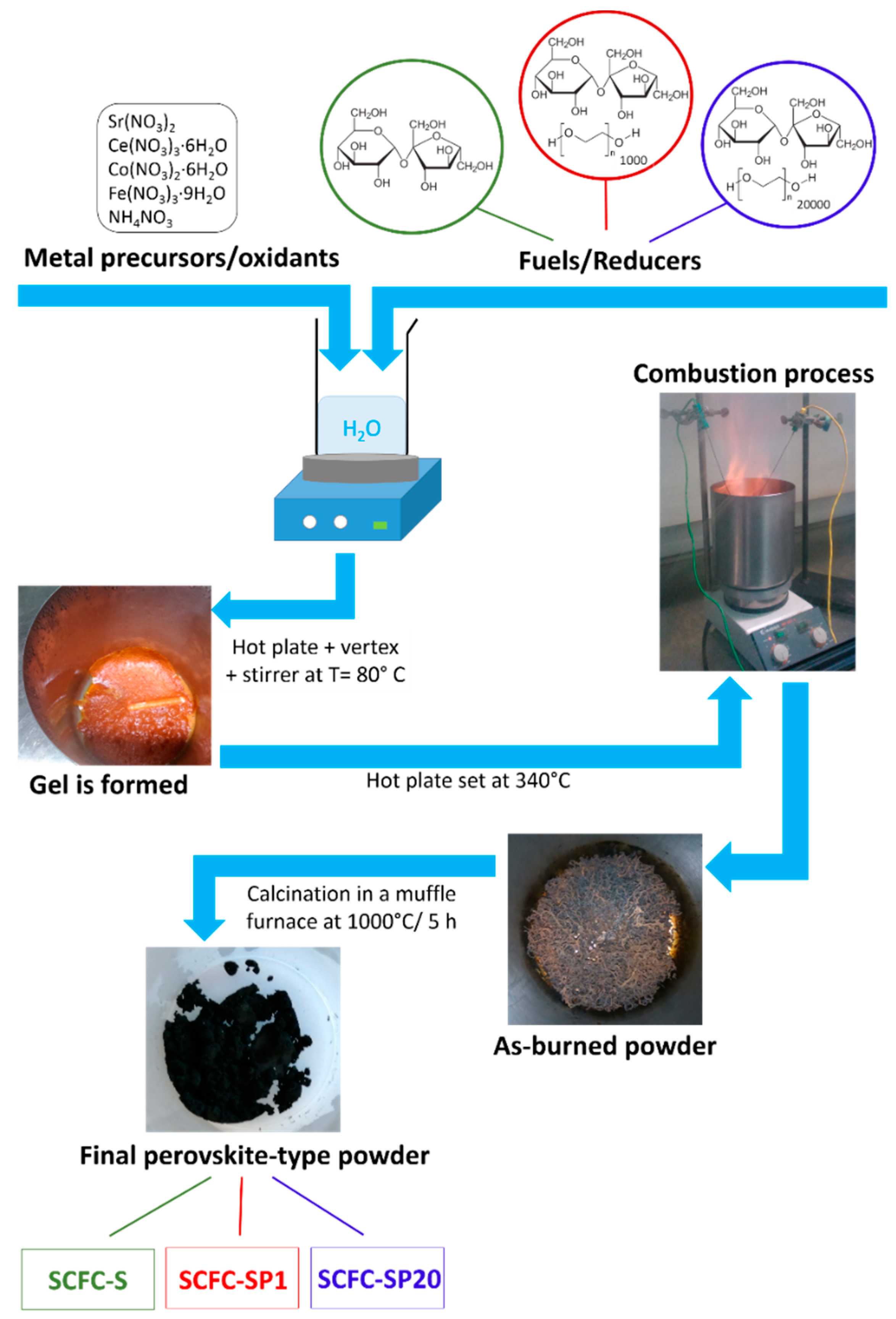
2.1. The Combustion Process
2.2. Structure and Microstructure of the Powders
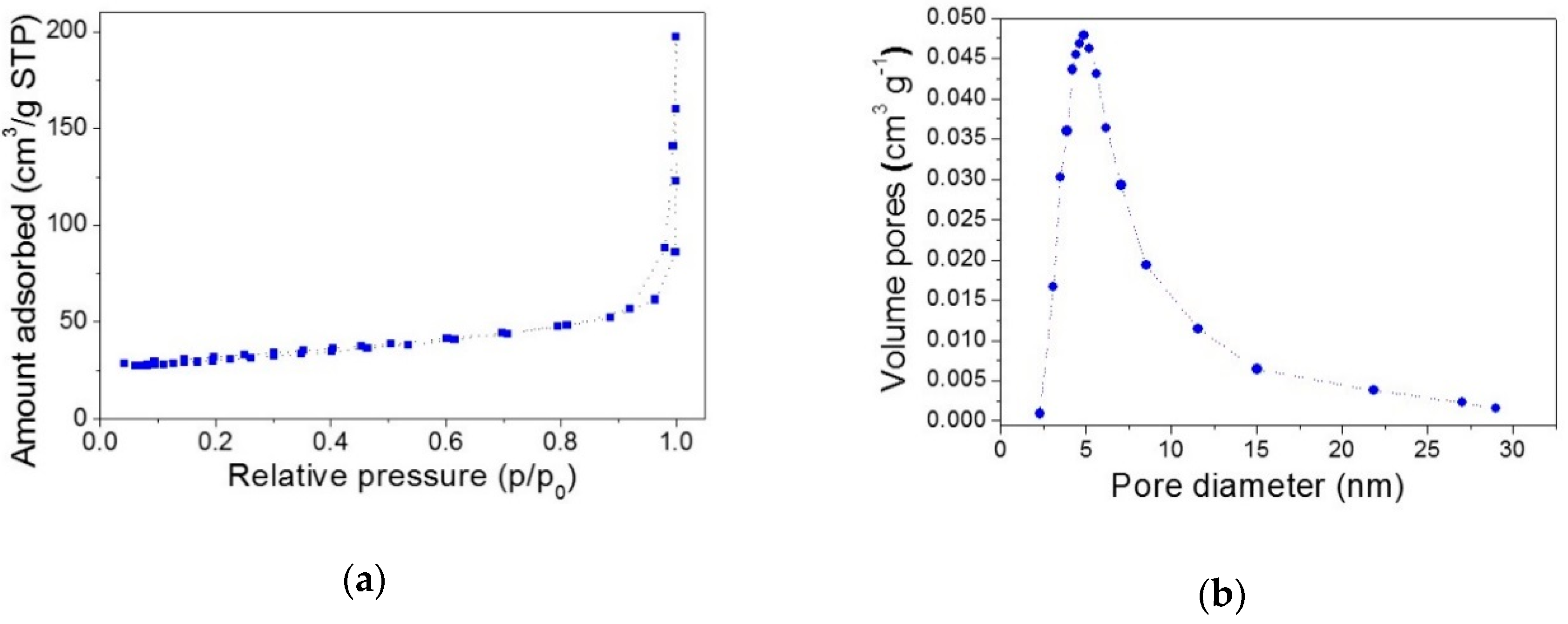
2.3. Textural Properties of the Powders
2.4. Morphological Properties of the Powders
2.5. Redox Properties of the Powders
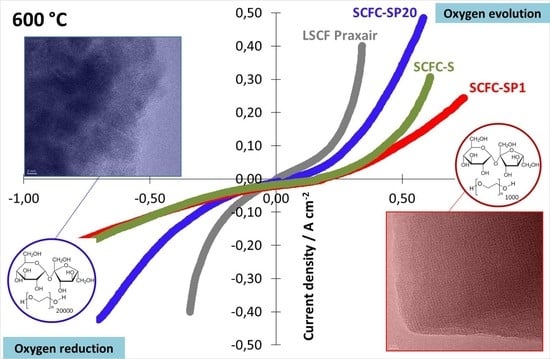
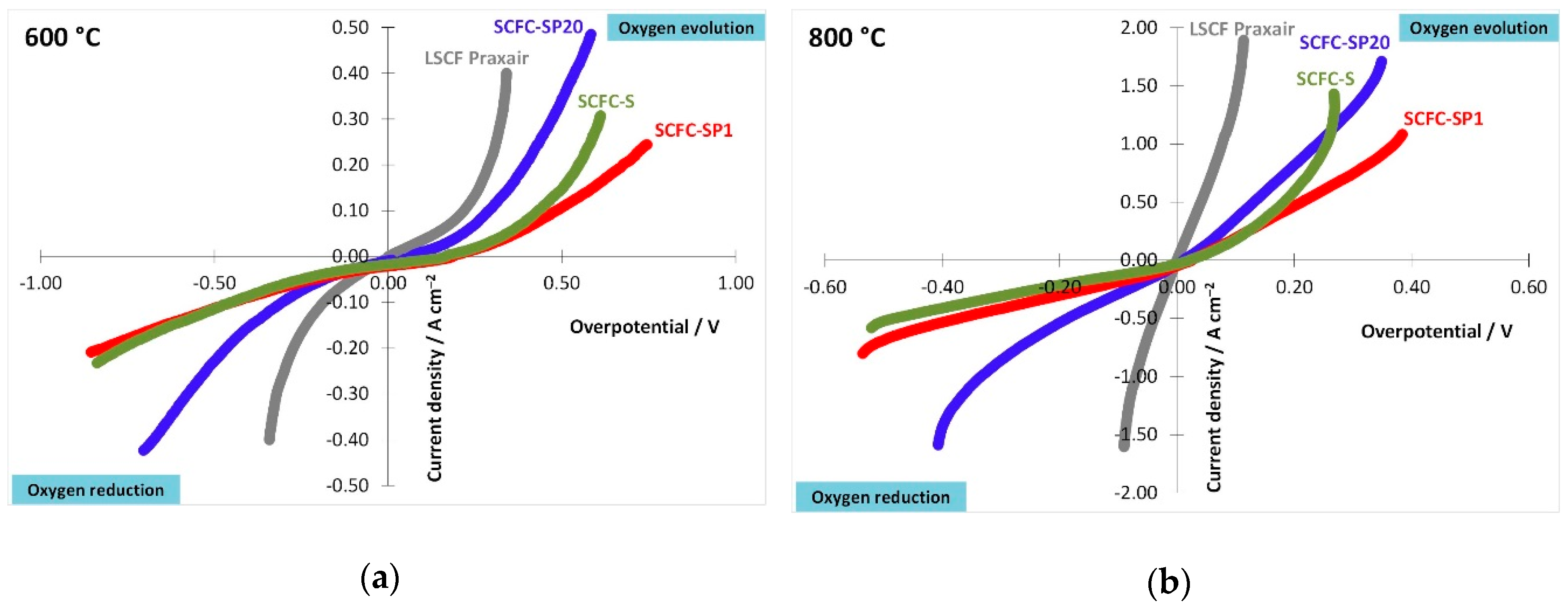
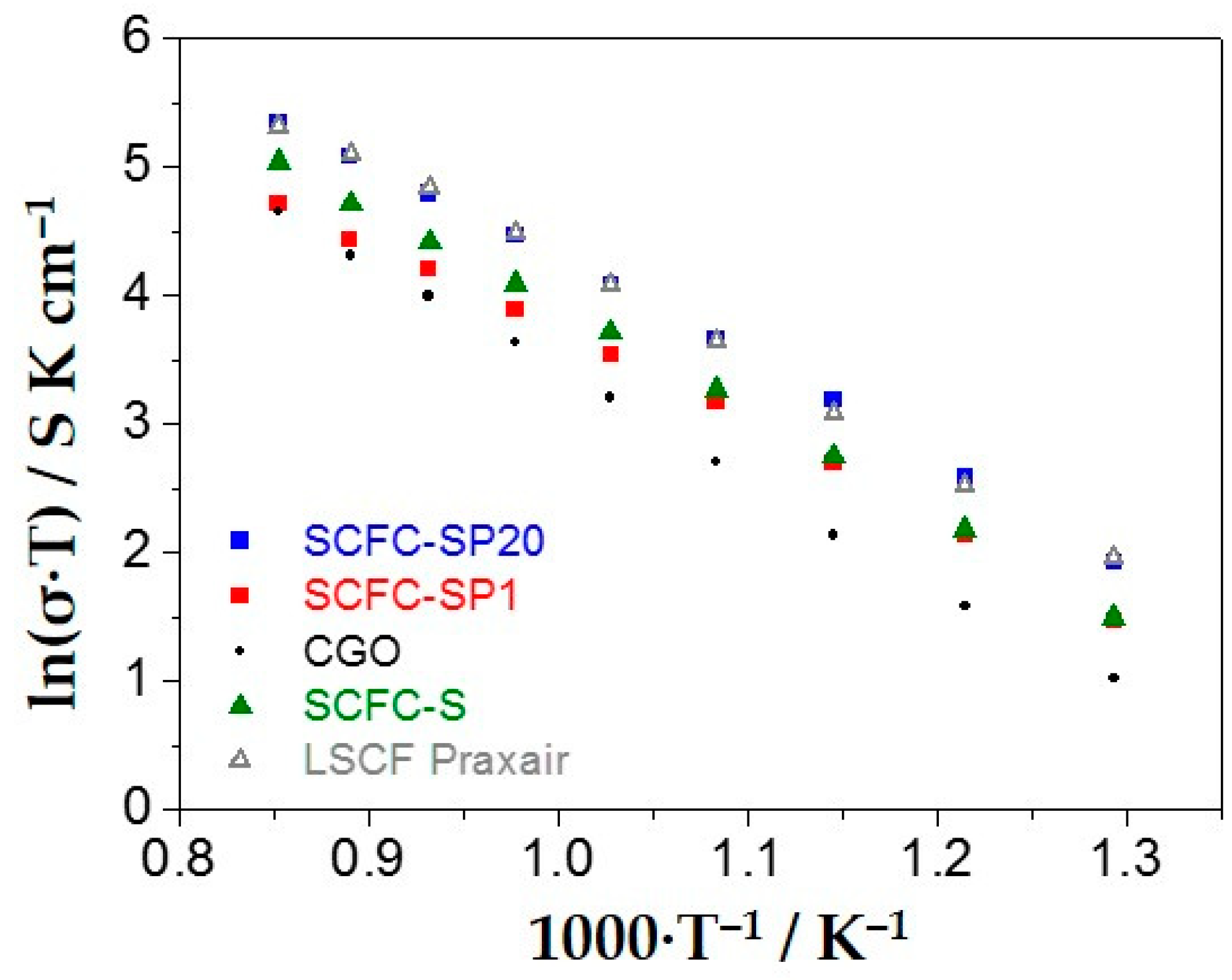
2.6. Electrochemical Properties of the Powders Deposited on the Electrolyte in Half-Cell Configuration
3. Materials and Methods
3.1. Powders Preparation
3.2. Physicochemical Characterization
3.3. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Varma, A.; Mukasyan, A.S.; Rogachev, A.S.; Manukyan, K.V. Solution Combustion Synthesis of Nanoscale Materials. Chem. Rev. 2016, 116, 14493–14586. [Google Scholar] [CrossRef]
- Deganello, F.; Tyagi, A.K. Solution combustion synthesis, energy and environment: Best parameters for better materials. Prog. Cryst. Growth Charact. Mater. 2018, 64, 23–61. [Google Scholar] [CrossRef]
- Deganello, F.; Testa, M.L.; La Parola, V.; Longo, A.; Tavares, A.C. LaFeO3-based nanopowders prepared by a soft-hard templating approach: The effect of silica texture. J. Mater. Chem. A 2014, 2, 8438–8447. [Google Scholar] [CrossRef]
- Thoda, O.; Xanthopoulou, G.; Vekinis, G.; Chroneos, A. Review of Recent Studies on Solution Combustion Synthesis of Nanostructured Catalysts. Adv. Eng. Mater. 2018, 20, 1–30. [Google Scholar] [CrossRef]
- Deganello, F. Nanomaterials for environmental and energy applications prepared by solution combustion based-methodologies: Role of the fuel. Mater. Today Proc. 2017, 4, 5507–5516. [Google Scholar] [CrossRef]
- Podbolotov, K.B.; Khort, A.A.; Tarasov, A.B.; Trusov, G.V.; Roslyakov, S.I.; Mukasyan, A.S. Solution Combustion Synthesis of Copper Nanopowders: The Fuel Effect. Combust. Sci. Technol. 2017, 189, 1878–1890. [Google Scholar] [CrossRef]
- Aliotta, C.; Liotta, L.F.; La Parola, V.; Martorana, A.; Muccillo, E.N.S.; Muccillo, R.; Deganello, F. Ceria-based electrolytes prepared by solution combustion synthesis: The role of fuel on the materials properties. Appl. Catal. B Environ. 2016, 197, 14–22. [Google Scholar] [CrossRef]
- Amarilla, J.M.; Rojas, R.M.; Rojo, J.M. Understanding the sucrose-assisted combustion method: Effects of the atmosphere and fuel amount on the synthesis and electrochemical performances of LiNi0.5Mn1.5O4 spinel. J. Power Sources 2011, 196, 5951–5959. [Google Scholar] [CrossRef]
- Lakshmi, R.; Velmurugan, V.; Sasikumar, S. Preparation and Phase Evolution of Wollastonite by Sol-Gel Combustion Method Using Sucrose as the Fuel. Combust. Sci. Technol. 2013, 185, 1777–1785. [Google Scholar] [CrossRef]
- Wang, B.; Zeng, L.; Huang, W.; Melkonyan, F.S.; Sheets, W.C.; Chi, L.; Bedzyk, M.J.; Marks, T.J.; Facchetti, A. Carbohydrate-Assisted Combustion Synthesis to Realize High-Performance Oxide Transistors. J. Am. Chem. Soc. 2016, 138, 7067–7074. [Google Scholar] [CrossRef]
- Da Conceião, L.; Silva, A.M.; Ribeiro, N.F.P.; Souza, M.M.V.M. Combustion synthesis of La0.7Sr0.3Co0.5Fe0.5O3 (LSCF) porous materials for application as cathode in IT-SOFC. Mater. Res. Bull. 2011, 46, 308–314. [Google Scholar] [CrossRef]
- Sihaib, Z.; Puleo, F.; Pantaleo, G.; La Parola, V.; Valverde, J.L.; Gil, S.; Liotta, L.F.; Fendler, A.G. The effect of citric acid concentration on the properties of LaMnO3 as a catalyst for hydrocarbon oxidation. Catalysts 2019, 9, 226. [Google Scholar] [CrossRef] [Green Version]
- Gabal, M.A.; Al-Juaid, A.A.; El-Rashed, S.; Hussein, M.A. Synthesis and characterization of nano-sized CoFe2O4 via facile methods: A comparative study. Mater. Res. Bull. 2017, 89, 68–78. [Google Scholar] [CrossRef]
- Mohan, E.H.; Siddhartha, V.; Gopalan, R.; Rao, T.N.; Rangappa, D. Urea and sucrose assisted combustion synthesis of LiFePO4/C nano-powder for lithium-ion battery cathode application. AIMS Mater. Sci. 2014, 1, 191–201. [Google Scholar] [CrossRef]
- Li, S.; Bergman, B.; Zhao, Z. Synthesis and characterization of lanthanum aluminate powders via a polymer complexing plus combustion route. Mater. Chem. Phys. 2012, 132, 309–315. [Google Scholar] [CrossRef]
- Chen, J.; Spear, S.K.; Huddleston, J.G.; Rogers, R.D. Polyethylene glycol and solutions of polyethylene glycol as green reaction media. Green Chem. 2005, 7, 64–82. [Google Scholar]
- El Khalfaouy, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Solution combustion synthesis of LiMnPO4/C cathode material: The effect of four fuel sources on the electrochemical performances. Int. J. Hydrogen Energy 2019, 44, 18272–18282. [Google Scholar] [CrossRef]
- Bu, S.; Jin, Z.; Liu, X.; Du, H.; Cheng, Z. Preparation and formation mechanism of porous TiO2 films using PEG and alcohol solvent as double-templates. J. Sol-Gel Sci. Technol. 2004, 30, 239–248. [Google Scholar] [CrossRef]
- Abdollahifar, M.; Huang, S.S.; Lin, Y.H.; Lin, Y.C.; Shih, B.Y.; Sheu, H.S.; Liao, Y.F.; Wu, N.L. High-performance carbon-coated ZnMn2O4 nanocrystallite supercapacitors with tailored microstructures enabled by a novel solution combustion method. J. Power Sources 2018, 378, 90–97. [Google Scholar] [CrossRef]
- Zeng, M.; Fang, Z.; Xu, C. Novel method of preparing microporous membrane by selective dissolution of chitosan/polyethylene glycol blend membrane. J. Appl. Polym. Sci. 2004, 91, 2840–2847. [Google Scholar] [CrossRef]
- Tarragó, D.P.; Malfatti, C.F.; de Sousa, V.C. Influence of fuel on morphology of LSM powders obtained by solution combustion synthesis. Powder Technol. 2015, 269, 481–487. [Google Scholar] [CrossRef]
- Deganello, F.; Liotta, L.F.; Marcì, G.; Fabbri, E.; Traversa, E. Strontium and iron-doped barium cobaltite prepared by solution combustion synthesis: Exploring a mixed-fuel approach for tailored intermediate temperature solid oxide fuel cell cathode materials. Mater. Renew. Sustain. Energy 2013, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Kalantari Bolaghi, Z.; Hasheminiasari, M.; Masoudpanah, S.M. Solution combustion synthesis of ZnO powders using mixture of fuels in closed system. Ceram. Int. 2018, 44, 12684–12690. [Google Scholar] [CrossRef]
- Giuliano, A.; Carpanese, M.P.; Clematis, D.; Boaro, M.; Pappacena, A.; Deganello, F.; Liotta, L.F.; Barbucci, A. Infiltration, overpotential and ageing effects on cathodes for solid oxide fuel cells: La0.6Sr0.4Co0.2Fe0.8O3-δ versus Ba0.5Sr0.5Co0.8Fe0.2O3-δ. J. Electrochem. Soc. 2017, 164, F3114–F3122. [Google Scholar] [CrossRef]
- Kamata, K. Perovskite oxide catalysts for liquid-phase organic reactions. Bull. Chem. Soc. Jpn. 2019, 92, 133–151. [Google Scholar] [CrossRef]
- Polo-Garzon, F.; Wu, Z. Acid-base catalysis over perovskites: A review. J. Mater. Chem. A 2018, 6, 2877–2894. [Google Scholar] [CrossRef]
- Shin, H.H.; Mullins, C.B.; Goodenough, J.B. Oxygen-Electrode Catalysis on Oxoperovskites at 700 °C versus 20 °C. Chem. Mater. 2018, 30, 629–635. [Google Scholar] [CrossRef]
- Li, C.; Soh, K.C.K.; Wu, P. Formability of ABO3 perovskites. J. Alloys Compd. 2004, 372, 40–48. [Google Scholar] [CrossRef]
- Sunarso, J.; Hashim, S.S.; Zhu, N.; Zhou, W. Perovskite oxides applications in high temperature oxygen separation, solid oxide fuel cell and membrane reactor: A review. Prog. Energy Combust. Sci. 2017, 61, 57–77. [Google Scholar] [CrossRef]
- Brett, D.J.L.; Atkinson, A.; Brandon, N.P.; Skinner, S.J. Intermediate temperature solid oxide fuel cells. Chem. Soc. Rev. 2008, 37, 1568–1578. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Hossain, S.; Azad, A.T.; Petra, P.M.I.; Begum, F.; Eriksson, S.G.; Azad, A.K. Nanomaterials for solid oxide fuel cells: A review. Renew. Sustain. Energy Rev. 2018, 82, 353–368. [Google Scholar] [CrossRef]
- Molenda, J.; Świerczek, K.; Zajac, W. Functional materials for the IT-SOFC. J. Power Sources 2007, 173, 657–670. [Google Scholar] [CrossRef]
- Clematis, D.; Barbucci, A.; Presto, S.; Viviani, M.; Carpanese, M.P. Electrocatalytic activity of perovskite-based cathodes for solid oxide fuel cells. Int. J. Hydrogen Energy 2019, 44, 6212–6222. [Google Scholar] [CrossRef]
- Lo Faro, M.; Aricò, A.S. Electrochemical behaviour of an all-perovskite-based intermediate temperature solid oxide fuel cell. Int. J. Hydrogen Energy 2013, 38, 14773–14778. [Google Scholar] [CrossRef]
- Li, Z.; Li, S.; Tseng, C.J.; Tao, S.; Xie, K. Redox-reversible perovskite ferrite cathode for high temperature solid oxide steam electrolyser. Electrochim. Acta 2017, 229, 48–54. [Google Scholar] [CrossRef]
- Su, C.; Wang, W.; Liu, M.; Tadé, M.O.; Shao, Z. Progress and Prospects in Symmetrical Solid Oxide Fuel Cells with Two Identical Electrodes. Adv. Energy Mater. 2015, 5, 1–19. [Google Scholar]
- Dos Santos-Gómez, L.; Compana, J.M.; Bruque, S.; Losilla, E.R.; Marrero-López, D. Symmetric electrodes for solid oxide fuel cells based on Zr-doped SrFeO3-δ. J. Power Sources 2015, 279, 419–427. [Google Scholar] [CrossRef]
- Jiang, S.P. Development of lanthanum strontium cobalt ferrite perovskite electrodes of solid oxide fuel cells–A review. Int. J. Hydrogen Energy 2019, 44, 7448–7493. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Jin, C.; Li, C.; Li, X.; Li, Y.; Yang, R.; Liu, M. Enhanced overall water electrolysis on a bifunctional perovskite oxide through interfacial engineering. Electrochim. Acta 2019, 318, 120–129. [Google Scholar] [CrossRef]
- Deganello, F.; Liotta, L.F.; Longo, A.; Casaletto, M.P.; Scopelliti, M. Cerium effect on the phase structure, phase stability and redox properties of Ce-doped strontium ferrates. J. Solid State Chem. 2006, 179, 3406–3419. [Google Scholar] [CrossRef]
- Ikeda, H.; Nikata, S.; Hirakawa, E.; Tsuchida, A.; Miura, N. Oxygen sorption/desorption behavior and crystal structural change for SrFeO3-δ. Chem. Eng. Sci. 2016, 147, 166–172. [Google Scholar] [CrossRef]
- Jiang, S.; Sunarso, J.; Zhou, W.; Shen, J.; Ran, R.; Shao, Z. Cobalt-free SrNbxFe1-xO3-δ (x = 0.05, 0.1 and 0.2) perovskite cathodes for intermediate temperature solid oxide fuel cells. J. Power Sources 2015, 298, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Ropero, A.J.; Porras-Vázquez, J.M.; Cabeza, A.; Slater, P.R.; Marrero-López, D.; Losilla, E.R. High valence transition metal doped strontium ferrites for electrode materials in symmetrical SOFCs. J. Power Sources 2014, 249, 405–413. [Google Scholar] [CrossRef] [Green Version]
- Deganello, F.; Liotta, L.F.; Leonardi, S.G.; Neri, G. Electrochemical properties of Ce-doped SrFeO3 perovskites-modified electrodes towards hydrogen peroxide oxidation. Electrochim. Acta 2016, 190, 939–947. [Google Scholar] [CrossRef]
- Tummino, M.L.; Laurenti, E.; Deganello, F.; Bianco Prevot, A.; Magnacca, G. Revisiting the catalytic activity of a doped SrFeO3 for water pollutants removal: Effect of light and temperature. Appl. Catal. B Environ. 2017, 207, 174–181. [Google Scholar] [CrossRef]
- Markov, A.A.; Nikitin, S.S.; Leonidov, I.A.; Patrakeev, M.V. Oxygen and electron transport in Ce0.1Sr0.9FeO3−δ. Solid State Ion. 2020, 344, 115131. [Google Scholar] [CrossRef]
- Istomin, S.Y.; Antipov, E. V Cathode materials based on perovskite-like transition metal oxides for intermediate temperature solid oxide fuel cells. Russ. Chem. Rev. 2013, 82, 686–700. [Google Scholar] [CrossRef]
- Choi, H.; Fuller, A.; Davis, J.; Wielgus, C.; Ozkan, U.S. Ce-doped strontium cobalt ferrite perovskites as cathode catalysts for solid oxide fuel cells: Effect of dopant concentration. Appl. Catal. B Environ. 2012, 127, 336–341. [Google Scholar] [CrossRef]
- Wiik, K.; Aasland, S.; Hansen, H.; Tangen, I.; Ødegård, R. Oxygen permeation in the system SrFeO3−x–SrCoO3−y. Solid State Ion. 2002, 152, 675–680. [Google Scholar] [CrossRef]
- Choi, H.; Fuller, A.; Dogu, D.; Binkley, K.E.; Davis, J.; Co, A.; Ozkan, U.S. Effect of Ce Doping on the Performance and Stability of Strontium Cobalt Ferrite Perovskites as SOFC Anode Catalysts. Top. Catal. 2015, 58, 359–374. [Google Scholar] [CrossRef]
- Gwon, O.; Yoo, S.; Shin, J.; Kim, G. Optimization of La1-xSrxCoO3-δ perovskite cathodes for intermediate temperature solid oxide fuel cells through the analysis of crystal structure and electrical properties. Int. J. Hydrogen Energy 2014, 39, 20806–20811. [Google Scholar] [CrossRef]
- Xu, X.; Pan, Y.; Zhou, W.; Chen, Y.; Zhang, Z.; Shao, Z. Toward Enhanced Oxygen Evolution on Perovskite Oxides Synthesized from Different Approaches: A Case Study of Ba0.5Sr0.5Co0.8Fe0.2O3−δ. Electrochim. Acta 2016, 219, 553–559. [Google Scholar] [CrossRef]
- Li, S.; Hao, X.; Abudula, A.; Guan, G. Nanostructured Co-based bifunctional electrocatalysts for energy conversion and storage: Current status and perspectives. J. Mater. Chem. A 2019, 7, 18674–18707. [Google Scholar] [CrossRef]
- Baharuddin, N.A.; Muchtar, A.; Somalu, M.R. Short review on cobalt-free cathodes for solid oxide fuel cells. Int. J. Hydrogen Energy 2017, 42, 9149–9155. [Google Scholar] [CrossRef]
- Gao, L.; Zhu, M.; Li, Q.; Sun, L.; Zhao, H.; Grenier, J.C. Electrode properties of Cu-doped Bi0.5Sr0.5FeO3−δ cobalt-free perovskite as cathode for intermediate-temperature solid oxide fuel cells. J. Alloys Compd. 2017, 700, 29–36. [Google Scholar] [CrossRef]
- Trofimenko, N.E.; Ullmann, H. Oxygen stoichiometry and mixed ionic-electronic conductivity of Sr1-aCeaFe1-bCobO3-x perovskite-type oxides. J. Eur. Ceram. Soc. 2000, 20, 1241–1250. [Google Scholar] [CrossRef]
- Hansen, K.K. Evaluation of LSF based SOFC cathodes using cone-shaped electrodes and EIS. Solid State Ion. 2020, 344, 115096. [Google Scholar] [CrossRef]
- Bogan, M.J.; Agnes, G.R. Poly (ethylene glycol) doubly and singly cationized by different alkali metal ions: Relative cation affinities and cation-dependent resolution in a quadrupole ion trap mass spectrometer. J. Am. Soc. Mass Spectrom. 2002, 13, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Nikolić, A.S.; Jović, N.; Rogan, J.; Kremenović, A.; Ristić, M.; Meden, A.; Antić, B. Carboxylic acids and polyethylene glycol assisted synthesis of nanocrystalline nickel ferrites. Ceram. Int. 2013, 39, 6681–6688. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, H.; Sun, C.; Liu, L.; Alonso, J.A.; Fernández-Díaz, M.T.; Chen, L. Insight into the structure and functional application of the Sr0.95Ce0.05CoO3-δ cathode for solid oxide fuel cells. Inorg. Chem. 2015, 54, 3477–3484. [Google Scholar] [CrossRef]
- Tiwari, S.; Balasubramanian, N.; Biring, S.; Sen, S. Synthesis, structure and characterization of Co doped CeO2 nanoparticles. IOP Conf. Ser. Mater. Sci. Eng. 2018, 396, 012035. [Google Scholar] [CrossRef]
- ahsanzadeh-Vadeqani, M.; Razavi, R.S.; Barekat, M.; Borhani, G.H.; Mishra, A.K. Preparation of yttria nanopowders for use in transparent ceramics by dry ball-milling technique. J. Eur. Ceram. Soc. 2017, 37, 2169–2177. [Google Scholar] [CrossRef]
- Carvalho, M.D.; Ferreira, L.P.; Colomer, M.T.; Gaczyński, P.; Cruz, M.M.; Waerenborgh, J.C.; Godinho, M. Magnetic studies on Sr0.8Ce0.1Fe0.7Co0.3O3-δ perovskite. Solid State Sci. 2006, 8, 444–449. [Google Scholar] [CrossRef]
- Deganello, F.; Tummino, M.L.; Calabrese, C.; Testa, M.L.; Avetta, P.; Fabbri, D.; Prevot, A.B.; Montoneri, E.; Magnacca, G. A new, sustainable LaFeO3 material prepared from biowaste-sourced soluble substances. New J. Chem. 2015, 39, 877–885. [Google Scholar] [CrossRef]
- Magnacca, G.; Spezzati, G.; Deganello, F.; Testa, M.L. A new in situ methodology for the quantification of the oxygen storage potential in perovskite-type materials. RSC Adv. 2013, 3, 26352–26360. [Google Scholar] [CrossRef]
- Royer, S.; Bérubé, F.; Kaliaguine, S. Effect of the synthesis conditions on the redox and catalytic properties in oxidation reactions of LaCo1-xFexO3. Appl. Catal. A Gen. 2005, 282, 273–284. [Google Scholar] [CrossRef]
- Lan, R.; Cowin, P.I.; Sengodan, S.; Tao, S. A perovskite oxide with high conductivities in both air and reducing atmosphere for use as electrode for solid oxide fuel cells. Sci. Rep. 2016, 6, 4–11. [Google Scholar] [CrossRef] [Green Version]
- Ganopadhyay, S.; Masunov, A.E.; Inerbaev, T.; Mesit, J.; Guha, R.K.; Sleiti, A.K.; Kapat, J.S. Understanding oxygen vacancy migration and clustering in barium strontiumcobalt iron oxide. Solid State Ion. 2010, 181, 1067–1073. [Google Scholar] [CrossRef]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 24, 210–213. [Google Scholar] [CrossRef] [Green Version]
- Toby, B.H. R factors in Rietveld analysis: How good is good enough? Powder Diffr. 2006, 21, 67–70. [Google Scholar] [CrossRef] [Green Version]
- Jansen, E.; Schaefer, W.; Will, G. R values in analysis of powder diffraction data using Rietveld refinement. J. Appl. Crystallogr. 1994, 27, 492–496. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 2, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Dunyushkina, L.A.; Lu, Y.; Adler, S.B. Microelectrode array for isolation of electrode polarization on planar solid electrolytes. J. Electrochem. Soc. 2005, 152. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tummino, M.L.; Liotta, L.F.; Magnacca, G.; Lo Faro, M.; Trocino, S.; Campagna Zignani, S.; Aricò, A.S.; Deganello, F. Sucrose-Assisted Solution Combustion Synthesis of Doped Strontium Ferrate Perovskite-Type Electrocatalysts: Primary Role of the Secondary Fuel. Catalysts 2020, 10, 134. https://doi.org/10.3390/catal10010134
Tummino ML, Liotta LF, Magnacca G, Lo Faro M, Trocino S, Campagna Zignani S, Aricò AS, Deganello F. Sucrose-Assisted Solution Combustion Synthesis of Doped Strontium Ferrate Perovskite-Type Electrocatalysts: Primary Role of the Secondary Fuel. Catalysts. 2020; 10(1):134. https://doi.org/10.3390/catal10010134
Chicago/Turabian StyleTummino, Maria Laura, Leonarda Francesca Liotta, Giuliana Magnacca, Massimiliano Lo Faro, Stefano Trocino, Sabrina Campagna Zignani, Antonino Salvatore Aricò, and Francesca Deganello. 2020. "Sucrose-Assisted Solution Combustion Synthesis of Doped Strontium Ferrate Perovskite-Type Electrocatalysts: Primary Role of the Secondary Fuel" Catalysts 10, no. 1: 134. https://doi.org/10.3390/catal10010134