BiOCOOH Microflowers Decorated with Ag/Ag2CrO4 Nanoparticles as Highly Efficient Photocatalyst for the Treatment of Toxic Wastewater
Abstract
:1. Introduction
2. Results and Discussion
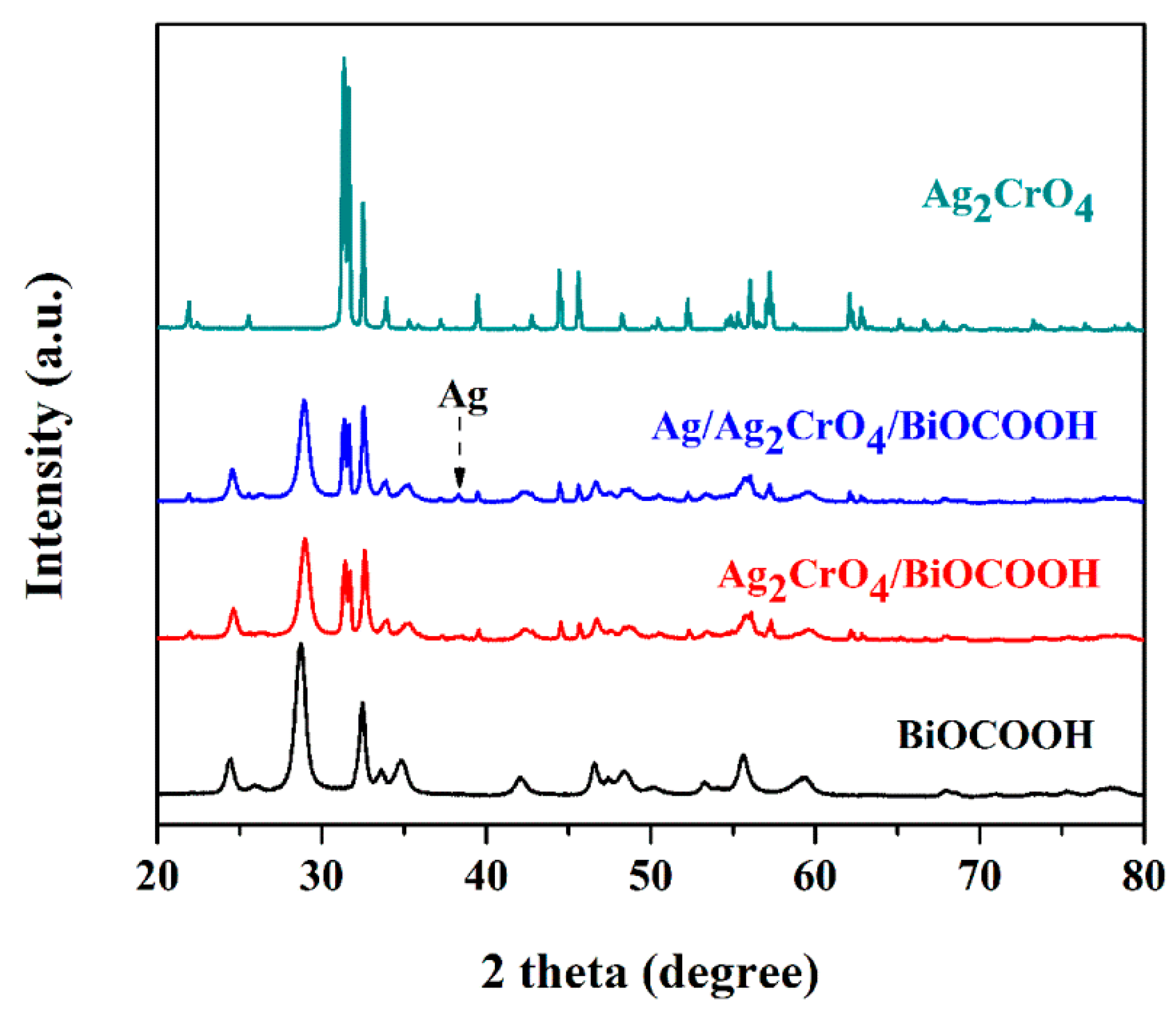
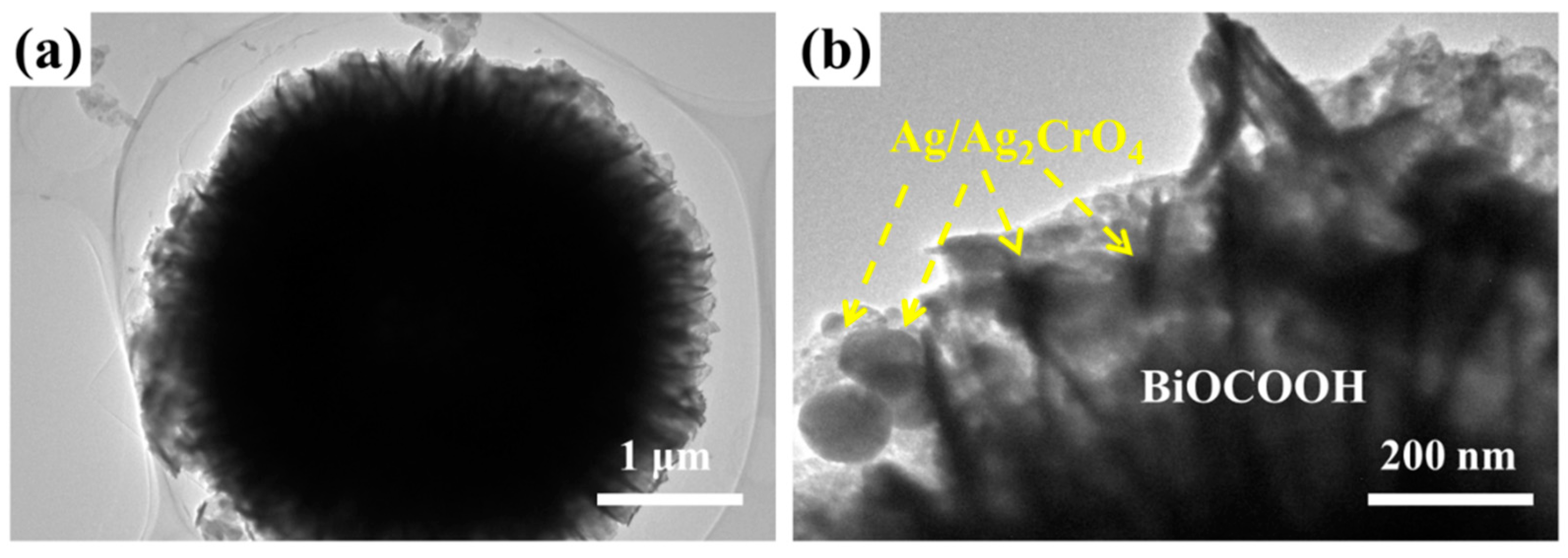
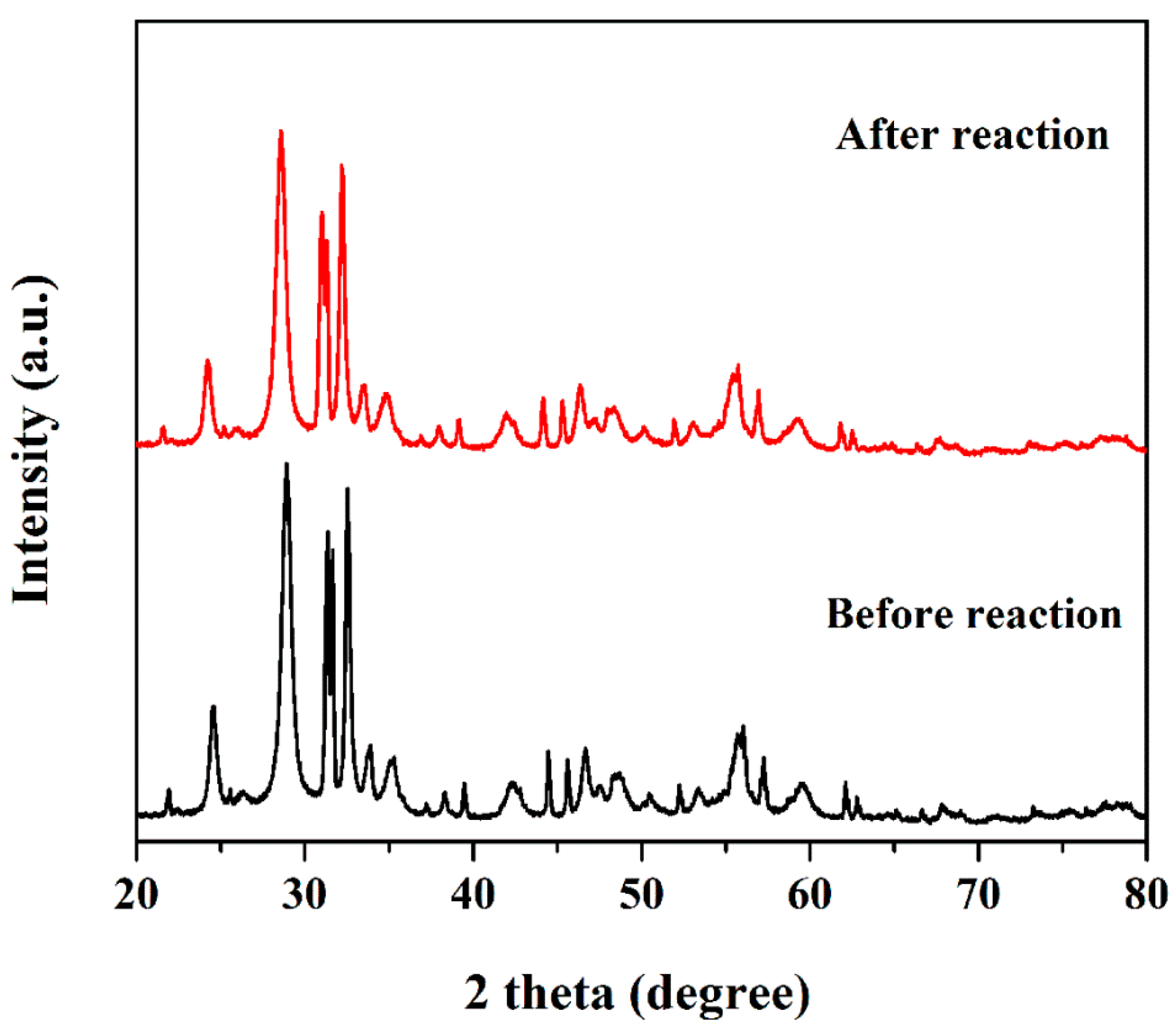
2.1. Structure and Morphology
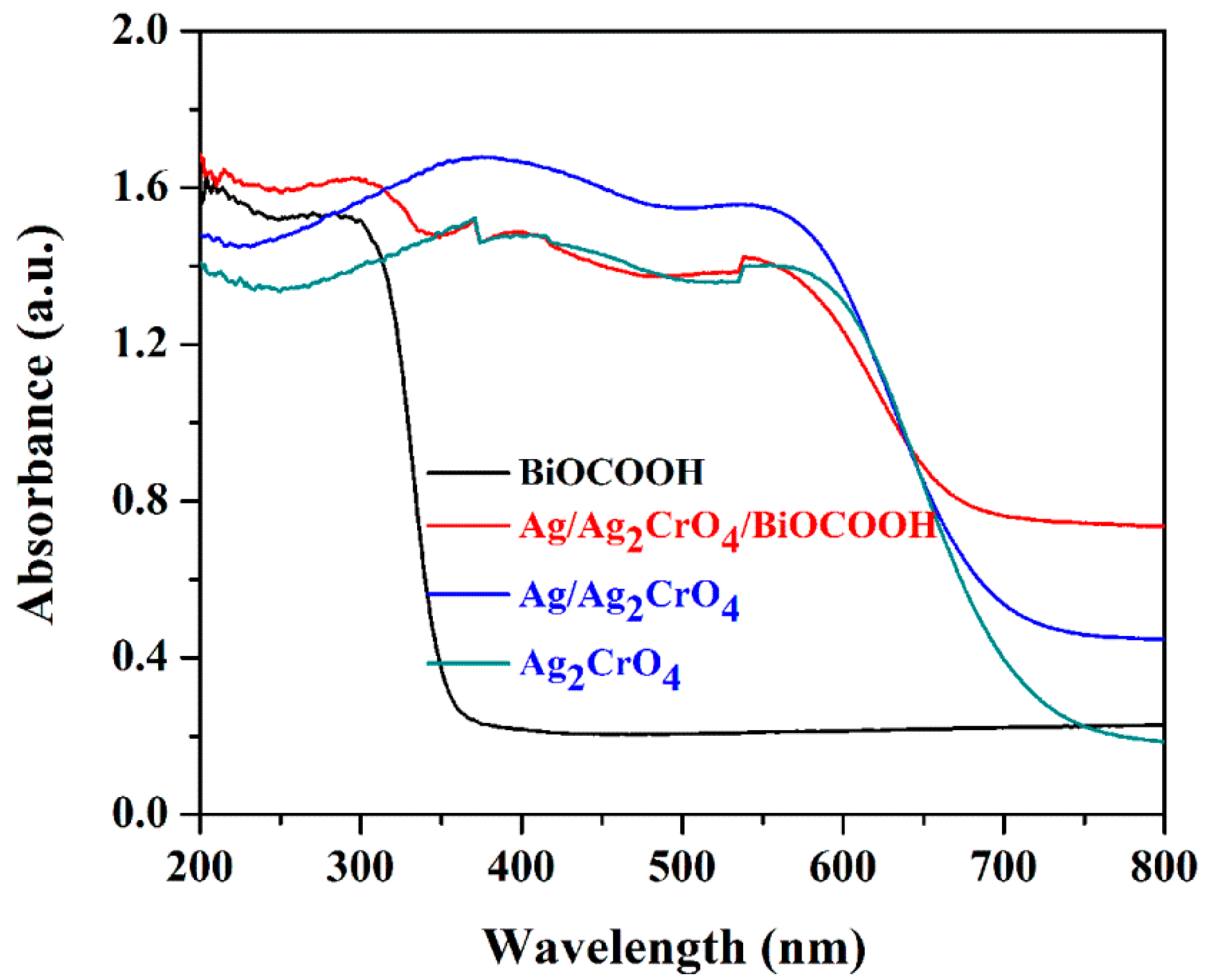
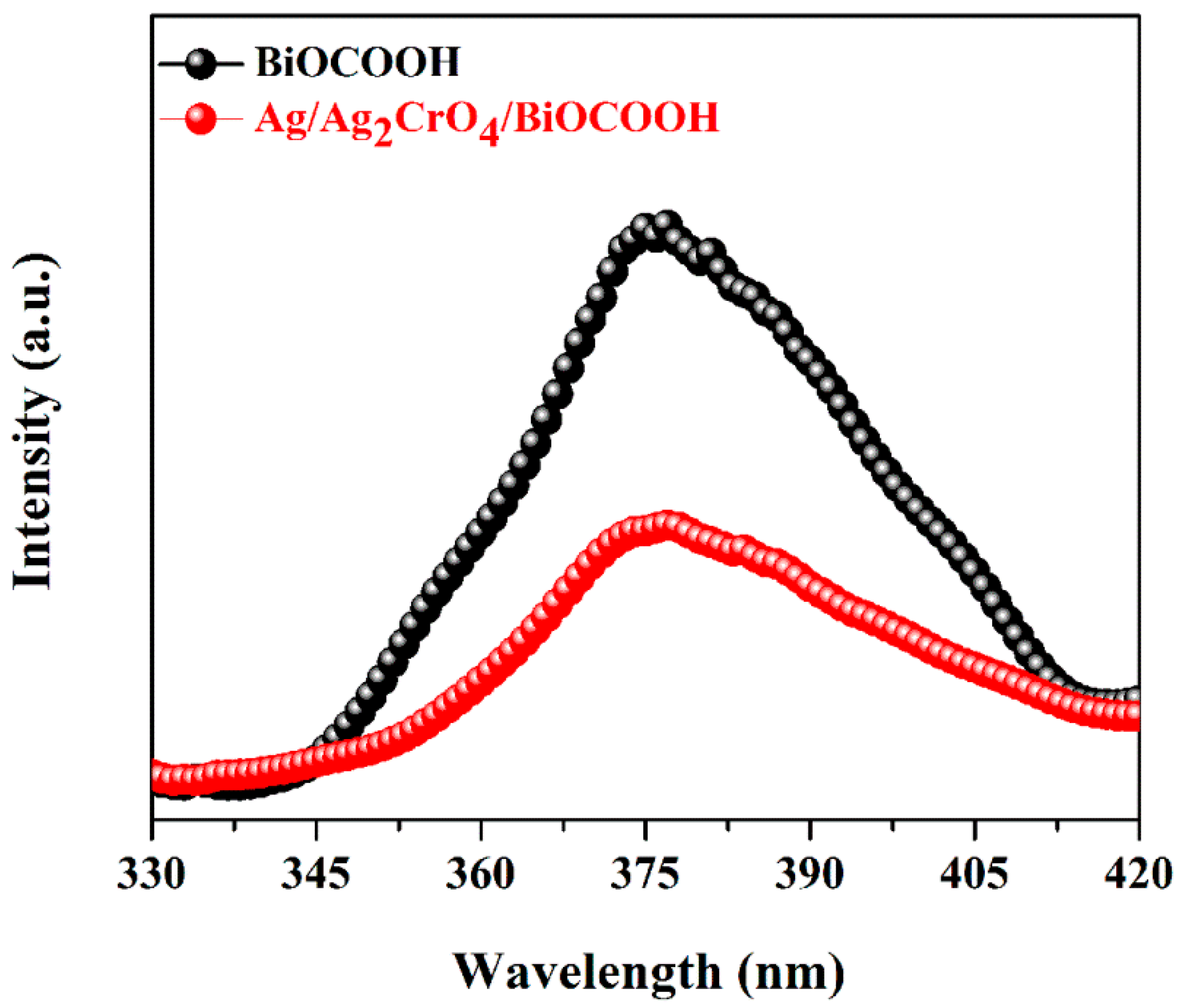
2.2. Optical Properties
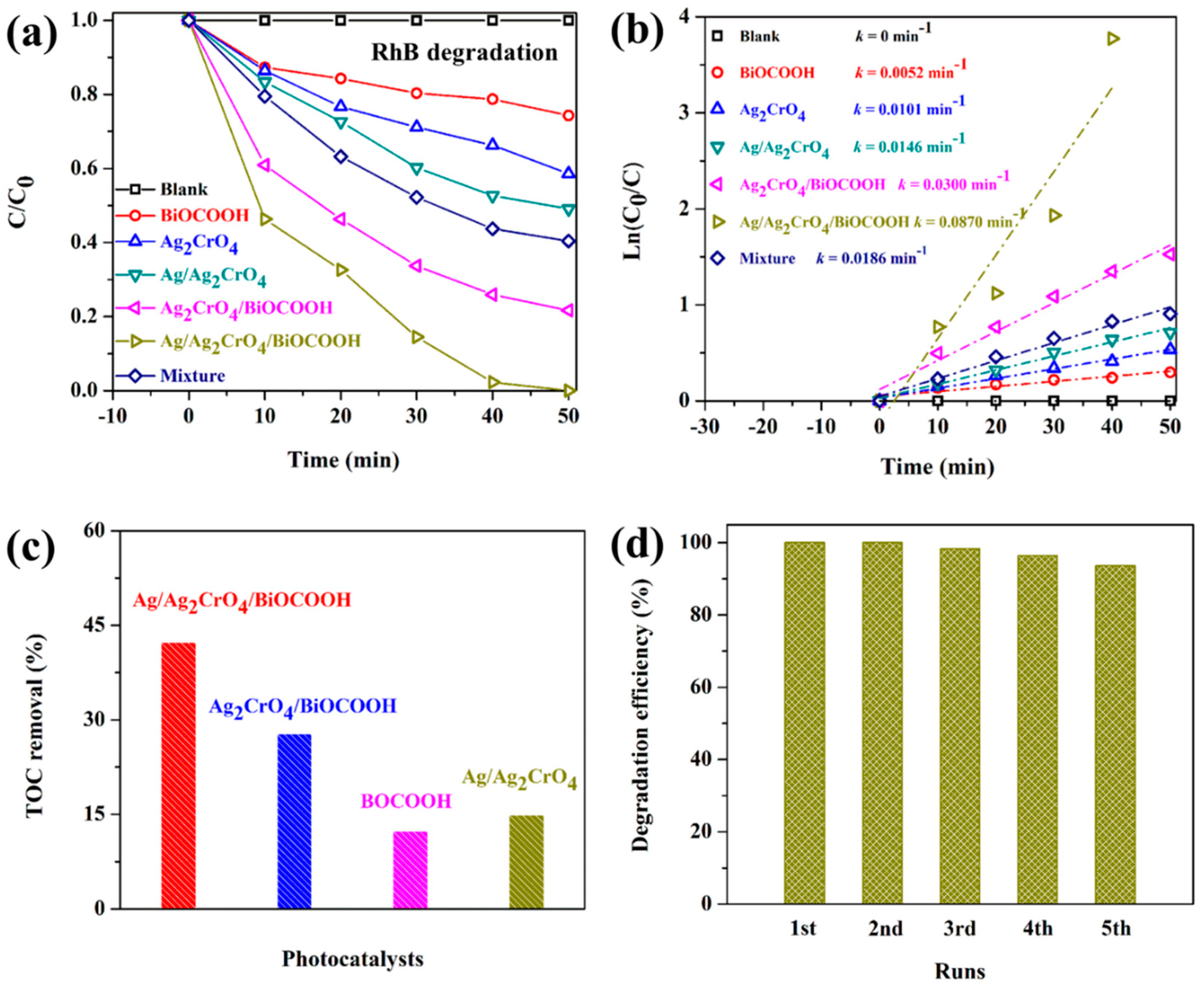
2.3. Photocatalytic Activity
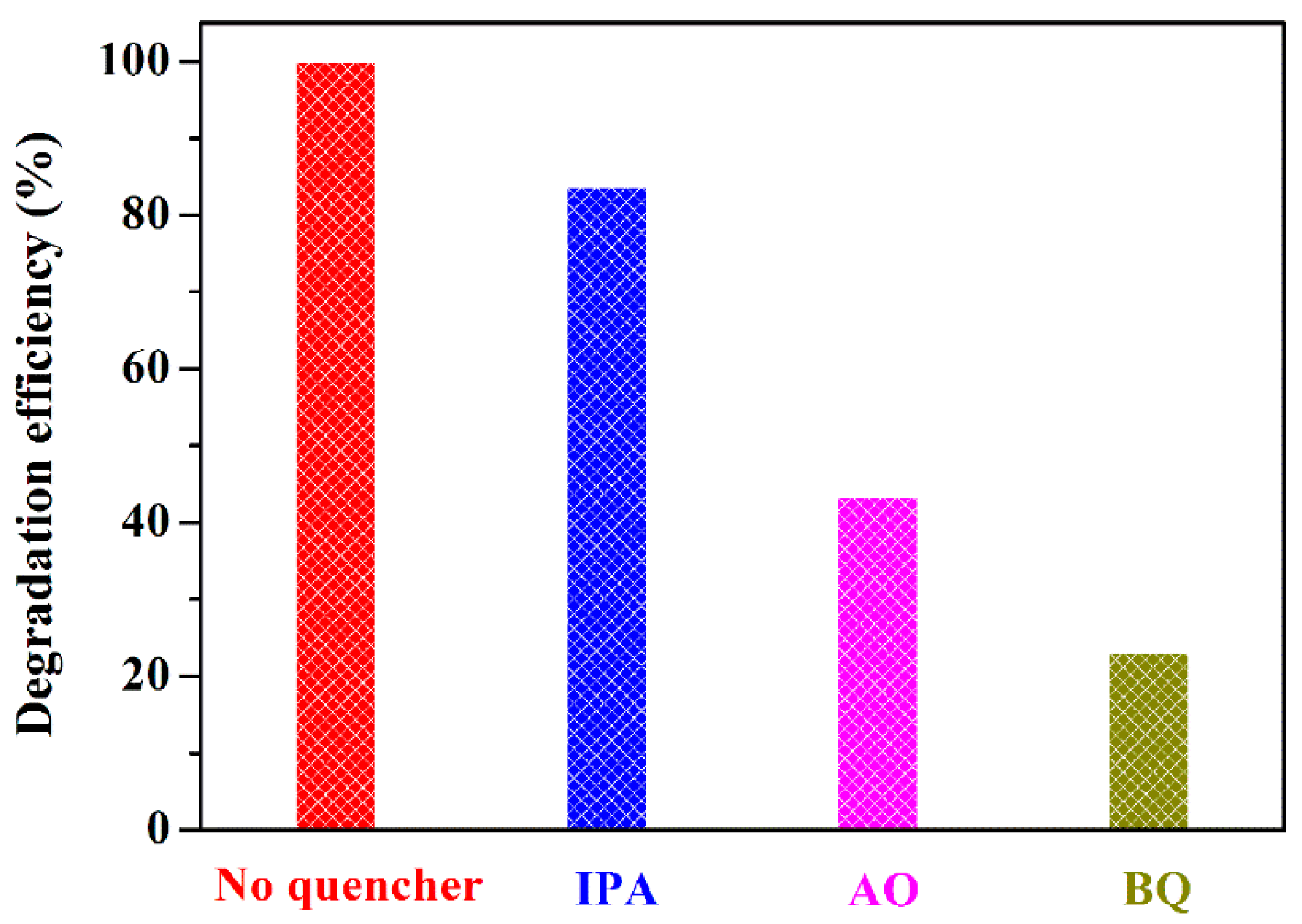
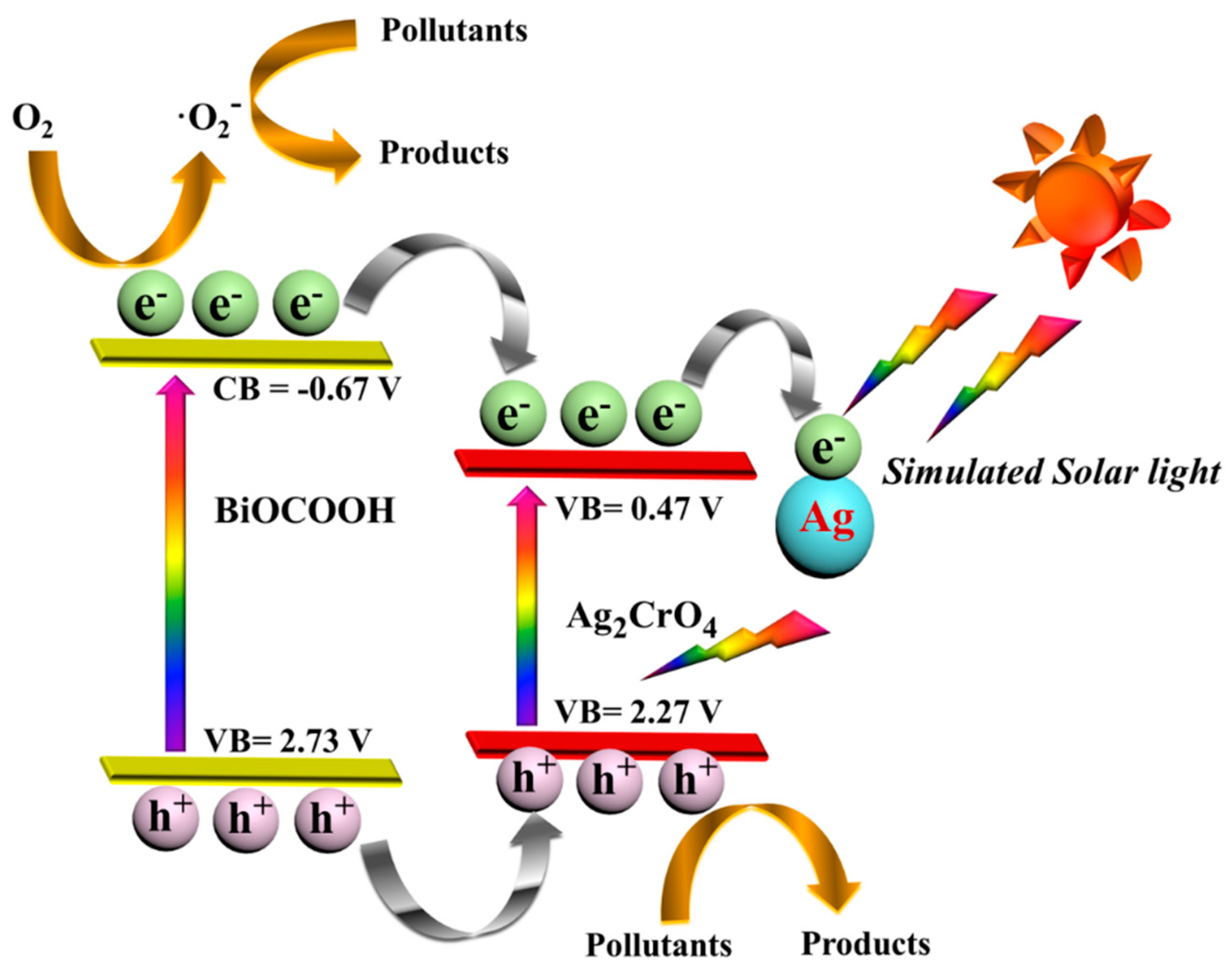
2.4. Photocatalytic Mechanism
3. Materials and Methods
3.1. Chemicals
3.2. Photocatalysts Fabrication
3.3. Characterization
3.4. Photocatalytic Activity Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, L.; Lin, C.; Zhang, D.; Gong, L.; Zhu, Y.; Xu, Q.; Li, H.; Xia, Z. Guiding principles for designing highly efficient metal-free carbon catalysts. Adv. Mater. 2019, 31, 1805252. [Google Scholar] [CrossRef]
- Xin, X.; Li, S.-H.; Zhang, N.; Tang, Z.-R.; Xu, Y.-J. 3D graphene/AgBr/Ag cascade aerogel for efficient photocatalytic disinfection. Appl. Catal. B 2019, 245, 343–350. [Google Scholar] [CrossRef]
- Wang, R.; Li, D.; Wang, H.; Liu, C.; Xu, L. Preparation, characterization, and performance analysis of S-doped Bi2MoO6 nanosheets. Nanomaterials 2019, 9, 1341. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Li, J.; Feng, X.; Li, J.; Hao, Y.; Zhang, J.; Wang, H.; Yin, A.; Zhou, J.; Ma, X.; et al. Metal-organic frameworks with photocatalytic bactericidal activity for integrated air cleaning. Nat. Commun. 2019, 10, 2177. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhang, N.; Tang, Z.-R.; Xu, Y.-J. Adaptive geometry regulation strategy for 3D graphene materials: Towards advanced hybrid photocatalysts. Chem. Sci. 2018, 9, 8876–8882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, L.; Ambrosi, A.; Zafir, M.; Guan, J.; Pumera, M. Smart robots: Self-propelled 3D-printed “Aircraft Carrier” of light-powered smart micromachines for large-volume nitroaromatic explosives removal. Adv. Funct. Mater. 2019, 29, 190387. [Google Scholar]
- Li, S.; Shen, X.; Liu, J.; Zhang, L. Synthesis of Ta3N5/Bi2MoO6 core-shell fiber-shaped heterojunctions as efficient and easily recyclable photocatalysts. Environ. Sci. Nano 2017, 4, 1155–1167. [Google Scholar] [CrossRef]
- Li, S.; Hu, S.; Jiang, W.; Liu, Y.; Zhou, Y.; Liu, Y.; Mo, L. Hierarchical architectures of bismuth molybdate nanosheets onto nickel titanate nanofibers: Facile synthesis and efficient photocatalytic removal of tetracycline hydrochloride. J. Colloid Interface Sci. 2018, 521, 42–49. [Google Scholar] [CrossRef]
- Pirhashemi, M.; Habibi-Yangjeh, A.; Pouran, S.R. Review on the criteria anticipated for the fabrication of highly efficient ZnO-based visible-light-driven photocatalysts. J. Ind. Eng. Chem. 2018, 62, 1–25. [Google Scholar] [CrossRef]
- Simeonidis, K.; Mourdikoudis, S.; Kaprara, E.; Mitrakas, M.; Polavarapu, L. Inorganic engineered nanoparticles in drinking water treatment: A critical review. Environ. Sci. Water Res. Technol. 2016, 2, 43–70. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Tian, Y.; Li, M.; Ma, H.; Ma, C.; Dong, X.; Zhang, X. Fabrication and photo-electrocatalytic activity of black TiO2 embedded Ti/PbO2 electrode. J. Appl. Electrochem. 2017, 47, 1045–1056. [Google Scholar] [CrossRef]
- Zhao, M.; Fu, Y.; Ma, H.; Ma, C.; Dong, X.; Zhang, X. Synthesis, characterization and photoreactivity of hierarchically N-doped (BiO)2CO3/Bi2S3 with highly exposed {001} facets. Mater. Des. 2016, 93, 1–8. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Shuai, D.; Shen, Y.; Wang, D. Progress and challenges in photocatalytic disinfection of waterborne Viruses: A review to fill current knowledge gaps. Chem. Eng. J. 2019, 335, 399–415. [Google Scholar] [CrossRef]
- Jiang, Z.J.; Sun, H.; Wang, T.; Wang, B.; Wei, W.; Li, H.; Yuan, S.; An, T.; Zhao, H.; Yu, J.; et al. Nature-inspired catalyst for visible-light-driven photocatalytic CO2 reduction. Energy Environ. Sci. 2018, 11, 2382–2389. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Chen, J.; Hu, S.; Jiang, W.; Liu, Y.; Liu, J. A novel 3D Z-scheme heterojunction photocatalyst: Ag6Si2O7 anchored on flower-like Bi2WO6 and its excellent photocatalytic performance for the degradation of toxic pharmaceutical antibiotics. Inorg. Chem. Front. 2020. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, K.-S.; Sorcar, S.; Razzaq, A.; Grimesc, C.A.; In, S.-I. Wastewater treatment and electricity generation from a sunlight-powered single chamber microbial fuel cell. J. Photochem. Photobiol. A Chem. 2018, 358, 432–440. [Google Scholar] [CrossRef]
- Han, N.; Wang, Y.; Yang, H.; Deng, J.; Wu, J.; Li, Y.; Li, Y. Ultrathin bismuth nanosheets from in situ topotactic transformation for selective electrocatalytic CO2 reduction to formate. Nat. Commun. 2019, 9, 1320. [Google Scholar] [CrossRef]
- Zhang, G.; Lin, L.; Li, G.; Zhang, Y.; Savateev, A.; Zafeiratos, S.; Wang, X.; Antonietti, M. Ionothermal Synthesis of Triazine-Heptazine Based Co-frameworks with Apparent Quantum Yields of 60% at 420 nm for Solar Hydrogen Production from “Sea Water”. Angew. Chem. Int. Ed. 2018, 57, 9372–9376. [Google Scholar] [CrossRef]
- Qiming, S.; Wang, N.; Yu, J.; Yu, J.C. A hollow porous CdS photocatalyst. Adv. Mater. 2018, 30, 1804368. [Google Scholar]
- She, H.; Sun, Y.; Li, S.; Wang, Q. Synthesis of Non-noble Metal Nickel Doped Sulfide Solid Solution for Improved Photocatalytic Performance. Appl. Catal. B 2019, 245, 439–447. [Google Scholar] [CrossRef]
- Xu, B.; An, Y.; Liu, Y.; Qin, X.; Zhang, X.; Dai, Y.; Wang, Z.; Wang, P.; Whangbo, M.-H.; Huang, B. Enhancing the photocatalytic activity of BiOX (X = Cl, Br, I), (BiO)2CO3 and Bi2O3 by modifying their surfaces with polar organic anions, 4-substituted thiophenolates. J. Mater. Chem. A 2017, 5, 14406–14414. [Google Scholar] [CrossRef]
- Li, S.; Hu, S.; Jiang, W.; Zhou, Y.; Liu, J.; Wang, Z. Facile synthesis of cerium oxide nanoparticles decorated flower-like bismuth molybdate for enhanced photocatalytic activity toward organic pollutant degradation. J. Colloid Interface Sci. 2018, 530, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, E.; Gionco, C.; Paganini, M.C.; Giamello, E.; Albanese, E.; Pacchioni, G. Origin of Visible Light Photoactivity of the CeO2/ZnO Heterojunction. ACS Appl. Energy Mater. 2018, 1, 4247–4260. [Google Scholar] [CrossRef]
- Pei, L.; Yuan, Y.; Zhong, J.; Li, T.; Yang, T.; Yan, S.; Ji, Z.; Zou, Z. Ta3N5 nanorods encapsulated into 3D hydrangea-like MoS2 for enhanced photocatalytic hydrogen evolution under visible light irradiation. Dalton Trans. 2019, 48, 13176–13183. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.L.; Han, Q.F.; Wang, X.; Zhu, J.W. Highly efficient removal of aqueous chromate and organic dyes by ultralong HCOOBiO nanowires. Chem. Eng. J. 2015, 262, 169–178. [Google Scholar] [CrossRef]
- Xiong, J.Y.; Cheng, G.; Lu, Z.; Tang, J.L.; Yu, X.L.; Chen, R. BiOCOOH hierarchical nanostructures: Shape-controlled solvothermal synthesis and photocatalytic degradation performances. CrystEngComm 2011, 13, 2381–2390. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, Q.; Su, Y.; Shen, L.; Wang, F.; Liu, H.; Liu, Y.; Cai, Z.; Lv, W.; Liu, G. Accelerated photocatalytic degradation of diclofenac by a novel CQDs/BiOCOOH hybrid material under visible-light irradiation: Dechloridation, detoxicity, and a new superoxide radical model study. Chem. Eng. J. 2018, 332, 737–748. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, X.; Zhang, H.; Cheng, Q.; Cheng, X. Construction of BiOCOOH/g-C3N4 composite photocatalyst and its enhanced visible light photocatalytic degradation of amido black 10B. Sep. Purif. Technol. 2019, 210, 125–134. [Google Scholar] [CrossRef]
- Li, S.; Chen, J.; Liu, Y.; Xu, K.; Liu, J. In situ anion exchange strategy to construct flower-like BiOCl/BiOCOOH p-n heterojunctions for efficiently photocatalytic removal of aqueous toxic pollutants under solar irradiation. J. Alloys Compd. 2019, 781, 582–588. [Google Scholar] [CrossRef]
- Li, S.; Chen, J.; Jiang, W.; Liu, Y.; Ge, Y.; Liu, J. Facile construction of flower-like bismuth oxybromide/bismuth oxide formate p-n heterojunctions with significantly enhanced photocatalytic performance under visible light. J. Colloid Interface Sci. 2019, 548, 12–19. [Google Scholar] [CrossRef]
- Li, S.; Mo, L.; Liu, Y.; Zhang, H.; Ge, Y.; Zhou, Y. Ag2CO3 decorating BiOCOOH microspheres with enhanced full-spectrum photocatalytic activity for the degradation of toxic pollutants. Nanomaterials 2018, 8, 914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajorowicz, B.; Kobylański, M.P.; Gołąbiewska, A.; Nadolna, J.; Zaleska-Medynska, A.; Malankowska, A. Quantum dot-decorated semiconductor micro- and nanoparticles: A review of their synthesis, characterization and application in photocatalysis. Adv. Colloid Interface Sci. 2018, 256, 352–372. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.; Li, Z.; Ouyang, Z.; Yu, T.; Ye, J.; Zou, Z. Correlation of crystal structures, electronic structures, and photocatalytic properties in a series of Ag-based oxides: AgAlO2, AgCrO2, and Ag2CrO4. J. Phys. Chem. C 2008, 112, 3134–3141. [Google Scholar] [CrossRef]
- Zou, X.; Dong, Y.; Li, S.; Ke, J.; Cui, Y. Facile anion exchange to construct uniform AgX (X = Cl, Br, I)/Ag2CrO4 NR hybrids for efficient visible light driven photocatalytic activity. Sol. Energy 2018, 169, 392–400. [Google Scholar] [CrossRef]
- Xu, D.; Cheng, B.; Wang, W.; Jiang, C.; Yu, J. Ag2CrO4/g-C3N4/graphene oxide ternary nanocomposite Z-scheme photocatalyst with enhanced CO2 reduction activity. Appl. Catal. B 2018, 231, 368–380. [Google Scholar] [CrossRef]
- Wu, X.-F.; Sun, Y.; Li, H.; Wang, Y.-J.; Zhang, C.-X.; Zhang, J.-R.; Su, J.-Z.; Wang, Y.-W.; Zhang, Y.; Wang, C.; et al. In-situ synthesis of novel p-n heterojunction of Ag2CrO4-Bi2Sn2O7 hybrids for visible-light-driven photocatalysis. J. Alloys Compd. 2018, 740, 1197–1203. [Google Scholar] [CrossRef]
- Azami, M.; Haghighi, M.; Allahyari, S. Sono-precipitation of Ag2CrO4-C composite enhanced by carbon-based materials (AC, GO, CNT and C3N4) and its activity in photocatalytic degradation of acid orange 7 in water. Ultrason. Sonochem. 2018, 40, 505–516. [Google Scholar] [CrossRef]
- Kushwaha, A.K.; Ugur, S.; Akbudak, S.; Ugur, G. Investigation of structural, elastic, electronic, optical and vibrational properties of silver chromate spinels: Normal (CrAg2O4) and inverse (Ag2CrO4). J. Alloys Compd. 2017, 704, 101–108. [Google Scholar] [CrossRef]
- Gong, Y.; Quan, X.; Yu, H.; Chen, S. Synthesis of Z-scheme Ag2CrO4/Ag/g-C3N4 composite with enhanced visible-light photocatalytic activity for 2,4-dichlorophenol degradation. Appl. Catal. B 2017, 219, 439–449. [Google Scholar] [CrossRef]
- Li, X.; Xiong, J.; Xu, Y.; Feng, Z.; Huang, J. Defect-assisted surface modification enhances the visible light photocatalytic performance of g-C3N4@C-TiO2 direct Z-scheme heterojunction. Chin. J. Catal. 2019, 40, 424–443. [Google Scholar] [CrossRef]
- Pei, L.; Li, T.; Yuan, Y.; Yang, T.; Zhong, J.; Ji, Z.; Yan, S.; Zou, Z. Schottky junction effect enhanced plasmonic photocatalysis by TaON@Ni NP heterostructures. Chem. Commun. 2019, 55, 11754–11757. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.-J.; Niu, C.-G.; Huang, D.-W.; Zhang, L.; Liang, C.; Zeng, G.-M. Study of the photocatalytic degradation pathway of norfloxacin and mineralization activity using a novel ternary Ag/AgCl-CeO2 photocatalyst. J. Catal. 2017, 355, 73–86. [Google Scholar] [CrossRef]
- Zhang, M.; Qi, Y.; Zhang, Z. AgBr/BiOBr Nano-Heterostructure-Decorated Polyacrylonitrile Nanofibers: A Recyclable High-Performance Photocatalyst for Dye Degradation under Visible-Light Irradiation. Polymers 2019, 11, 1718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Hu, S.; Jiang, W.; Zhang, J.; Xu, K.; Wang, Z. In situ construction of WO3 nanoparticles decorated Bi2MoO6 microspheres for boosting photocatalytic degradation of refractory pollutants. J. Colloid Interface Sci. 2019, 556, 335–344. [Google Scholar] [CrossRef]
- Li, X.; Fang, S.; Ge, L.; Han, C.; Qiu, P.; Liu, W. Synthesis of flower-like Ag/AgCl-Bi2MoO6 plasmonic photocatalysts with enhanced visible-light photocatalytic performance. Appl. Catal. B 2015, 176–177, 62–69. [Google Scholar] [CrossRef]
- Asadzadeh-Khaneghah, S.; Habibi-Yangjeh, A.; Abedi, M. Decoration of carbon dots and AgCl over g-C3N4 nanosheets: Novel photocatalysts with substantially improved activity under visible light. Sep. Purif. Technol. 2018, 199, 64–77. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, H.; Zou, Y.; Wang, R.; Lyu, Y.; Wang, S. Efficiency and stability of narrow-gap semiconductor-based photoelectrodes. Energy Environ. Sci. 2019, 12, 2345–2374. [Google Scholar] [CrossRef]
- Liu, Z.; Leow, W.R.; Chen, X. Bio-inspired plasmonic photocatalysts. Small Methods 2019, 3, 1800295. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, Y.; Duoerkun, G.; Shi, Z.; Liu, J.; Chen, Z.; Wong, P.K.; Zhang, L. Vis-NIR light-responsive photocatalytic activity of C3N4-Ag-Ag2O heterojunction-decorated carbon-fiber cloth as efficient filter-membrane-shaped photocatalyst. ChemCatChem 2019, 11, 1362–1373. [Google Scholar] [CrossRef]
- Duan, F.; Zheng, Y.; Liu, L.; Chen, M.Q.; Xie, Y. Synthesis and photocatalytic behaviour of 3D flowerlike bismuth oxide formate architectures. Mater. Lett. 2010, 64, 1566–1569. [Google Scholar] [CrossRef]
- Li, S.; Hu, S.; Zhang, J.; Jiang, W.; Liu, J. Facile synthesis of Fe2O3 nanoparticles anchored on Bi2MoO6 microflowers with improved visible light photocatalytic activity. J. Colloid Interface Sci. 2017, 497, 93–101. [Google Scholar] [CrossRef] [PubMed]



| Photocatalysts | Light | Photocatalytic Activity | Ref |
|---|---|---|---|
| Ag2CO3/BiOCOOH (ACO/BOCH-30) | 300W-Xe lamp | The RhB removal efficiency reach 89.4% within 60 min over 30 mg of catalysts | [29] |
| BiOBr/BiOCOOH (0.6Br-Bi) | 300W-Xe lamp | The RhB removal efficiency reach 100% within 50 min over 50 mg of catalysts | [28] |
| Ag/Ag2CrO4/BiOCOOH | 300W-Xe lamp | The RhB removal efficiency reach 100% within 50 min over 40 mg of catalysts | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Xue, B.; Chen, J.; Jiang, W.; Liu, Y. BiOCOOH Microflowers Decorated with Ag/Ag2CrO4 Nanoparticles as Highly Efficient Photocatalyst for the Treatment of Toxic Wastewater. Catalysts 2020, 10, 93. https://doi.org/10.3390/catal10010093
Li S, Xue B, Chen J, Jiang W, Liu Y. BiOCOOH Microflowers Decorated with Ag/Ag2CrO4 Nanoparticles as Highly Efficient Photocatalyst for the Treatment of Toxic Wastewater. Catalysts. 2020; 10(1):93. https://doi.org/10.3390/catal10010093
Chicago/Turabian StyleLi, Shijie, Bing Xue, Jialin Chen, Wei Jiang, and Yanping Liu. 2020. "BiOCOOH Microflowers Decorated with Ag/Ag2CrO4 Nanoparticles as Highly Efficient Photocatalyst for the Treatment of Toxic Wastewater" Catalysts 10, no. 1: 93. https://doi.org/10.3390/catal10010093
APA StyleLi, S., Xue, B., Chen, J., Jiang, W., & Liu, Y. (2020). BiOCOOH Microflowers Decorated with Ag/Ag2CrO4 Nanoparticles as Highly Efficient Photocatalyst for the Treatment of Toxic Wastewater. Catalysts, 10(1), 93. https://doi.org/10.3390/catal10010093






