Perovskites in the Energy Grid and CO2 Conversion: Current Context and Future Directions
Abstract
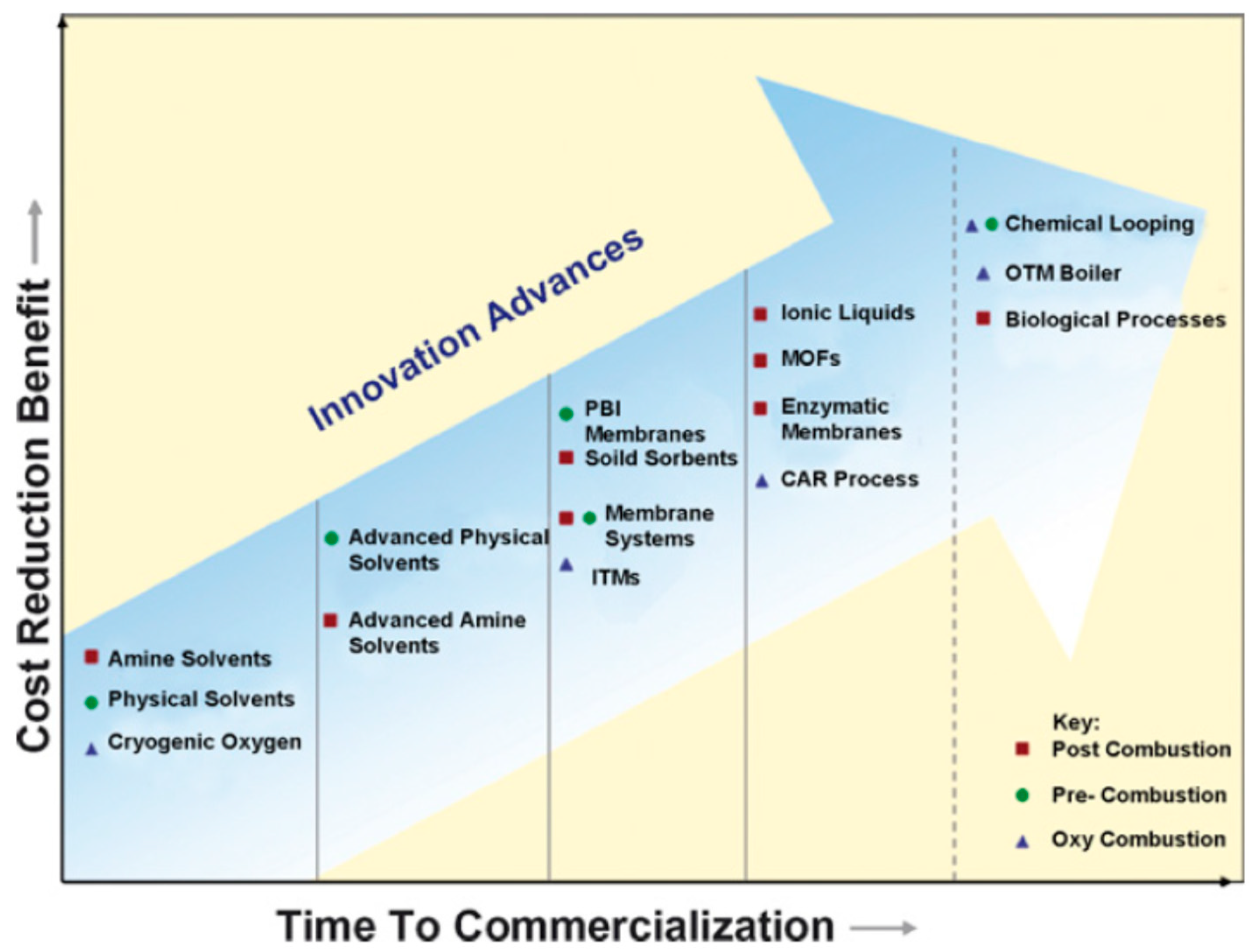
:1. Introduction
2. Perovskite Structures and Properties
3. Chemical Looping
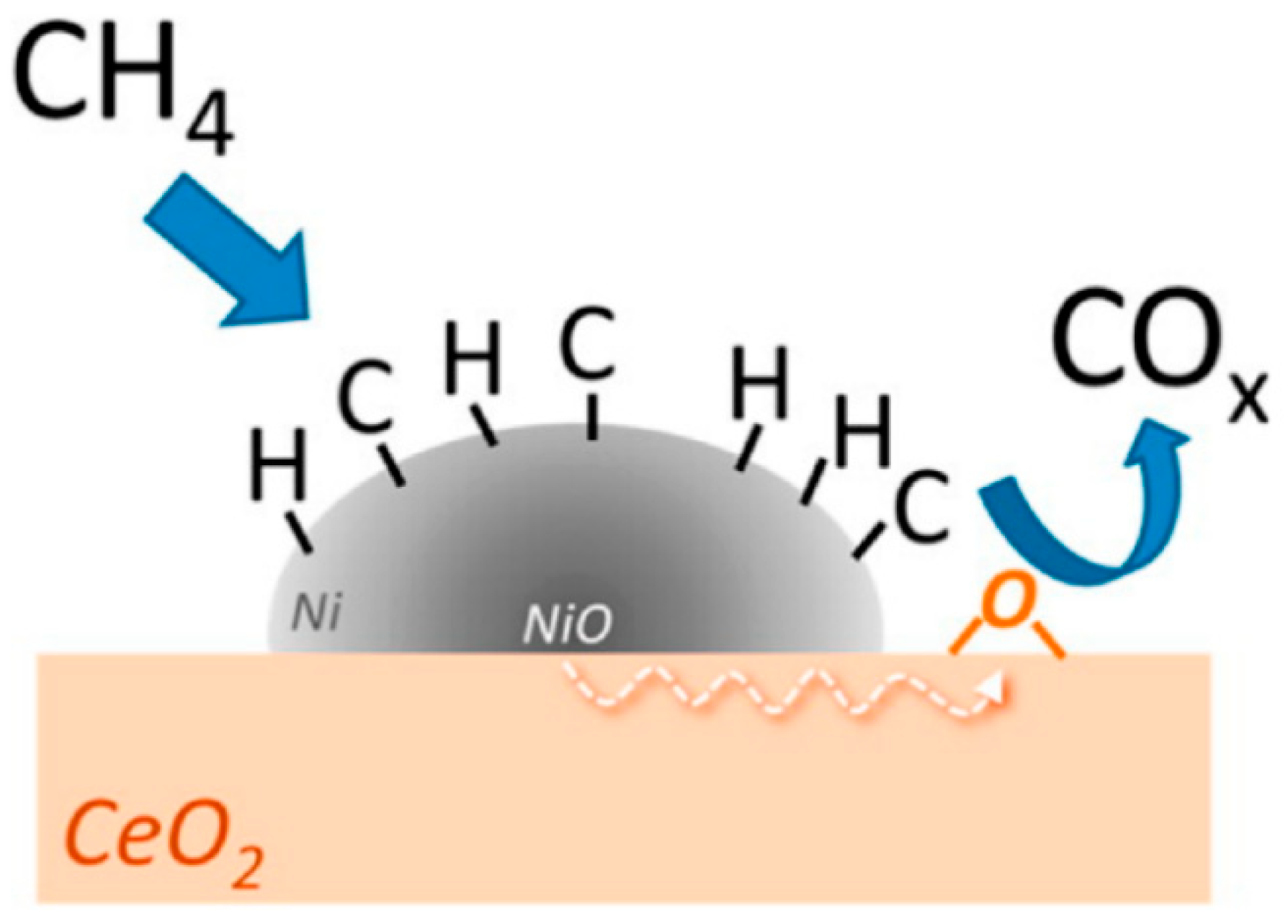
4. Engineering of Oxygen Carriers
5. CO2-Derived Fuels
5.1. Electrolysis
5.2. Thermochemical and Photoelectrochemical Conversions
6. Photon Capture
7. Artificial Photosynthesis
8. Solar Fuels
| Catalyst | Reactor | Reduction Temperature (°C) | Reducing Atmosphere | Oxidizing Atmosphere | CO Yield (μmol/g) | O2 Yield (μmol/g) | Reference |
|---|---|---|---|---|---|---|---|
| LaMn0.5Co0.5O3 | TGA | 1400 | <2 ppm O2 premixed Ar | 50% CO2/Ar | 145 | 83 | Nair et al. [199] |
| Ba0.5Sr0.5FeO3 | TGA | 1000 | <2 ppm O2 premixed Ar | 50% CO2/Ar | 136 | 582 | Nair et al. [199] |
| La0.5Sr0.5MnO3 | TGA | 1400 | <2 ppm O2 premixed Ar | 50% CO2/Ar | 269 | 248 | Nair et al. [199] |
| La0.5Sr0.5MnO3 | TGA | 1500 | 10 ppm O2 premixed N2 | 1 atm CO2 | 230 | 111.6 | Dey et al. [200] |
| Y0.5Sr0.5MnO3 | TGA | 1400 | 10 ppm O2 premixed N2 | 1 atm CO2 | 196.4 | 108 | Dey et al. [200] |
| La0.6Sr0.4Mn0.6Al0.4O3 | TGA | 1400 | <2 ppm O2 premixed Ar | 50% CO2/Ar | 205 | 210 | Nair et al. [201] |
| La0.25Sr0.75MnO3 | TGA | 1400 | N2 | CO2/air | 786.32 ± 41.6 | 516.24 ± 19.55 | Riaz et al. [202] |
| La0.6Sr0.4Cr0.75Mn0.25O3 | TGA | 1400 | Ar | 0.5 atm CO2 | ~7 * | ~1.2 * | Carrillo et al. [203] |
| La0.6Sr0.4MnO3 | TGA | 1350 | N2 | CO2 | 469.1 | 348.8 | Luciani et al. [205] |
| La0.6Sr0.4Mn0.8Fe0.2O3 | TGA | 1350 | N2 | CO2 | 329.9 | 286.0 | Luciani et al. [205] |
| La0.6Ca0.4Mn0.6Al0.4O3 | TGA | 1375 | 3 × 10−5 bar O2 mixed Ar | 50% CO2/Ar | ca. 420 | - | Cooper et al. [207] |
| La0.6r0.4Mn0.6Al0.4O3 | Fixed bed reactor | 1250 | Ar | 5% CO2/He | 114 | 266 | Sastre et al. [208] |
| La0.6Sr0.4Cr0.8Co0.2O3−δ | TGA | 1200 | Ar | 50% CO2/Ar | 157 | - | Bork et al. [209] |
| SiO2-supported La0.75Sr0.25FeO3 | Quartz microreactor connected with Cirrus MKS MS | 950 | 10% H2/He | 10% CO2/He | 1700 | - | Hare et al. [211] |
| La0.75Ca0.25MnO3 | Quartz microreactor connected with Cirrus MKS MS | 950 | 10% H2/He | 10% CO2/He | 1680 | - | Hare et al. [212] |
| La0.5Ca0.5Fe0.25Mn0.75O3 | Quartz microreactor connected with Cirrus MKS MS | 600 | 10% H2/He | 10% CO2/He | 1450 | - | Hare et al. [212] |
| LaCo0.5Fe0.25Mn0.25O3 | Quartz U-tube reactor connected with Cirrus MKS MS | 550 | 10% H2/He | 10% CO2/He | 1780 | - | Ramos et al. [213] |
| Y0.5Sr0.5MnO3 | TGA | 1400 | Ar | 40% CO2/Ar | 757 | 483 | Dey et al. [214] |
| Y0.5Ca0.5MnO3 | TGA | 1400 | Ar | 40% CO2/Ar | 671 | 575 | Dey et al. [214] |
| Pr0.18Sr0.80Mn0.99O2.951 | TGA | 1400 | Ar | CO2/Ar | 637.6 | 255.0 | Takalkar et al. [216] |
| La0.5Sr0.5MnO3−δ | TGA | 1400 | Ar | 50% CO2/Ar | 338.7 | 684.3 | Takalkar et al. [217] |
| Catalyst | Light Source | Solvent | Products | CO Yield (μmol/g) | CH4 Yield (μmol/g) | H2 Yield (μmol/g) | Reference |
|---|---|---|---|---|---|---|---|
| CsPbBr3 quantum dots (3.05–8.65 nm) | 100 W Xe lamp with an AM 1.5 G filter | Ethyl acetate | CO, CH4, H2 | 49.5 | 22.9 | 1.07 | Xu et al. [220] |
| CsPbBr3 quantum dots/GO composite | 100 W Xe lamp with an AM 1.5 G filter | Ethyl acetate | CO, CH4, H2 | 58.7 | 29.6 | 1.58 | Xu et al. [220] |
| CsPbBr3 quantum dots (3–12 nm) | 300 W Xe lamp with an AM 1.5 G filter | Ethyl acetate/H2O | CO, CH4, H2 | 34.1 ± 0.1 | 12.2 ± 0.1 | 0.80 ± 0.03 | Hou et al. [221] |
| CsPb(Br0.5/Cl0.5)3 nanocrystals | 300 W Xe lamp with an AM 1.5 G filter | Ethyl acetate | CO, CH4 | ca. 750 | ca. 125 | - | Guo et al. [222] |
| Cs2AgBiBr6 nanocrystals | 100 W Xe lamp with an AM 1.5 G filter | Ethyl acetate | CO, CH4 | 14.1 | 9.6 | - | Zhou et al. [223] |
| CsPbBr3 nanocrystals/Pd nanosheet composite | 150 W Xe lamp with with a 420 nm optical filter | H2O | CO, CH4, H2 | 12.633 | 3.935 | 1.167 | Xu et al. [224] |
| CsPbBr3 quantum dots/UiO-66 (NH2) composite | 300 W Xe lamp with a 420 nm UV-cut filter | Ethyl acetate/H2O | CO, CH4 | 98.57 | 3.08 | - | Wan et al. [225] |
| PbBiO2Br/carbonized polymer dots composite | 300 W Xe lamp | H2O | CO | ca. 48 | - | - | Wang et al. [226] |
| Boron-doped SrTiO3 | 300 W Xe arc lamp | H2O | CO, CH4, O2 | 21 * | 14 * | - | Shan et al. [227] |
| Ag-loaded H2SrTa2O7 | 300 W Xe lamp with λ > 200 nm | H2O | CO, H2 | 0.39 * | - | 0.25 * | Wang et al. [228] |
| Sodium tantalate nanocubes | 300 W Xe lamp with λ > 400 nm | H2O | CO, CH4 | 75 | 26 | - | Hou et al. [230] |
| N-doped graphene quantum dots-grafted sodium tantalate nanocubes | 300 W Xe lamp with λ > 400 nm | H2O | CO, CH4 | 180 | 45 | - | Hou et al. [230] |
9. Challenges and Future Directions
10. Conclusions
Funding
Conflicts of Interest
References
- Li, K.; Peng, B.; Peng, T. Recent advances in heterogeneous photocatalytic CO2 conversion to solar fuels. ACS Catal. 2016, 6, 7485–7527. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, W.; Li, Y.; Chen, J.; Yu, B.; Wang, J.; Zhang, L.; Zhang, J. Energy related CO2 conversion and utilization: Advanced materials/nanomaterials, reaction mechanisms and technologies. Nano Energy 2017, 40, 512–539. [Google Scholar] [CrossRef]
- Ahmad, W.; Khan, J.; Niu, G.; Tang, J. Inorganic CsPbI3 perovskite-based solar cells: A choice for a tandem device. Sol. RRL 2017, 1, 1700048. [Google Scholar] [CrossRef]
- Andrews, E.M.; Flake, J.; Fang, Y. CO2 electrocatalytic reduction at gold and copper electrodes: Role of particle size and surface chemistry. ECS Trans. 2015, 66, 67–70. [Google Scholar] [CrossRef]
- Baghel, R.S.; Mantri, V.A.; Reddy, C. A new wave of research interest in marine macroalgae for chemicals and fuels: Challenges and potentials. In Fuels, Chemicals and Materials from the Oceans and Aquatic Sources; Kerton, F.M., Yan, N., Eds.; John Wiley & Sons Ltd.: Hoboken, NY, USA, 2017; pp. 43–63. [Google Scholar]
- Anastasiou, S.; Bhoria, N.; Pokhrel, J.; Reddy, K.S.K.; Srinivasakannan, C.; Wang, K.; Karanikolos, G.N. Metal-organic framework/graphene oxide composite fillers in mixed-matrix membranes for CO2 separation. Mater. Chem. Phys. 2018, 212, 513–522. [Google Scholar] [CrossRef]
- Pokhrel, J.; Bhoria, N.; Anastasiou, S.; Tsoufis, T.; Gournis, D.; Romanos, G.; Karanikolos, G.N. CO2 adsorption behavior of amine-functionalized ZIF-8, graphene oxide, and ZIF-8/graphene oxide composites under dry and wet conditions. Microporous Mesoporous Mater. 2018, 267, 53–67. [Google Scholar] [CrossRef]
- Varghese, A.; Mittal, V. Performance of various adsorbents towards diverse gases: A comparative study. In Functional Nanomaterials and Nanotechnologies: Applications for Energy & Environment; Central West Publishing: Orange, NSW, Australia, 2018; pp. 305–404. [Google Scholar]
- Behar, O. Solar thermal power plants—A review of configurations and performance comparison. Renew. Sustain. Energy Rev. 2018, 92, 608–627. [Google Scholar] [CrossRef]
- Bhati, M.; Rai, R. Commercialization of large-scale perovskite solar energy technology and scaling-up issues. In Perovskite Photovoltaics; Elsevier: Amsterdam, The Netherlands, 2018; pp. 387–445. [Google Scholar]
- Bijarniya, J.P.; Sudhakar, K.; Baredar, P. Concentrated solar power technology in India: A review. Renew. Sustain. Energy Rev. 2016, 63, 593–603. [Google Scholar] [CrossRef]
- Bisquert, J.; Garcia-Belmonte, G.; Guerrero, A. Impedance characteristics of hybrid organometal halide perovskite solar cells. In Organic-Inorganic Halide Perovskite Photovoltaics: From Fundamentals to Device Architectures; Springer: Cham, Switzerland, 2016; pp. 163–199. [Google Scholar]
- Brendelberger, S.; Sattler, C. Concept analysis of an indirect particle-based redox process for solar-driven H2O/CO2 splitting. Sol. Energy 2015, 113, 158–170. [Google Scholar] [CrossRef]
- Calió, L.; Kazim, S.; Grätzel, M.; Ahmad, S. Hole-transport materials for perovskite solar cells. Angew. Chem. Int. Ed. 2016, 55, 14522–14545. [Google Scholar] [CrossRef]
- Chatzichristodoulou, C.; Chen, M.; Hendriksen, P.V.; Jacobsen, T.; Mogensen, M.B. Understanding degradation of solid oxide electrolysis cells through modeling of electrochemical potential profiles. Electrochimica Acta 2016, 189, 265–282. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Yang, S. Carbon-based perovskite solar cells without hole transport materials: The front runner to the market? Adv. Mater. 2017, 29, 1603994. [Google Scholar] [CrossRef]
- Chen, P.; Bai, Y.; Lyu, M.; Yun, J.H.; Hao, M.; Wang, L. Progress and perspective in low-dimensional metal halide perovskites for optoelectronic applications. Sol. RRL 2018, 2, 1700186. [Google Scholar] [CrossRef]
- Chen, S.; Pan, B.; Zeng, L.; Luo, S.; Wang, X.; Su, W. La2Sn2O7 enhanced photocatalytic CO2 reduction with H2O by deposition of Au co-catalyst. RSC Adv. 2017, 7, 14186–14191. [Google Scholar] [CrossRef] [Green Version]
- Ezbiri, M.; Takacs, M.; Stolz, B.; Lungthok, J.; Steinfeld, A.; Michalsky, R. Design principles of perovskites for solar-driven thermochemical splitting of CO2. J. Mater. Chem. A 2017, 5, 15105–15115. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Waterhouse, G.I.; Chen, G.; Xiong, X.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. Two-dimensional-related catalytic materials for solar-driven conversion of COx into valuable chemical feedstocks. Chem. Soc. Rev. 2019, 48, 1972–2010. [Google Scholar] [CrossRef]
- Salhi, B.; Wudil, Y.; Hossain, M.; Al-Ahmed, A.; Al-Sulaiman, F. Review of recent developments and persistent challenges in stability of perovskite solar cells. Renew. Sustain. Energy Rev. 2018, 90, 210–222. [Google Scholar] [CrossRef]
- Wang, J.; Di Giacomo, F.; Brüls, J.; Gorter, H.; Katsouras, I.; Groen, P.; Janssen, R.A.; Andriessen, R.; Galagan, Y. Highly efficient perovskite solar cells using non-toxic industry compatible solvent system. Sol. RRL 2017, 1, 1700091. [Google Scholar] [CrossRef]
- Shi, R.; Waterhouse, G.I.; Zhang, T. Recent progress in photocatalytic CO2 reduction over perovskite oxides. Sol. RRL 2017, 1, 1700126. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, Z.; Zhou, L.; Chen, X.; Chen, J.; Zhou, Y.; Roy, V.; Han, S.-T. Emerging perovskite materials for high density data storage and artificial synapses. J. Mater. Chem. C 2018, 6, 1600–1617. [Google Scholar] [CrossRef]
- Tu, W.; Zhou, Y.; Zou, Z. Photocatalytic conversion of CO2 into renewable hydrocarbon fuels: State-of-the-art accomplishment, challenges, and prospects. Adv. Mater. 2014, 26, 4607–4626. [Google Scholar] [CrossRef] [PubMed]
- Kubicek, M.; Bork, A.H.; Rupp, J.L. Perovskite oxides—A review on a versatile material class for solar-to-fuel conversion processes. J. Mater. Chem. A 2017, 5, 11983–12000. [Google Scholar] [CrossRef]
- Risch, M. Perovskite electrocatalysts for the oxygen reduction reaction in alkaline media. Catalysts 2017, 7, 154. [Google Scholar] [CrossRef] [Green Version]
- Yun, S.; Qin, Y.; Uhl, A.R.; Vlachopoulos, N.; Yin, M.; Li, D.; Han, X.; Hagfeldt, A. New-generation integrated devices based on dye-sensitized and perovskite solar cells. Energy Environ. Sci. 2018, 11, 476–526. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, J.; Yu, B.; Zhang, W.; Chen, J.; Qiao, J.; Zhang, J. A review of high temperature co-electrolysis of H2O and CO2 to produce sustainable fuels using solid oxide electrolysis cells (SOECs): Advanced materials and technology. Chem. Soc. Rev. 2017, 46, 1427–1463. [Google Scholar] [CrossRef]
- Yang, M.-Q.; Xu, Y.-J. Photocatalytic conversion of CO2 over graphene-based composites: Current status and future perspective. Nanoscale Horiz. 2016, 1, 185–200. [Google Scholar] [CrossRef]
- Sahara, G.; Kumagai, H.; Maeda, K.; Kaeffer, N.; Artero, V.; Higashi, M.; Abe, R.; Ishitani, O. Photoelectrochemical reduction of CO2 coupled to water oxidation using a photocathode with a Ru(II)–Re(I) complex photocatalyst and a CoOx/TaON Photoanode. J. Am. Chem. Soc. 2016, 138, 14152–14158. [Google Scholar] [CrossRef]
- Costentin, C.; Robert, M.; Savéant, J.-M. Catalysis of the electrochemical reduction of carbon dioxide. Chem. Soc. Rev. 2013, 42, 2423–2436. [Google Scholar] [CrossRef]
- Ganesh, I. Electrochemical conversion of carbon dioxide into renewable fuel chemicals—The role of nanomaterials and the commercialization. Renew. Sustain. Energy Rev. 2016, 59, 1269–1297. [Google Scholar] [CrossRef]
- Atsonios, K.; Panopoulos, K.D.; Kakaras, E. Thermocatalytic CO2 hydrogenation for methanol and ethanol production: Process improvements. Int. J. Hydrogen Energy 2016, 41, 792–806. [Google Scholar] [CrossRef]
- Smestad, G.P.; Steinfeld, A. photochemical and thermochemical production of solar fuels from H2O and CO2 using metal oxide catalysts. Ind. Eng. Chem. Res. 2012, 51, 11828–11840. [Google Scholar] [CrossRef]
- Wei-Guang, D.E.; Chao-Yu, C.P. Perovskite Solar Cells: Principle, Materials and Devices; World Scientific: Singapore, 2017; Volume 1. [Google Scholar]
- Dolganov, A.; Tanaseichuk, B.; Ivantsova, P.; Tsebulaeva, Y.; Kostrukov, S.; Moiseeva, D.; Shmelkova, N.; Yurova, V.; Balakireva, O.; Trushkova, N. Metal-free electrocatalyst for hydrogen production from water. Int. J. Electrochem. Sci. 2016, 11, 9559–9565. [Google Scholar] [CrossRef]
- Kanhere, P.; Chen, Z. A review on visible light active perovskite-based photocatalysts. Molecules 2014, 19, 19995–20022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Tadé, M.O.; Shao, Z. Research progress of perovskite materials in photocatalysis-and photovoltaics-related energy conversion and environmental treatment. Chem. Soc. Rev. 2015, 44, 5371–5408. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, D.; Ye, M.; Zhang, C.; Xu, B.; Lin, C.; Sun, L.; Lin, Z. One-dimensional densely aligned perovskite-decorated semiconductor heterojunctions with enhanced photocatalytic activity. Small 2015, 11, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Zhou, W.; Ren, Z.; Du, S.; Meng, X.; Tian, G.; Pan, K.; Wang, G.; Fu, H. Facile preparation of porous NiTiO3 nanorods with enhanced visible-light-driven photocatalytic performance. J. Mater. Chem. 2012, 22, 16471–16476. [Google Scholar] [CrossRef]
- Döscher, H.; Geisz, J.; Deutsch, T.; Turner, J. Sunlight absorption in water–efficiency and design implications for photoelectrochemical devices. Energy Environ. Sci. 2014, 7, 2951–2956. [Google Scholar] [CrossRef]
- Fan, R.; Zhou, N.; Zhang, L.; Yang, R.; Meng, Y.; Li, L.; Guo, T.; Chen, Y.; Xu, Z.; Zheng, G. Toward full solution processed perovskite/Si monolithic tandem solar device with PCE exceeding 20%. Sol. RRL 2017, 1, 1700149. [Google Scholar] [CrossRef]
- Zhang, M.; Lyu, M.; Chen, P.; Hao, M.; Yun, J.H.; Wang, L. Recent advances in low-toxic lead-free metal halide perovskite materials for solar cell application. Asia-Pac. J. Chem. Eng. 2016, 11, 392–398. [Google Scholar] [CrossRef]
- Zhang, Z.; Qin, J.; Shi, W.; Liu, Y.; Zhang, Y.; Liu, Y.; Gao, H.; Mao, Y. Enhanced power conversion efficiency of perovskite solar cells with an up-conversion material of Er3+-Yb3+-Li+ tri-doped TiO2. Nanoscale Res. Lett. 2018, 13, 147. [Google Scholar] [CrossRef] [Green Version]
- Maiti, D.; Hare, B.J.; Daza, Y.A.; Ramos, A.E.; Kuhn, J.N.; Bhethanabotla, V.R. Earth abundant perovskite oxides for low temperature CO2 conversion. Energy Environ. Sci. 2018, 11, 648–659. [Google Scholar] [CrossRef]
- Ibn-Mohammed, T.; Koh, S.; Reaney, I.; Acquaye, A.; Schileo, G.; Mustapha, K.; Greenough, R. Perovskite solar cells: An integrated hybrid lifecycle assessment and review in comparison with other photovoltaic technologies. Renew. Sustain. Energy Rev. 2017, 80, 1321–1344. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, Y. Reactivity and efficiency of ceria-based oxides for solar CO2 splitting via isothermal and near-isothermal cycles. Energy Fuels 2017, 32, 736–746. [Google Scholar] [CrossRef]
- Guo, Z.; Gao, L.; Zhang, C.; Xu, Z.; Ma, T. Low-temperature processed non-TiO2 electron selective layers for perovskite solar cells. J. Mater. Chem. A 2018, 6, 4572–4589. [Google Scholar] [CrossRef]
- Gupta, K.; Bersani, M.; Darr, J.A. Highly efficient electro-reduction of CO2 to formic acid by nano-copper. J. Mater. Chem. A 2016, 4, 13786–13794. [Google Scholar] [CrossRef] [Green Version]
- Hathaway, B.J.; Bala Chandran, R.; Gladen, A.C.; Chase, T.R.; Davidson, J.H. Demonstration of a solar reactor for carbon dioxide splitting via the isothermal ceria redox cycle and practical implications. Energy Fuels 2016, 30, 6654–6661. [Google Scholar] [CrossRef]
- He, J.; Bi, E.; Tang, W.; Wang, Y.; Zhou, Z.; Yang, X.; Chen, H.; Han, L. Ligand-free, highly dispersed NiOx nanocrystal for efficient, stable, low-temperature processable perovskite solar cells. Sol. RRL 2018, 2, 1800004. [Google Scholar] [CrossRef]
- Hori, Y. CO2 Reduction using electrochemical approach. In Solar to Chemical Energy Conversion: Theory and Application; Springer: Cham, Switzerland, 2016; pp. 191–211. [Google Scholar]
- Hossain, M.I.; Qarony, W.; Jovanov, V.; Tsang, Y.H.; Knipp, D. Nanophotonic design of perovskite/silicon tandem solar cells. J. Mater. Chem. A 2018, 6, 3625–3633. [Google Scholar] [CrossRef]
- Huang, C.; Li, Z.; Zou, Z. A perspective on perovskite oxide semiconductor catalysts for gas phase photoreduction of carbon dioxide. MRS Commun. 2016, 6, 216–225. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Cheng, Q.; Fan, R.; Zhou, H. Recent development of organic–inorganic perovskite-based tandem solar cells. Sol. RRL 2017, 1, 1700045. [Google Scholar] [CrossRef]
- Di Giacomo, F.; Galagan, Y.; Shanmugam, S.; Gorter, H.; van den Bruele, F.; Kirchner, G.; de Vries, I.; Fledderus, H.; Lifka, H.; Veenstra, S. Up-scaling perovskite solar cell manufacturing from sheet-to-sheet to roll-to-roll: Challenges and solutions. In Proceedings of the Organic, Hybrid, and Perovskite Photovoltaics XVIII, San Diego, CA, USA, 6–10 August 2017. [Google Scholar]
- Dias, P.; Mendes, A. Hydrogen production from photoelectrochemical water splitting. In Fuel Cells and Hydrogen Production: A Volume in the Encyclopedia of Sustainability Science and Technology, 2nd ed.; Lipman, T.E., Weber, A., Eds.; Springer-Verlag: New York, NY, USA, 2019; pp. 1003–1053. [Google Scholar]
- Williams, S.T.; Chueh, C.C.; Jen, A.K.Y. Navigating organo-lead halide perovskite phase space via nucleation kinetics toward a deeper understanding of perovskite phase transformations and structure–property relationships. Small 2015, 11, 3088–3096. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, Z.; Ming, N. Perovskite oxide nanotubes: Synthesis, structural characterization, properties and applications. J. Mater. Chem. 2010, 20, 4015–4030. [Google Scholar] [CrossRef]
- Choi, J.J.; Yang, X.; Norman, Z.M.; Billinge, S.J.; Owen, J.S. Structure of methylammonium lead iodide within mesoporous titanium dioxide: Active material in high-performance perovskite solar cells. Nano Lett. 2013, 14, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Colonna, S.; De Rossi, S.; Faticanti, M.; Pettiti, I.; Porta, P. Zirconia supported La, Co oxides and LaCoO3 perovskite: Structural characterization and catalytic CO oxidation. J. Mol. Catal. A Chem. 2002, 180, 161–168. [Google Scholar] [CrossRef]
- Crumlin, E.J.; Mutoro, E.; Liu, Z.; Grass, M.E.; Biegalski, M.D.; Lee, Y.-L.; Morgan, D.; Christen, H.M.; Bluhm, H.; Shao-Horn, Y. Surface strontium enrichment on highly active perovskites for oxygen electrocatalysis in solid oxide fuel cells. Energy Environ. Sci. 2012, 5, 6081–6088. [Google Scholar] [CrossRef]
- Keav, S.; Matam, S.; Ferri, D.; Weidenkaff, A. Structured perovskite-based catalysts and their application as three-way catalytic converters—A review. Catalysts 2014, 4, 226–255. [Google Scholar] [CrossRef] [Green Version]
- Avila, P.; Montes, M.; Miró, E.E. Monolithic reactors for environmental applications: A review on preparation technologies. Chem. Eng. J. 2005, 109, 11–36. [Google Scholar] [CrossRef]
- Tanaka, H.; Taniguchi, M.; Uenishi, M.; Kajita, N.; Tan, I.; Nishihata, Y.; Mizuki, J.I.; Narita, K.; Kimura, M.; Kaneko, K. Self-regenerating Rh-and Pt-based perovskite catalysts for automotive-emissions control. Angew. Chem. Int. Ed. 2006, 45, 5998–6002. [Google Scholar] [CrossRef]
- Abdelhady, A.L.; Saidaminov, M.I.; Murali, B.; Adinolfi, V.; Voznyy, O.; Katsiev, K.; Alarousu, E.; Comin, R.; Dursun, I.; Sinatra, L. Heterovalent dopant incorporation for bandgap and type engineering of perovskite crystals. J. Phys. Chem. Lett. 2016, 7, 295–301. [Google Scholar] [CrossRef]
- Kueh, B.; Kapsi, M.; Veziri, C.M.; Athanasekou, C.; Pilatos, G.; Reddy, K.S.K.; Raj, A.; Karanikolos, G.N. Asphaltene-derived activated carbon and carbon nanotube membranes for CO2 separation. Energy Fuels 2018, 32, 11718–11730. [Google Scholar] [CrossRef]
- Kontos, A.G.; Likodimos, V.; Veziri, C.M.; Kouvelos, E.; Moustakas, N.; Karanikolos, G.N.; Romanos, G.E.; Falaras, P. CO2 captured in zeolitic imidazolate frameworks: Raman spectroscopic analysis of uptake and host–guest interactions. ChemSusChem 2014, 7, 1696–1702. [Google Scholar] [CrossRef] [PubMed]
- Stoeger, J.A.; Palomino, M.; Agrawal, K.V.; Zhang, X.; Karanikolos, G.N.; Valencia, S.; Corma, A.; Tsapatsis, M. Oriented CoSAPO-5 membranes by microwave-enhanced growth on TiO2-coated porous alumina. Angew. Chem. Int. Ed. 2012, 51, 2470–2473. [Google Scholar] [CrossRef] [PubMed]
- Langørgen, Ø.; Saanum, I.; Haugen, N.E.L. Chemical looping combustion of methane using a copper-based oxygen carrier in a 150 kW reactor system. Energy Procedia 2017, 114, 352–360. [Google Scholar] [CrossRef]
- Nadarajah, A. Fabrication and Processing of Next-Generation Oxygen Carrier Materials for Chemical Looping Combustion; Technical Report No. DE-FE0008774; University of Toledo: Toledo, OH, USA, 2017. [Google Scholar]
- Pishahang, M.; Larring, Y.; Sunding, M.; Jacobs, M.; Snijkers, F. Performance of perovskite-type oxides as oxygen-carrier materials for chemical looping combustion in the presence of H2S. Energy Technol. 2016, 4, 1305–1316. [Google Scholar] [CrossRef]
- Figueroa, J.D.; Fout, T.; Plasynski, S.; McIlvried, H.; Srivastava, R.D. Advances in CO2 capture technology—The US Department of Energy’s Carbon Sequestration Program. Int. J. Greenh. Gas Control 2008, 2, 9–20. [Google Scholar] [CrossRef]
- Goel, M. CO2 Capture and utilization for the energy industry: Outlook for capability development to address climate change in India. In Carbon Utilization—Applications for the Energy Industry; Springer: Singapore, 2017; pp. 3–33. [Google Scholar]
- Yeh, S.; Rubin, E.S. A review of uncertainties in technology experience curves. Energy Econ. 2012, 34, 762–771. [Google Scholar] [CrossRef]
- Fan, L.-S.; Zeng, L.; Wang, W.; Luo, S. Chemical looping processes for CO2 capture and carbonaceous fuel conversion—Prospect and opportunity. Energy Environ. Sci. 2012, 5, 7254–7280. [Google Scholar] [CrossRef]
- Han, L.; Lin, M.; Haussener, S. Reliable performance characterization of mediated photocatalytic water-splitting half reactions. ChemSusChem 2017, 10, 2158–2166. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Galvita, V.; Poelman, H.; Marin, G. Advanced chemical looping materials for CO2 utilization: A review. Materials 2018, 11, 1187. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.S.; Zeng, L.; Luo, S. Chemical-looping technology platform. AIChE J. 2015, 61, 2–22. [Google Scholar] [CrossRef]
- Bhavsar, S.; Veser, G.T. Reducible supports for Ni-based oxygen carriers in chemical looping combustion. Energy Fuels 2013, 27, 2073–2084. [Google Scholar] [CrossRef]
- Liu, G.; He, D.; Yao, R.; Zhao, Y.; Li, J. Amorphous NiFeB nanoparticles realizing highly active and stable oxygen evolving reaction for water splitting. Nano Res. 2018, 11, 1664–1675. [Google Scholar] [CrossRef]
- Jamesh, M.I. Recent progress on earth abundant hydrogen evolution reaction and oxygen evolution reaction bifunctional electrocatalyst for overall water splitting in alkaline media. J. Power Sources 2016, 333, 213–236. [Google Scholar] [CrossRef]
- Serrano, A.; García-Labiano, F.; de Diego, L.; Gayán, P.; Abad, A.; Adánez, J. Chemical Looping Combustion of liquid fossil fuels in a 1 kWth unit using a Fe-based oxygen carrier. Fuel Process. Technol. 2017, 160, 47–54. [Google Scholar] [CrossRef]
- Thursfield, A.; Murugan, A.; Franca, R.; Metcalfe, I.S. Chemical looping and oxygen permeable ceramic membranes for hydrogen production—A review. Energy Environ. Sci. 2012, 5, 7421–7459. [Google Scholar] [CrossRef]
- Kwak, B.S.; Park, N.-K.; Ryu, S.O.; Baek, J.-I.; Ryu, H.-J.; Kang, M. Improved reversible redox cycles on MTiOx (M = Fe, Co, Ni, and Cu) particles afforded by rapid and stable oxygen carrier capacity for use in chemical looping combustion of methane. Chem. Eng. J. 2017, 309, 617–627. [Google Scholar] [CrossRef]
- Ito, S. Inorganic hole-transporting materials for perovskite solar cell. In Organic-Inorganic Halide Perovskite Photovoltaics; Park, N.-G., Grätzel, M., Miyasaka, T., Eds.; Springer: Cham, Switzerland, 2016; pp. 343–366. [Google Scholar]
- Gayán, P.; Luis, F.; García-Labiano, F.; Adánez, J.; Abad, A.; Dueso, C. Effect of support on reactivity and selectivity of Ni-based oxygen carriers for chemical-looping combustion. Fuel 2008, 87, 2641–2650. [Google Scholar] [CrossRef] [Green Version]
- Dueso, C.; Ortiz, M.; Abad, A.; García-Labiano, F.; Luis, F.; Gayán, P.; Adánez, J. Reduction and oxidation kinetics of nickel-based oxygen-carriers for chemical-looping combustion and chemical-looping reforming. Chem. Eng. J. 2012, 188, 142–154. [Google Scholar] [CrossRef] [Green Version]
- Rydén, M.; Lyngfelt, A.; Mattisson, T. Chemical-looping combustion and chemical-looping reforming in a circulating fluidized-bed reactor using Ni-based oxygen carriers. Energy Fuels 2008, 22, 2585–2597. [Google Scholar] [CrossRef] [Green Version]
- Luis, F.; Ortiz, M.; Adánez, J.; García-Labiano, F.; Abad, A.; Gayán, P. Synthesis gas generation by chemical-looping reforming in a batch fluidized bed reactor using Ni-based oxygen carriers. Chem. Eng. J. 2008, 144, 289–298. [Google Scholar]
- Ipsakis, D.; Heracleous, E.; Silvester, L.; Bukur, D.; Lemonidou, A. Kinetic modeling of NiO-based oxygen carriers for the sorption enhanced chemical looping steam CH4 reforming. Mater. Today Proc. 2018, 5, 27353–27361. [Google Scholar] [CrossRef]
- Tijani, M.M.A.A. Study of the Methane Chemical Looping Combustion Process Using Supported Metal Carriers for Carbon Capture. Ph.D. Thesis, University of Calgary, Calgary, AB, Canada, November 2018. [Google Scholar]
- Park, J.H.; Hwang, R.H.; ur Rasheed, H.; Baek, J.I.; Ryu, H.J.; Yi, K.B. Kinetics of the reduction and oxidation of Mg added NiO/Al2O3 for chemical looping combustion. Chem. Eng. Res. Des. 2019, 141, 481–491. [Google Scholar] [CrossRef]
- Choudhary, V.; Uphade, B.; Mamman, A. Oxidative conversion of methane to syngas over nickel supported on commercial low surface area porous catalyst carriers precoated with alkaline and rare earth oxides. J. Catal. 1997, 172, 281–293. [Google Scholar] [CrossRef]
- Wang, S.; Lu, G. A comprehensive study on carbon dioxide reforming of methane over Ni/γ-Al2O3 catalysts. Ind. Eng. Chem. Res. 1999, 38, 2615–2625. [Google Scholar] [CrossRef]
- Chen, S.; Lior, N.; Xiang, W. Coal gasification integration with solid oxide fuel cell and chemical looping combustion for high-efficiency power generation with inherent CO2 capture. Appl. Energy 2015, 146, 298–312. [Google Scholar] [CrossRef]
- Gayán, P.; Dueso, C.; Abad, A.; Adanez, J.; Luis, F.; García-Labiano, F. NiO/Al2O3 oxygen carriers for chemical-looping combustion prepared by impregnation and deposition–precipitation methods. Fuel 2009, 88, 1016–1023. [Google Scholar] [CrossRef] [Green Version]
- Sedor, K.E.; Hossain, M.M.; de Lasa, H.I. Reactivity and stability of Ni/Al2O3 oxygen carrier for chemical-looping combustion (CLC). Chem. Eng. Sci. 2008, 63, 2994–3007. [Google Scholar] [CrossRef]
- Yu, L.; Song, M.; Williams, P.T.; Wei, Y. Alumina-supported spinel Ni Al2O4 as a catalyst for reforming pyrolysis gas. Ind. Eng. Chem. Res. 2019, 58, 11770–11778. [Google Scholar] [CrossRef]
- Beierlein, D.; Häussermann, D.; Pfeifer, M.; Schwarz, T.; Stöwe, K.; Traa, Y.; Klemm, E. Is the CO2 methanation on highly loaded Ni-Al2O3 catalysts really structure-sensitive? Appl. Catal. B Environ. 2019, 247, 200–219. [Google Scholar] [CrossRef]
- Baek, J.-I.; Kim, U.; Jo, H.; Eom, T.H.; Lee, J.B.; Ryu, H.-J. Effect of Mg–Al layered double hydroxide use on the performance of a spray-dried NiO oxygen carrier. J. Chem. Eng. Jpn. 2018, 51, 596–604. [Google Scholar] [CrossRef]
- Adánez, J.; García-Labiano, F.; Abad, A.; de Diego, L.; Gayán, P.; Dueso, C. NiO-based oxygen carriers impregnated on Al2O3-based materials for chemical-looping combustion. In Carbon Dioxide Capture for Storage in Deep Geological Formations—Results from the CO2 Capture Project; Eide, L.I., Ed.; CPL Press: Newbury, UK, 2009; Volume 3, pp. 85–94. [Google Scholar]
- Shervedani, R.K.; Torabi, M.; Yaghoobi, F. Binder-free prickly nickel nanostructured/reduced graphene oxide composite: A highly efficient electrocatalyst for hydrogen evolution reaction in alkaline solutions. Electrochim. Acta 2017, 244, 230–238. [Google Scholar] [CrossRef]
- Roux, S.; Bensakhria, A.; Antonini, G. Study and improvement of the regeneration of metallic oxides used as oxygen carriers for a new combustion process. Int. J. Chem. React. Eng. 2006, 4, 1542–6580. [Google Scholar] [CrossRef]
- Wei, Y. Syngas generation from methane using a chemical-looping concept: A review of oxygen carriers. J. Chem. 2013, 2013, 294817. [Google Scholar]
- Ahmed, W.; Awadallah, A.E.; Aboul-Enein, A.A. Ni/CeO2–Al2O3 catalysts for methane thermo-catalytic decomposition to COx-free H2 production. Int. J. Hydrogen Energy 2016, 41, 18484–18493. [Google Scholar] [CrossRef]
- Yonggang, W.; Hua, W.; Kongzhai, L.; Xing, Z.; Yunpeng, D. Preparation and characterization of Ce1−x NixO2 as oxygen carrier for selective oxidation methane to syngas in absence of gaseous oxygen. J. Rare Earths 2010, 28, 357–361. [Google Scholar]
- Du, X.; Zhang, D.; Shi, L.; Gao, R.; Zhang, J. Morphology dependence of catalytic properties of Ni/CeO2 nanostructures for carbon dioxide reforming of methane. J. Phys. Chem. C 2012, 116, 10009–10016. [Google Scholar] [CrossRef]
- Hossain, M.M. Solid-state kinetics of reduction of NiO/Ce–γAl2O3 oxygen carriers for chemical-looping combustion. Arab. J. Sci. Eng. 2018, 43, 2281–2290. [Google Scholar] [CrossRef]
- Zhao, X.; Ngo, H.T.; Walker, D.M.; Weber, D.; Maiti, D.; Cimenler, U.; Petrov, A.D.; Joseph, B.; Kuhn, J.N. Tri-reforming of surrogate biogas over Ni/Mg/ceria–zirconia/alumina pellet catalysts. Chem. Eng. Commun. 2018, 205, 1129–1142. [Google Scholar] [CrossRef]
- Abad, A.; Mattisson, T.; Lyngfelt, A.; Johansson, M. The use of iron oxide as oxygen carrier in a chemical-looping reactor. Fuel 2007, 86, 1021–1035. [Google Scholar] [CrossRef]
- Ma, S.; Chen, S.; Soomro, A.; Xiang, W. Effects of supports on hydrogen production and carbon deposition of Fe-based oxygen carriers in chemical looping hydrogen generation. Int. J. Hydrogen Energy 2017, 42, 11006–11016. [Google Scholar] [CrossRef]
- Bhui, B.; Vairakannu, P. Experimental and kinetic studies on in-situ CO2 gasification based chemical looping combustion of low ash coal using Fe2O3 as the oxygen carrier. J. CO2 Util. 2019, 29, 103–116. [Google Scholar] [CrossRef]
- Hu, S.; Chen, Q.; Xiang, J.; Su, S.; Sun, L.; Wang, Y.; Xu, B.; Chi, H. Modification of iron oxide to promote reaction property for chemical looping combustion with CO. Combust. Sci. Technol. 2016, 188, 1319–1330. [Google Scholar] [CrossRef]
- De Diego, L.F.; Serrano, A.; García-Labiano, F.; García-Díez, E.; Abad, A.; Gayán, P.; Adánez, J. Bioethanol combustion with CO2 capture in a 1 kWth Chemical Looping Combustion prototype: Suitability of the oxygen carrier. Chem. Eng. J. 2016, 283, 1405–1413. [Google Scholar] [CrossRef]
- Karimi, E.; Forutan, H.; Saidi, M.; Rahimpour, M.; Shariati, A. Experimental study of chemical-looping reforming in a fixed-bed reactor: Performance investigation of different oxygen carriers on Al2O3 and TiO2 support. Energy Fuels 2014, 28, 2811–2820. [Google Scholar] [CrossRef]
- Hafizi, A.; Rahimpour, M.; Hassanajili, S. Calcium promoted Fe/Al2O3 oxygen carrier for hydrogen production via cyclic chemical looping steam methane reforming process. Int. J. Hydrogen Energy 2015, 40, 16159–16168. [Google Scholar] [CrossRef]
- Siriwardane, R.; Tian, H.; Miller, D.; Richards, G. Fluidized bed testing of commercially prepared MgO-promoted hematite and CuO–Fe2O3 mixed metal oxide oxygen carriers for methane and coal chemical looping combustion. Appl. Energy 2015, 157, 348–357. [Google Scholar] [CrossRef]
- Hafizi, A.; Rahimpour, M.; Hassanajili, S. High purity hydrogen production via sorption enhanced chemical looping reforming: Application of 22Fe2O3/MgAl2O4 and 22Fe2O3/Al2O3 as oxygen carriers and cerium promoted CaO as CO2 sorbent. Appl. Energy 2016, 169, 629–641. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Ku, Y.; Tseng, Y.-H.; Lee, H.-Y.; Kuo, Y.-L. Fabrication of Fe2O3/TiO2 oxygen carriers for chemical looping combustion and hydrogen generation. Aerosol Air Qual. Res 2016, 16, 2023–2032. [Google Scholar] [CrossRef] [Green Version]
- Tang, M.; Liu, K.; Roddick, D.M.; Fan, M. Enhanced lattice oxygen reactivity over Fe2O3/Al2O3 redox catalyst for chemical-looping dry (CO2) reforming of CH4: Synergistic La-Ce effect. J. Catal. 2018, 368, 38–52. [Google Scholar] [CrossRef]
- Hu, J.; Galvita, V.V.; Poelman, H.; Detavernier, C.; Marin, G.B. A core-shell structured Fe2O3/ZrO2@ZrO2 nanomaterial with enhanced redox activity and stability for CO2 conversion. J. CO2 Util. 2017, 17, 20–31. [Google Scholar] [CrossRef]
- Pérez-Vega, R.; Abad, A.; Gayán, P.; de Diego, L.; García-Labiano, F.; Adánez, J. Development of (Mn0.77Fe0.23)2O3 particles as an oxygen carrier for coal combustion with CO2 capture via in-situ gasification chemical looping combustion (iG-CLC) aided by oxygen uncoupling (CLOU). Fuel Process. Technol. 2017, 164, 69–79. [Google Scholar]
- Pérez-Vega, R.; Abad, A.; Izquierdo, M.; Gayán, P.; de Diego, L.; Adánez, J. Evaluation of Mn-Fe mixed oxide doped with TiO2 for the combustion with CO2 capture by Chemical Looping assisted by Oxygen Uncoupling. Appl. Energy 2019, 237, 822–835. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Jung, H.S.; Kang, Y.T. A review: Effect of nanostructures on photocatalytic CO2 conversion over metal oxides and compound semiconductors. J. CO2 Util. 2017, 20, 163–177. [Google Scholar] [CrossRef]
- Liu, F.; Chen, L.; Neathery, J.K.; Saito, K.; Liu, K. Cerium oxide promoted iron-based oxygen carrier for chemical looping combustion. Ind. Eng. Chem. Res. 2014, 53, 16341–16348. [Google Scholar] [CrossRef]
- Kang, D.; Lee, M.; Lim, H.S.; Lee, J.W. Chemical looping partial oxidation of methane with CO2 utilization on the ceria-enhanced mesoporous Fe2O3 oxygen carrier. Fuel 2018, 215, 787–798. [Google Scholar] [CrossRef]
- Hafizi, A.; Jafari, M.; Rahimpour, M.; Hassanajili, S. Experimental investigation of sorption enhanced chemical looping reforming for high purity hydrogen production using CeO2–CaO CO2 sorbent and 15Fe–5Ca/Al2O3 oxygen carrier. J. Taiwan Inst. Chem. Eng. 2016, 65, 185–196. [Google Scholar] [CrossRef]
- Kuo, Y.-L.; Huang, W.-C.; Hsu, W.-M.; Tseng, Y.-H.; Ku, Y. Use of spinel nickel aluminium ferrite as self-supported oxygen carrier for chemical looping hydrogen generation process. Aerosol Air Qual. Res. 2015, 15, 2700–2708. [Google Scholar] [CrossRef] [Green Version]
- Zaabout, A.; Dahl, P.I.; Ugwu, A.; Tolchard, J.R.; Cloete, S.; Amini, S. Gas Switching Reforming (GSR) for syngas production with integrated CO2 capture using iron-based oxygen carriers. Int. J. Greenh. Gas Control 2019, 81, 170–180. [Google Scholar] [CrossRef]
- Hu, J.; Buelens, L.; Theofanidis, S.-A.; Galvita, V.V.; Poelman, H.; Marin, G.B. CO2 conversion to CO by auto-thermal catalyst-assisted chemical looping. J. CO2 Util. 2016, 16, 8–16. [Google Scholar] [CrossRef]
- Huang, X.; Wang, X.; Fan, M.; Wang, Y.; Adidharma, H.; Gasem, K.A.; Radosz, M. A cost-effective approach to reducing carbon deposition and resulting deactivation of oxygen carriers for improvement of energy efficiency and CO2 capture during methane chemical-looping combustion. Appl. Energy 2017, 193, 381–392. [Google Scholar] [CrossRef]
- Pérez-Vega, R.; Adánez-Rubio, I.; Gayán, P.; Izquierdo, M.T.; Abad, A.; García-Labiano, F.; Luis, F.; Adánez, J. Sulphur, nitrogen and mercury emissions from coal combustion with CO2 capture in chemical looping with oxygen uncoupling (CLOU). Int. J. Greenh. Gas Control 2016, 46, 28–38. [Google Scholar] [CrossRef]
- Adánez-Rubio, I.; Abad, A.; Gayán, P.; Luis, F.; Adánez, J. CLOU process performance with a Cu-Mn oxygen carrier in the combustion of different types of coal with CO2 capture. Fuel 2018, 212, 605–612. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Li, J.; Zhu, Q.; Peng, W.; Li, H. Fabrication of hierarchical Co/MgO catalyst for enhanced CO2 reforming of CH4 in a fluidized-bed reactor. AIChE J. 2019, 65, 120–131. [Google Scholar] [CrossRef] [Green Version]
- Moldenhauer, P.; Hallberg, P.; Biermann, M.; Snijkers, F.; Albertsen, K.; Mattisson, T.; Lyngfelt, A. Oxygen carrier development of calcium manganite-based materials with perovskite structure for chemical looping combustion of methane. In Proceedings of the 42nd International Technical Conference on Clean Energy, Clearwater, FL, USA, 11–15 June 2017. [Google Scholar]
- Zhu, Y.; Liu, R.; Sun, X.; Ma, X.; Wang, X.; Tian, H. Metal modified hexaaluminates for syngas generation and CO2 utilization via chemical looping. Int. J. Hydrogen Energy 2019, 44, 10218–10231. [Google Scholar] [CrossRef]
- Huang, F.; Tian, M.; Zhu, Y.; Wang, X.; Wang, A.; Li, L.; Lin, J.; Wang, J. Fe-substituted Ba-hexaaluminate with enhanced oxygen mobility for CO2 capture by chemical looping combustion of methane. J. Energy Chem. 2019, 29, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Pecchi, G.; Escalona, N.; Ghampson, I.T.; Morales, R. Energy production, decontamination, and hydrogenation reactions over perovskite-type oxide catalyst. In Perovskite Materials—Synthesis, Characterisation, Properties, and Applications; Pan, L., Zhu, G., Eds.; IntechOpen: London, UK, 2016. [Google Scholar]
- Santinacci, L. ALD for photoelectrochemical water splitting. In Atomic Layer Deposition in Energy Conversion Applications; Bachmann, J., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016. [Google Scholar]
- Radha, R.; Sakar, M.; Bharathkumar, S.; Balakumar, S. Sunlight driven photocatalytic water splitting using nanostructured bismuth tungstate (Bi2WO6). AIP Conf. Proc. 2017, 1832, 050031. [Google Scholar]
- Sathre, R.; Greenblatt, J.B.; Walczak, K.; Sharp, I.D.; Stevens, J.C.; Ager, J.W.; Houle, F.A. Opportunities to improve the net energy performance of photoelectrochemical water-splitting technology. Energy Environ. Sci. 2016, 9, 803–819. [Google Scholar] [CrossRef] [Green Version]
- Sivula, K.; Van De Krol, R. Semiconducting materials for photoelectrochemical energy conversion. Nat. Rev. Mater. 2016, 1, 15010. [Google Scholar] [CrossRef]
- Takata, T.; Domen, K. Development of non-oxide semiconductors as light harvesting materials in photocatalytic and photoelectrochemical water splitting. Dalton Trans. 2017, 46, 10529–10544. [Google Scholar] [CrossRef]
- Smith, W.A. Photoelectrochemical cell design, efficiency, definitions, standards, and protocols. In Photoelectrochemical Solar Fuel Production; Giménez, S., Bisquert, J., Eds.; Springer: New York, NY, USA, 2016; pp. 163–197. [Google Scholar]
- Sadeghi, N.; Sharifnia, S.; Arabi, M.S. A porphyrin-based metal organic framework for high rate photoreduction of CO2 to CH4 in gas phase. J. CO2 Util. 2016, 16, 450–457. [Google Scholar] [CrossRef]
- Tuller, H.L. Solar to fuels conversion technologies: A perspective. Mater. Renew. Sustain. Energy 2017, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Toniolo, F.S.; Schmal, M. Improvement of catalytic performance of perovskites by partial substitution of cations and supporting on high surface area materials. In Perovskite Materials—Synthesis, Characterisation, Properties, and Applications; Pan, L., Zhu, G., Eds.; IntechOpen: London, UK, 2016. [Google Scholar]
- Tobías, I.; Luque, A. Emerging high efficiency concepts for concentrator solar cells. In Handbook of Concentrator Photovoltaic Technology; Algora, C., Rey-Stolle, I., Eds.; John Wiley & Sons Ltd.: Hoboken, NY, USA, 2016; p. 137. [Google Scholar]
- Block, T.; Knoblauch, N.; Schmücker, M. The cobalt-oxide/iron-oxide binary system for use as high temperature thermochemical energy storage material. Thermochim. Acta 2014, 577, 25–32. [Google Scholar] [CrossRef]
- Takata, T.; Hisatomi, T.; Domen, K. Solar hydrogen production on some water splitting photocatalysts. In Proceedings of the Solar Hydrogen and Nanotechnology XI, San Diego, CA, USA, 28 August–1 September 2016; International Society for Optics and Photonics: Washington, DC, USA; p. 99350O. [Google Scholar]
- Ussiri, D.A.; Lal, R. The role of bioenergy in mitigating climate change. In Carbon Sequestration for Climate Change Mitigation and Adaptation; Springer: New York, NY, USA, 2017; pp. 433–495. [Google Scholar]
- Yang, W.; Moon, J. Recent advances in earth-abundant photocathodes for photoelectrochemical water splitting. ChemSusChem 2018, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, F.; Zhu, C.; Li, C.; Zhang, S.; Zhang, X.; Chen, Y. Highly stable three-dimensional nickel–iron oxyhydroxide catalysts for oxygen evolution reaction at high current densities. Electrochim. Acta 2017, 245, 770–779. [Google Scholar] [CrossRef]
- Vermaas, D.A.; Smith, W.A. Applications of bipolar membranes for electrochemical and photoelectrochemical water splitting. In Advances in Photoelectrochemical Water Splitting: Theory, Experiment and Systems Analysis; Tilley, S.D., Lany, S., van de Krol, R., Eds.; Royal Society of Chemistry: London, UK, 2018; pp. 208–238. [Google Scholar]
- Waldie, K.M.; Kim, S.K.; Ingram, A.J.; Waymouth, R.M. Cyclopentadienyl cobalt complexes as precatalysts for electrocatalytic hydrogen evolution. Eur. J. Inorg. Chem. 2017, 2017, 2755–2761. [Google Scholar] [CrossRef]
- Wu, P.; Liu, Z.; Chen, D.; Zhou, M.; Wei, J. Flake-like NiO/WO3 pn heterojunction photocathode for photoelectrochemical water splitting. Appl. Surf. Sci. 2018, 440, 1101–1106. [Google Scholar] [CrossRef]
- Zhang, Y.; Knibbe, R.; Sunarso, J.; Zhong, Y.; Zhou, W.; Shao, Z.; Zhu, Z. Solid-oxide fuel cells: Recent progress on advanced materials for solid-oxide fuel cells operating below 500 °C. Adv. Mater. 2017, 29, 1770345. [Google Scholar] [CrossRef] [Green Version]
- Meda, L.; Abbondanza, L. Materials for photo-electrochemical water splitting. Rev. Adv. Sci. Eng. 2013, 2, 200–207. [Google Scholar] [CrossRef]
- Schreier, M.; Curvat, L.; Giordano, F.; Steier, L.; Abate, A.; Zakeeruddin, S.M.; Luo, J.; Mayer, M.T.; Grätzel, M. Efficient photosynthesis of carbon monoxide from CO2 using perovskite photovoltaics. Nat. Commun. 2015, 6, 7326. [Google Scholar] [CrossRef] [Green Version]
- Jang, Y.J.; Jeong, I.; Lee, J.; Lee, J.; Ko, M.J.; Lee, J.S. Unbiased sunlight-driven artificial photosynthesis of carbon monoxide from CO2 using a ZnTe-based photocathode and a perovskite solar cell in tandem. ACS Nano 2016, 10, 6980–6987. [Google Scholar] [CrossRef]
- Kalamaras, E.; Maroto-Valer, M.M.; Shao, M.; Xuan, J.; Wang, H. Solar carbon fuel via photoelectrochemistry. Catal. Today 2018, 317, 56–75. [Google Scholar] [CrossRef]
- Marquez, O.; Márquez, J. Synthesis of electrocatalysts for electrochemistry in energy. In Advanced Solid Catalysts for Renewable Energy Production; IGI Global: Hershey, PA, USA, 2018; pp. 300–385. [Google Scholar]
- Marchi, M.; Niccolucci, V.; Pulselli, R.M.; Marchettini, N. Environmental policies for GHG emissions reduction and energy transition in the medieval historic centre of Siena (Italy): The role of solar energy. J. Clean. Prod. 2018, 185, 829–840. [Google Scholar] [CrossRef]
- Li, F.; Bashir, S.; Liu, J.L. Nanostructured Materials for Next-Generation Energy Storage and Conversion: Fuel Cells; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Ning, X.; Li, W.; Meng, Y.; Qin, D.; Chen, J.; Mao, X.; Xue, Z.; Shan, D.; Devaramani, S.; Lu, X. New insight into procedure of interface electron transfer through cascade system with enhanced photocatalytic activity. Small 2018, 14, 1703989. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, B. Energy Sources: Fundamentals of Chemical Conversion Processes and Applications; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Sugiyama, M.; Fujii, K.; Nakamura, S. Solar to chemical energy conversion. In Lecture Notes Energy; Springer: Basel, Switzerland, 2016; Volume 32, p. 253. [Google Scholar]
- Thorpe, D.; Martin, C.L.; Goswami, D.Y. Solar Energy Pocket Reference; Routledge: London, UK, 2017. [Google Scholar]
- Lari, M.O.; Sahin, A.Z. Design, performance and economic analysis of a nanofluid-based photovoltaic/thermal system for residential applications. Energy Convers. Manag. 2017, 149, 467–484. [Google Scholar] [CrossRef]
- May, M.M.; Lewerenz, H.-J.; Lackner, D.; Dimroth, F.; Hannappel, T. Efficient direct solar-to-hydrogen conversion by in situ interface transformation of a tandem structure. Nat. Commun. 2015, 6, 8286. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Sun, L. Inorganic hole-transporting materials for perovskite solar cells. Small Methods 2018, 2, 1700280. [Google Scholar] [CrossRef]
- Reinders, A.; Verlinden, P.; Van Sark, W.; Freundlich, A. Photovoltaic Solar Energy: From Fundamentals to Applications; Reinders, A., Verlinden, P., van Sark, W., Freundlich, A., Eds.; John Wiley & Sons, Inc.: Hoboken, NY, USA, 2017. [Google Scholar]
- Yu, Y.; Gao, P. Development of electron and hole selective contact materials for perovskite solar cells. Chin. Chem. Lett. 2017, 28, 1144–1152. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, P.; Yao, L.; Deng, L.; Ren, X.; Li, Y. Recent research progress on lead-free or less-lead perovskite solar cells. Int. J. Electrochem. Sci. 2017, 12, 4915–4927. [Google Scholar] [CrossRef]
- Severance, C.A. A practical, affordable (and least business risk) plan to achieve “80% clean electricity” by 2035. Electr. J. 2011, 24, 8–26. [Google Scholar] [CrossRef]
- Hagerman, G.; Polagye, B.; Bedard, R.; Previsic, M. Methodology for Estimating Tidal Current Energy Resources and Power Production by Tidal In-Stream Energy Conversion (TISEC) Devices; Technical Report No. EPRI–TP–001 NA Rev 2; EPRI: Palo Alto, CA, USA, 2006. [Google Scholar]
- Li, R. Latest progress in hydrogen production from solar water splitting via photocatalysis, photoelectrochemical, and photovoltaic-photoelectrochemical solutions. Chin. J. Catal. 2017, 38, 5–12. [Google Scholar] [CrossRef]
- Du, H.; Liu, Y.-N.; Shen, C.-C.; Xu, A.-W. Nanoheterostructured photocatalysts for improving photocatalytic hydrogen production. Chin. J. Catal. 2017, 38, 1295–1306. [Google Scholar] [CrossRef]
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solangi, K.; Islam, M.; Saidur, R.; Rahim, N.; Fayaz, H. A review on global solar energy policy. Renew. Sustain. Energy Rev. 2011, 15, 2149–2163. [Google Scholar] [CrossRef]
- Timilsina, G.R.; Kurdgelashvili, L.; Narbel, P.A. Solar energy: Markets, economics and policies. Renew. Sustain. Energy Rev. 2012, 16, 449–465. [Google Scholar] [CrossRef]
- Aman, M.; Solangi, K.; Hossain, M.; Badarudin, A.; Jasmon, G.; Mokhlis, H.; Bakar, A.; Kazi, S.N. A review of Safety, Health and Environmental (SHE) issues of solar energy system. Renew. Sustain. Energy Rev. 2015, 41, 1190–1204. [Google Scholar] [CrossRef]
- Miller, E.L. Solar hydrogen production by photoelectrochemical water splitting: The promise and challenge. In On Solar Hydrogen and Nanotechnology; John Wiley & Sons (Asia) Pte. Ltd.: Singapore, 2009; pp. 3–35. [Google Scholar]
- Zhu, W.H.; Zhu, Y.; Davis, Z.; Tatarchuk, B.J. Energy efficiency and capacity retention of Ni–MH batteries for storage applications. Appl. Energy 2013, 106, 307–313. [Google Scholar] [CrossRef]
- Hoffmann, P. Tomorrow’s Energy: Hydrogen, Fuel Cells, and the Prospects for a Cleaner Planet; MIT Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Herron, J.A.; Kim, J.; Upadhye, A.A.; Huber, G.W.; Maravelias, C.T. A general framework for the assessment of solar fuel technologies. Energy Environ. Sci. 2015, 8, 126–157. [Google Scholar] [CrossRef]
- Fernández, J.; Abanades, J.; Murillo, R.; Grasa, G. Conceptual design of a hydrogen production process from natural gas with CO2 capture using a Ca–Cu chemical loop. Int. J. Greenh. Gas Control 2012, 6, 126–141. [Google Scholar] [CrossRef] [Green Version]
- Rostrup-Nielsen, J.R.; Sehested, J.; Nørskov, J.K. Hydrogen and synthesis gas by steam-and CO2 reforming. Adv. Catal. 2002, 47, 65–139. [Google Scholar] [CrossRef]
- Palzer, A.; Henning, H.M. A future German energy system with a dominating contribution from renewable energies: A holistic model based on hourly simulation. Energy Technol. 2014, 2, 13–28. [Google Scholar] [CrossRef]
- Palzer, A.; Henning, H.-M. A comprehensive model for the German electricity and heat sector in a future energy system with a dominant contribution from renewable energy technologies—Part II: Results. Renew. Sustain. Energy Rev. 2014, 30, 1019–1034. [Google Scholar] [CrossRef]
- Murugadoss, G.; Thangamuthu, R.; Kumar, S.M.S. Fabrication of CH3NH3PbI3 perovskite-based solar cells: Developing various new solvents for CuSCN hole transport material. Sol. Energy Mater. Sol. Cells 2017, 164, 56–62. [Google Scholar] [CrossRef]
- Nair, M.M.; Abanades, S.P. Tailoring hybrid nonstoichiometric ceria redox cycle for combined solar methane reforming and thermochemical conversion of H2O/CO2. Energy Fuels 2016, 30, 6050–6058. [Google Scholar] [CrossRef]
- Montoya, J.H.; Seitz, L.C.; Chakthranont, P.; Vojvodic, A.; Jaramillo, T.F.; Nørskov, J.K. Materials for solar fuels and chemicals. Nat. Mater. 2017, 16, 70. [Google Scholar] [CrossRef]
- Ohsato, H. Microwave dielectrics with perovskite-type structure. In Perovskite Materials—Synthesis, Characterisation, Properties, and Applications; Pan, L., Zhu, G., Eds.; IntechOpen: London, UK, 2016. [Google Scholar]
- Yamazaki, Y. Solar-Driven Thermochemical CO2 Reduction Using Nonstoichiometric Perovskite. 2016. Available online: https://dc.engconfintl.org/nonstoichiometric_vi/15 (accessed on 9 January 2020).
- Demont, A.; Abanades, S. Solar thermochemical conversion of CO2 into fuel via two-step redox cycling of non-stoichiometric Mn-containing perovskite oxides. J. Mater. Chem. A 2015, 3, 3536–3546. [Google Scholar] [CrossRef]
- Nair, M.M.; Abanades, S. Experimental screening of perovskite oxides as efficient redox materials for solar thermochemical CO2 conversion. Sustain. Energy Fuels 2018, 2, 843–854. [Google Scholar] [CrossRef]
- Dey, S.; Rao, C. Splitting of CO2 by manganite perovskites to generate CO by solar isothermal redox cycling. ACS Energy Lett. 2016, 1, 237–243. [Google Scholar] [CrossRef]
- Nair, M.M.; Abanades, S. Insights into the redox performance of non–stoichiometric lanthanum manganite perovskites for solar thermochemical CO2 splitting. ChemistrySelect 2016, 1, 4449–4457. [Google Scholar] [CrossRef]
- Riaz, A.; Kreider, P.; Kremer, F.; Tabassum, H.; Yeoh, J.S.; Lipiński, W.; Lowe, A. Electrospun manganese-based perovskites as efficient oxygen exchange redox materials for improved solar thermochemical CO2 splitting. ACS Appl. Energy Mater. 2019, 2, 2494–2505. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Bork, A.H.; Moser, T.; Sediva, E.; Hood, Z.D.; Rupp, J.L. Modifying La0.6Sr0.4MnO3 perovskites with Cr incorporation for fast isothermal CO2-splitting kinetics in solar-driven thermochemical cycles. Adv. Energy Mater. 2019, 9, 1803886. [Google Scholar] [CrossRef]
- Bork, A.H.; Povoden-Karadeniz, E.; Carrillo, A.J.; Rupp, J.L. Thermodynamic assessment of the solar-to-fuel performance of La0.6Sr0.4Mn1−YCryO3 perovskite solid solution series. Acta Mater. 2018, 178, 163–172. [Google Scholar] [CrossRef]
- Luciani, G.; Landi, G.; Aronne, A.; Di Benedetto, A. Partial substitution of B cation in La0.6Sr0.4MnO3 perovskites: A promising strategy to improve the redox properties useful for solar thermochemical water and carbon dioxide splitting. Sol. Energy 2018, 171, 1–7. [Google Scholar] [CrossRef]
- Ezbiri, M.; Becattini, V.; Hoes, M.; Michalsky, R.; Steinfeld, A. High redox capacity of Al-doped La1−xSrxMnO3−δ perovskites for splitting CO2 and H2O at Mn-enriched surfaces. ChemSusChem 2017, 10, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T.; Scheffe, J.R.; Galvez, M.E.; Jacot, R.; Patzke, G.; Steinfeld, A. Lanthanum manganite perovskites with Ca/Sr A-site and Al B-site doping as effective oxygen exchange materials for solar thermochemical fuel production. Energy Technol. 2015, 3, 1130–1142. [Google Scholar] [CrossRef]
- Sastre, D.; Carrillo, A.; Serrano, D.; Pizarro, P.; Coronado, J. Exploring the redox behavior of LaSrMnAlO perovskites for CO-splitting in thermochemical cycles. Top. Catal. 2017, 60, 1108–1118. [Google Scholar] [CrossRef]
- Bork, A.H.; Kubicek, M.; Struzik, M.; Rupp, J.L. Perovskite La0.6Sr0.4Cr1−x CoxO3−δ solid solutions for solar-thermochemical fuel production: Strategies to lower the operation temperature. J. Mater. Chem. A 2015, 3, 15546–15557. [Google Scholar] [CrossRef] [Green Version]
- Hare, B.J.; Maiti, D.; Daza, Y.A.; Bhethanabotla, V.R.; Kuhn, J.N. Enhanced CO2 conversion to CO by silica-supported perovskite oxides at low temperatures. ACS Catal. 2018, 8, 3021–3029. [Google Scholar] [CrossRef]
- Hare, B.J.; Maiti, D.; Ramani, S.; Ramos, A.E.; Bhethanabotla, V.R.; Kuhn, J.N. Thermochemical conversion of carbon dioxide by reverse water-gas shift chemical looping using supported perovskite oxides. Catal. Today 2019, 323, 225–232. [Google Scholar] [CrossRef]
- Hare, B.J.; Maiti, D.; Meier, A.; Bhethanabotla, V.R.; Kuhn, J.N. CO2 conversion performance of perovskite oxides designed with abundant metals. Ind. Eng. Chem. Res. 2019, 58, 12551–12560. [Google Scholar] [CrossRef]
- Ramos, A.E.; Maiti, D.; Daza, Y.A.; Kuhn, J.N.; Bhethanabotla, V.R. Co, Fe, and Mn in La-perovskite oxides for low temperature thermochemical CO2 conversion. Catal. Today 2019, 338, 52–59. [Google Scholar] [CrossRef]
- Dey, S.; Naidu, B.; Rao, C. Ln0.5A0.5MnO3 (Ln = Lanthanide, A = Ca, Sr) perovskites exhibiting remarkable performance in the thermochemical generation of CO and H2 from CO2 and H2O. Chem. A Eur. J. 2015, 21, 7077–7081. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.; Dey, S. Generation of H2 and CO by solar thermochemical splitting of H2O and CO2 by employing metal oxides. J. Solid State Chem. 2016, 242, 107–115. [Google Scholar] [CrossRef]
- Takalkar, G.; Bhosale, R.R. Solar thermocatalytic conversion of CO2 using PrxSr(1−x)MnO3−δ perovskites. Fuel 2019, 254, 115624. [Google Scholar] [CrossRef]
- Takalkar, G.; Bhosale, R.; AlMomani, F. Combustion synthesized A0.5Sr0.5MnO3−δ perovskites (where, a = La, Nd, Sm, Gd, Tb, Pr, Dy, and Y) as redox materials for thermochemical splitting of CO2. Appl. Surf. Sci. 2019. [Google Scholar] [CrossRef]
- Mulmi, S.; Chen, H.; Hassan, A.; Marco, J.F.; Berry, F.J.; Sharif, F.; Slater, P.R.; Roberts, E.P.; Adams, S.; Thangadurai, V. Thermochemical CO2 splitting using double perovskite-type Ba2Ca0.66Nb1.34−xFexO6−δ. J. Mater. Chem. A 2017, 5, 6874–6883. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Haribal, V.; Li, F. Perovskite nanocomposites as effective CO2-splitting agents in a cyclic redox scheme. Sci. Adv. 2017, 3, e1701184. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.-F.; Yang, M.-Z.; Chen, B.-X.; Wang, X.-D.; Chen, H.-Y.; Kuang, D.-B.; Su, C.-Y. A CsPbBr3 perovskite quantum dot/graphene oxide composite for photocatalytic CO2 reduction. J. Am. Chem. Soc. 2017, 139, 5660–5663. [Google Scholar] [CrossRef]
- Hou, J.; Cao, S.; Wu, Y.; Gao, Z.; Liang, F.; Sun, Y.; Lin, Z.; Sun, L. Inorganic colloidal perovskite quantum dots for robust solar CO2 reduction. Chem. Eur. J. 2017, 23, 9481–9485. [Google Scholar] [CrossRef]
- Guo, S.-H.; Zhou, J.; Zhao, X.; Sun, C.-Y.; You, S.-Q.; Wang, X.-L.; Su, Z.-M. Enhanced CO2 photoreduction via tuning halides in perovskites. J. Catal. 2019, 369, 201–208. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, Y.F.; Chen, B.X.; Kuang, D.B.; Su, C.Y. Synthesis and photocatalytic application of stable lead-free Cs2AgBiBr6 perovskite nanocrystals. Small 2018, 14, 1703762. [Google Scholar] [CrossRef]
- Xu, Y.-F.; Yang, M.-Z.; Chen, H.-Y.; Liao, J.-F.; Wang, X.-D.; Kuang, D.-B. Enhanced solar-driven gaseous CO2 conversion by CsPbBr3 nanocrystal/Pd nanosheet Schottky-junction photocatalyst. ACS Appl. Energy Mater. 2018, 1, 5083–5089. [Google Scholar] [CrossRef]
- Wan, S.; Ou, M.; Zhong, Q.; Wang, X. Perovskite-type CsPbBr3 quantum dots/UiO-66 (NH2) nanojunction as efficient visible-light-driven photocatalyst for CO2 reduction. Chem. Eng. J. 2019, 358, 1287–1295. [Google Scholar] [CrossRef]
- Wang, B.; Di, J.; Lu, L.; Yan, S.; Liu, G.; Ye, Y.; Li, H.; Zhu, W.; Li, H.; Xia, J. Sacrificing ionic liquid-assisted anchoring of carbonized polymer dots on perovskite-like PbBiO2Br for robust CO2 photoreduction. Appl. Catal. B Environ. 2019, 254, 551–559. [Google Scholar] [CrossRef]
- Shan, J.; Raziq, F.; Humayun, M.; Zhou, W.; Qu, Y.; Wang, G.; Li, Y. Improved charge separation and surface activation via boron-doped layered polyhedron SrTiO3 for co-catalyst free photocatalytic CO2 conversion. Appl. Catal. B Environ. 2017, 219, 10–17. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Chen, W.; Mao, L.; Shangguan, W. Ag loaded on layered perovskite H2SrTa2O7 to enhance the selectivity of photocatalytic CO2 reduction with H2O. J. Alloys Compd. 2019, 786, 149–154. [Google Scholar] [CrossRef]
- Hafez, A.M.; Zedan, A.F.; AlQaradawi, S.Y.; Salem, N.M.; Allam, N.K. Computational study on oxynitride perovskites for CO2 photoreduction. Energy Convers. Manag. 2016, 122, 207–214. [Google Scholar] [CrossRef]
- Hou, J.; Cao, S.; Wu, Y.; Liang, F.; Ye, L.; Lin, Z.; Sun, L. Perovskite-based nanocubes with simultaneously improved visible-light absorption and charge separation enabling efficient photocatalytic CO2 reduction. Nano Energy 2016, 30, 59–68. [Google Scholar] [CrossRef]
- Wang, Q.; Tao, L.; Jiang, X.; Wang, M.; Shen, Y. Graphene oxide wrapped CH3NH3PbBr3 perovskite quantum dots hybrid for photoelectrochemical CO2 reduction in organic solvents. Appl. Surf. Sci. 2019, 465, 607–613. [Google Scholar] [CrossRef]
- Yu, J.; Cheng, G.; Luo, W. 3D mesoporous rose-like nickel-iron selenide microspheres as advanced electrocatalysts for the oxygen evolution reaction. Nano Res. 2018, 11, 2149–2158. [Google Scholar] [CrossRef]
- Pilatos, G.; Perdikaki, A.V.; Sapalidis, A.; Pappas, G.S.; Giannakopoulou, T.; Tsoutsou, D.; Xenogiannopoulou, E.; Boukos, N.; Dimoulas, A.; Trapalis, C. Graphene by one-step chemical vapor deposition from ferrocene vapors: Properties and electrochemical evaluation. J. Appl. Phys. 2016, 119, 064303. [Google Scholar] [CrossRef]
- Vermisoglou, E.C.; Karanikolos, G.N.; Pilatos, G.; Devlin, E.; Romanos, G.E.; Veziri, C.U.; Kanellopoulos, N.K. Aligned carbon nanotubes with ferromagnetic behavior. Adv. Mater. 2010, 22, 473–477. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabish, A.; Varghese, A.M.; Wahab, M.A.; Karanikolos, G.N. Perovskites in the Energy Grid and CO2 Conversion: Current Context and Future Directions. Catalysts 2020, 10, 95. https://doi.org/10.3390/catal10010095
Tabish A, Varghese AM, Wahab MA, Karanikolos GN. Perovskites in the Energy Grid and CO2 Conversion: Current Context and Future Directions. Catalysts. 2020; 10(1):95. https://doi.org/10.3390/catal10010095
Chicago/Turabian StyleTabish, Ahmad, Anish Mathai Varghese, Md A. Wahab, and Georgios N. Karanikolos. 2020. "Perovskites in the Energy Grid and CO2 Conversion: Current Context and Future Directions" Catalysts 10, no. 1: 95. https://doi.org/10.3390/catal10010095
APA StyleTabish, A., Varghese, A. M., Wahab, M. A., & Karanikolos, G. N. (2020). Perovskites in the Energy Grid and CO2 Conversion: Current Context and Future Directions. Catalysts, 10(1), 95. https://doi.org/10.3390/catal10010095






