The Use of Tunable Optical Absorption Plasmonic Au and Ag Decorated TiO2 Structures as Efficient Visible Light Photocatalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Composition Analysis
2.2. Morphology Study
2.3. Optical Properties
2.4. Specific Surface Area Test
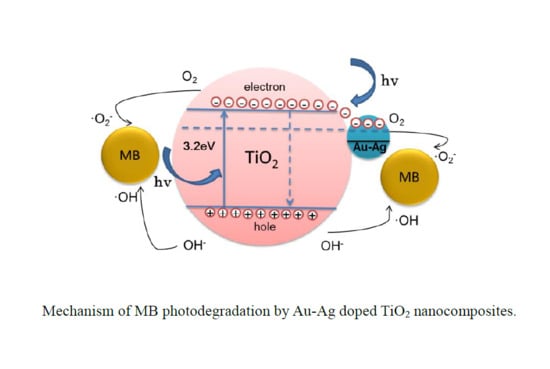
2.5. Photocatalytic Assessment
3. Experimental
3.1. Chemicals
3.2. Synthesis of TiO2 Nanoparticles
3.3. Synthesis of Au-Ag/TiO2 Composites
3.4. Characterizations
3.5. Photocatalytic Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fujishima, A.; Zhang, X.; Tryk, D. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Yao, W.F.; Zhang, B.; Huang, C.P.; Ma, C.; Song, X.L.; Xu, Q.J. Synthesis and characterization of high efficiency and stable Ag3PO4/TiO2 visible light photocatalyst for the degradation of methylene blue and rhodamine B solutions. J. Mater. Chem. 2012, 22, 4050–4055. [Google Scholar] [CrossRef]
- Babu, S.G.; Vinoth, R.; Kumar, D.P.; Shankar, M.V.; Chou, H.L.; Vinodgopal, K.; Neppolian, B. Influence of electron storing, transferring and shuttling assets of reduced graphene oxide at the interfacial copper doped TiO2 p–n heterojunction for increased hydrogen production. Nanoscale 2015, 7, 7849–7857. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.H.; Huang, L.L.; Wang, H.J.; Yu, H.; Peng, F. AgI/TiO2 nanobelts monolithic catalyst with enhanced visible light photocatalytic activity. J. Hazard. Mater. 2015, 284, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Pinho, L.; Mosquera, M.J. Photocatalytic activity of TiO2–SiO2 nanocomposites applied to buildings: Influence of particle size and loading. Appl. Catal. B Environ. 2013, 134, 205–221. [Google Scholar] [CrossRef]
- Primo, A.; Corma, A.; Garcia, H. Titania supported gold nanoparticles as photocatalyst. Phys. Chem. Chem. Phys. 2011, 13, 886–910. [Google Scholar] [CrossRef]
- Dong, F.; Guo, S.; Wang, H.Q.; Li, X.F.; Wu, Z.B. Enhancement of the Visible Light Photocatalytic Activity of C-Doped TiO2Nanomaterials Prepared by a Green Synthetic Approach. J. Phys. Chem. C 2011, 115, 13285–13292. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-Doped Titanium Dioxide as Visible-Light-Sensitive Photocatalyst: Designs, Developments, and Prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef]
- Zimbone, M.; Cacciato, G.; Spitaleri, L.; Egdell, R.G.; Grimaldi, M.G.; Gulino, A. Sb-Doped Titanium Oxide: A Rationale for Its Photocatalytic Activity for Environmental Remediation. ACS Omega 2018, 3, 11270–11277. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Shen, D.Y.; Xu, T.; Jiang, R.L. Photocatalytic degradation properties of V-doped TiO2 to automobile exhaust. Sci. Total Environ. 2017, 586, 347–354. [Google Scholar] [CrossRef]
- Banisharif, A.; Khodadadi, A.A.; Mortazavi, Y.; Firooz, A.A.; Beheshtian, J.; Agah, S.; Menbari, S. Highly active Fe2O3-doped TiO2 photocatalyst for degradation of trichloroethylene in air under UV and visible light irradiation: Experimental and computational studies. Appl. Catal. B Environ. 2015, 165, 209–221. [Google Scholar] [CrossRef]
- Cheng, C.; Amini, A.; Zhu, C.; Xu, Z.L.; Song, H.S.; Wang, N. Enhanced photocatalytic performance of TiO2-ZnO hybrid nanostructures. Sci. Rep. 2014, 4, 4181. [Google Scholar] [CrossRef] [PubMed]
- Zimbone, M.; Cacciato, G.; Boutinguiza, M.; Gulino, A.; Cantarella, M.; Privitera, V.; Grimaldi, M. Hydrogenated black-TiOx: A facile and scalable synthesis for environmental water purification. Catal. Today 2019, 146–157. [Google Scholar] [CrossRef]
- Zhou, X.M.; Liu, G.; Yu, J.G.; Fan, W.H. Surface plasmon resonance-mediated photocatalysis by noble metal-based composites under visible light. J. Mater. Chem. 2012, 22, 21337–21354. [Google Scholar] [CrossRef]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef]
- Zhu, Z.N.; Meng, H.F.; Liu, W.J.; Liu, X.F.; Gong, J.X.; Qiu, X.H.; Jiang, L.; Wang, D.; Tang, Z.Y. Superstructures and SERS Properties of Gold Nanocrystals with Different Shapes. Angew. Chem. 2011, 123, 1593–1596. [Google Scholar] [CrossRef]
- Khlebtsov, B.N.; Khlebtsov, N.G. Multipole Plasmons in Metal Nanorods: Scaling Properties and Dependence on Particle Size, Shape, Orientation, and Dielectric Environment. J. Phys. Chem. C 2007, 111, 11516–11527. [Google Scholar] [CrossRef]
- Rycenga, M.; Cobley, C.M.; Zeng, J.; Li, W.Y.; Moran, C.H.; Zhang, Q.; Qin, N.; Xia, Y.N. Controlling the Synthesis and Assembly of Silver Nanostructures for Plasmonic Applications. Chem. Rev. 2011, 111, 3669–3712. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.N.; Xiong, Y.J.; Lim, B.; Skrabalak, S.E. Shape-controlled synthesis of metal nanocrystals: Simple chemistry meets complex physics? Angew. Chem. Int. Ed. 2009, 48, 60–103. [Google Scholar] [CrossRef]
- Grzelczak, M.; Pérez-Juste, J.; Mulvaney, P.; Liz-Marzán, L.M. Shape control in gold nanoparticle synthesis. Chem. Soc. Rev. 2008, 37, 1783–1791. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich Method for Gold Nanoparticle Synthesis Revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Iglesias, A.; Grzelczak, M.; Perez-Juste, J.; Liz-Marzán, L.M. Binary Self-Assembly of Gold Nanowires with Nanospheres and Nanorods. Angew. Chem. Int. Ed. 2010, 49, 9985–9989. [Google Scholar] [CrossRef] [PubMed]
- Liz-Marzán, L.M. Tailoring Surface Plasmons through the Morphology and Assembly of Metal Nanoparticles. Langmuir 2006, 22, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, S.W.; Keulemans, M.; Martens, J.A.; Lenaerts, S. Predicting the Surface Plasmon Resonance Wavelength of Gold–Silver Alloy Nanoparticles. J. Phys. Chem. C 2013, 117, 19142–19145. [Google Scholar] [CrossRef]
- Ingram, D.B.; Christopher, P.; Bauer, J.L.; Linic, S. Predictive Model for the Design of Plasmonic Metal/Semiconductor Composite Photocatalysts. ACS Catal. 2011, 1, 1441–1447. [Google Scholar] [CrossRef]
- Sun, Y.G.; Xia, Y.N. Shape-Controlled Synthesis of Gold and Silver Nanoparticles. Science 2002, 298, 2176–2179. [Google Scholar] [CrossRef] [Green Version]
- Kim, F.; Song, J.H.; Yang, P.D. Photochemical Synthesis of Gold Nanorods. J. Am. Chem. Soc. 2002, 124, 14316–14317. [Google Scholar] [CrossRef]
- Wang, X.; Zhuang, J.; Peng, Q.; Li, Y.D. A general strategy for nanocrystal synthesis. Nature 2005, 437, 121–124. [Google Scholar] [CrossRef]
- Huang, J.L.; Li, Q.B.; Sun, D.H.; Lu, Y.H.; Su, Y.B.; Yang, X.; Wang, H.X.; Wang, Y.P.; Shao, W.Y.; He, N.; et al. Biosynthesis of silver and gold nanoparticles by novel sundriedCinnamomum camphoraleaf. Nanotechnology 2007, 18. [Google Scholar] [CrossRef]
- Mallin, M.P.; Murphy, C.J. Solution-Phase Synthesis of Sub-10 nm Au−Ag Alloy Nanoparticles. Nano Lett. 2002, 2, 1235–1237. [Google Scholar] [CrossRef]
- Malathi, S.; Ezhilarasu, T.; Abiraman, T.; Balasubramanian, S. One pot green synthesis of Ag, Au and Au–Ag alloy nanoparticles using isonicotinic acid hydrazide and starch. Carbohydr. Polym. 2014, 111, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yin, H.F.; Chan, R.; Peng, S.; Dai, S.; Sun, S.H. One-Pot Synthesis of Oleylamine Coated AuAg Alloy NPs and Their Catalysis for CO Oxidation. Chem. Mater. 2009, 21, 433–435. [Google Scholar] [CrossRef]
- Liu, S.; Chen, G.Y.; Prasad, P.N.; Swihart, M.T. Synthesis of Monodisperse Au, Ag, and Au–Ag Alloy Nanoparticles with Tunable Size and Surface Plasmon Resonance Frequency. Chem. Mater. 2011, 23, 4098–4101. [Google Scholar] [CrossRef]
- Fu, H.T.; Sun, S.Y.; Yang, X.H.; Li, W.F.; An, X.Z.; Zhang, H.; Dong, Y.; Jiang, X.C.; Yu, A.B. A facile coating method to construct uniform porous α-Fe2O3@TiO2 core-shell nanostructures with enhanced solar light photocatalytic activity. Powder Technol. 2018, 328, 389–396. [Google Scholar] [CrossRef]
- Qiu, G.Y.; Ng, S.P.; Wu, C.M.L. Bimetallic Au-Ag alloy nanoislands for highly sensitive localized surface plasmon resonance biosensing. Sens. Actuators B Chem. 2018, 265, 459–467. [Google Scholar] [CrossRef]
- Millesi, S.; Nigro, R.L.; Pedroni, M.; Speghini, A.; Gulino, A. Photoexcited Porphyrins Functionalizing TiO2 and SnO2 Nanocrystals. J. Phys. Chem. C 2015, 119, 23743–23751. [Google Scholar] [CrossRef]
- Taverner, A.E.; Gulino, A.; Egdell, R.G.; Tate, T.J. A photoemission study of electron states in Sb-ion implanted TiO2(110). Appl. Surf. Sci. 1995, 90, 383–387. [Google Scholar] [CrossRef]
- Kaminker, R.; Lahav, M.; Altman, M.; Evmenenko, G.; Dutta, P.; Gulino, A.; Van Der Boom, M.E. Surface-confined core–shell structures based on gold nanoparticles and metal–organic networks. Chem. Commun. 2014, 50, 4635–4638. [Google Scholar] [CrossRef] [Green Version]
- Yuan, C.L.; Wei, W.Y.; Mei, Y.X.; Luo, X.F.; Lei, W. A new approach for fabricating Au-Ag alloy nanoparticles confined in Al2O3 matrix. Mater. Lett. 2017, 190, 248–251. [Google Scholar] [CrossRef]
- Arrii, S.; Morfin, F.; Renouprez, A.J.; Rousset, J.L. Oxidation of CO on Gold Supported Catalysts Prepared by Laser Vaporization: Direct Evidence of Support Contribution. J. Am. Chem. Soc. 2004, 126, 1199–1205. [Google Scholar] [CrossRef]
- Wang, A.Q.; Liu, J.H.; Lin, S.D.; Lin, T.S.; Mou, C.Y. A novel efficient Au–Ag alloy catalyst system: Preparation, activity, and characterization. J. Catal. 2005, 233, 186–197. [Google Scholar] [CrossRef]
- Yang, X.H.; Fu, H.T.; Yu, A.B.; Jiang, X.C. Large-surface mesoporous TiO2 nanoparticles: Synthesis, growth and photocatalytic performance. J. Colloid Interface Sci. 2012, 387, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.Q.; Xu, L.; Song, J.; Zhou, C.Y.; Li, Q.L.; Liu, D.L.; Song, H.W. Preparation and Gas Sensing Properties of In2O3/Au Nanorods for Detection of Volatile Organic Compounds in Exhaled Breath. Sci. Rep. 2015, 5, 10717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, R.Q.; Li, Q.L.; Zhang, J.H.; Xia, L.; Song, J.; Xu, L.; Xie, Y.; Song, H.W. Au-modified three-dimensional In2O3 inverse opals: Synthesis and improved performance for acetone sensing toward diagnosis of diabetes. Nanoscale 2015, 7, 13051–13060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Wei, Z.; Wu, T.; Peng, Q.; Li, Y.D. Au−ZnO Hybrid Nanopyramids and Their Photocatalytic Properties. J. Am. Chem. Soc. 2011, 133, 5660–5663. [Google Scholar] [CrossRef] [PubMed]
- Afsari, M.; Youzbashi, A.A.; Nuranian, H.; Zahraee, S.M. Remarkable improvement of visible light photocatalytic activity of TiO2 nanotubes doped sequentially with noble metals for removing of organic and microbial pollutants. Mater. Res. Bull. 2017, 94, 15–21. [Google Scholar] [CrossRef]
- Singh, J.; Tripathi, N.; Mohapatra, S. Synthesis of Ag–TiO2 hybrid nanoparticles with enhanced photocatalytic activity by a facile wet chemical method. Nano Struct. Nano Objects 2019, 18, 100266. [Google Scholar] [CrossRef]
- Drunka, R.; Grabis, J.; Krumina, A. Preparation of Au, Pt, Pd and Ag Doped TiO2 Nanofibers and their Photocatalytic Properties under LED Illumination. Key Eng. Mater. 2018, 762, 283–287. [Google Scholar] [CrossRef]
- Lan, J.Y.; Zhou, X.M.; Liu, G.; Yu, J.G.; Zhang, J.C.; Zhi, L.J.; Nie, G.J. Enhancing photocatalytic activity of one-dimensional KNbO3 nanowires by Au nanoparticles under ultraviolet and visible-light. Nanoscale 2011, 3, 5161–5167. [Google Scholar] [CrossRef]
- Naya, S.I.; Teranishi, M.; Isobe, T.; Tada, H. Light wavelength-switchable photocatalytic reaction by gold nanoparticle-loaded titanium(iv) dioxide. Chem. Commun. 2010, 46, 815–817. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Pal, T. Interparticle Coupling Effect on the Surface Plasmon Resonance of Gold Nanoparticles: From Theory to Applications. Chem. Rev. 2007, 107, 4797–4862. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, G.; García-López, E.; Marci, G.; Loddo, V.; Yurdakal, S.; Augugliaro, V.; Palmisano, L. Advances in selective conversions by heterogeneous photocatalysis. Chem. Commun. 2010, 46, 7074–7089. [Google Scholar] [CrossRef] [PubMed]
- Christopher, P.; Xin, H.L.; Linic, S. Visible-light-enhanced catalytic oxidation reactions on plasmonic silver nanostructures. Nat. Chem. 2011, 3, 467–472. [Google Scholar] [CrossRef] [PubMed]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Wang, Y.; Zhang, L.; Fu, H.; He, P.; Han, D.; Lawson, T.; An, X. The Use of Tunable Optical Absorption Plasmonic Au and Ag Decorated TiO2 Structures as Efficient Visible Light Photocatalysts. Catalysts 2020, 10, 139. https://doi.org/10.3390/catal10010139
Yang X, Wang Y, Zhang L, Fu H, He P, Han D, Lawson T, An X. The Use of Tunable Optical Absorption Plasmonic Au and Ag Decorated TiO2 Structures as Efficient Visible Light Photocatalysts. Catalysts. 2020; 10(1):139. https://doi.org/10.3390/catal10010139
Chicago/Turabian StyleYang, Xiaohong, Yan Wang, Lingtong Zhang, Haitao Fu, Peng He, Dezhi Han, Tom Lawson, and Xizhong An. 2020. "The Use of Tunable Optical Absorption Plasmonic Au and Ag Decorated TiO2 Structures as Efficient Visible Light Photocatalysts" Catalysts 10, no. 1: 139. https://doi.org/10.3390/catal10010139
APA StyleYang, X., Wang, Y., Zhang, L., Fu, H., He, P., Han, D., Lawson, T., & An, X. (2020). The Use of Tunable Optical Absorption Plasmonic Au and Ag Decorated TiO2 Structures as Efficient Visible Light Photocatalysts. Catalysts, 10(1), 139. https://doi.org/10.3390/catal10010139