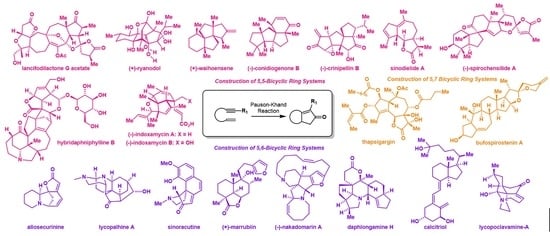
Evolution of Pauson-Khand Reaction: Strategic Applications in Total Syntheses of Architecturally Complex Natural Products (2016–2020)
Abstract
:1. Introduction
1.1. Classic PK Reaction Catalyzed by Co
1.2. PK Reaction Catalyzed by Rh and Pd
1.3. Hetero-Pauson-Khand Reaction
1.4. Summary
2. Recent Pauson-Khand Reaction Applications in Natural Products Total Syntheses
2.1. Construction of 5,5-Bicyclic Ring Systems
2.2. Construction of 5,6-Bicyclic Ring Systems
2.3. Construction of 5,7 Bicyclic Ring Systems
2.4. Construction of Macrocycles
3. Summary and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- D’yakonov, V.A.; Trapeznikova, O.A.; de Meijere, A.; Dzhemilev, U.M. Metal Complex Catalysis in the Synthesis of Spirocarbocycles. Chem. Rev. 2014, 114, 5775–5814. [Google Scholar] [CrossRef] [PubMed]
- Porcheddu, A.; Colacino, E.; De Luca, L.; Delogu, F. Metal-Mediated and Metal-Catalyzed Reactions Under Mechanochemical Conditions. ACS Catal. 2020, 10, 8344–8394. [Google Scholar] [CrossRef]
- Rodríguez, J.; Martínez, C.M. Transition-Metal-Mediated Modification of Biomolecules. Chem.-A Eur. J. 2020, 26, 9792–9813. [Google Scholar] [CrossRef] [PubMed]
- Khand, I.U.; Knox, G.R.; Pauson, P.L.; Watts, W.E.; Foreman, M.I. Organocobalt complexes. II. Reaction of acetylenehexacarbonyl dicobalt complexes, (RC2R1) Co2(CO)6, with norbornene and its derivatives. J. Chem. Soc. Perkin Trans. 1 1973, 9, 977–981. [Google Scholar] [CrossRef]
- Chung, Y.K. Transition metal alkyne complexes: The Pauson–Khand reaction. Coord. Chem. Rev. 1999, 188, 297–314. [Google Scholar] [CrossRef]
- Gibson, S.E.; Stevenazzi, A. The Pauson–Khand Reaction: The Catalytic Age Is Here. Angew. Chem. Int. Ed. 2003, 42, 1800–1810. [Google Scholar] [CrossRef] [PubMed]
- Urgoiti, J.B.; Anorbe, L.; Serrano, L.P.; Dominguez, G.; Castells, L.P. The Pauson–Khand reaction, a powerful synthetic tool for the synthesis of complex molecules. Chem. Soc. Rev. 2004, 33, 32–42. [Google Scholar] [CrossRef]
- Gibson, S.E.; Mainolfi, N. The Intermolecular Pauson–Khand Reaction. Angew. Chem. Int. Ed. 2005, 44, 3022–3037. [Google Scholar] [CrossRef]
- Kitagaki, S.; Inagaki, F.; Mukai, C. [2+2+1] Cyclization of allenes. Chem. Soc. Rev. 2014, 43, 2956–2978. [Google Scholar] [CrossRef]
- Lee, H.-W.; Kwong, F.-Y. A Decade of Advancements in Pauson–Khand-Type Reactions. Eur. J. Org. Chem. 2010, 2010, 789–811. [Google Scholar] [CrossRef]
- Shi, L.; Yang, Z. Exploring the Complexity-Generating Features of the Pauson–Khand Reaction from a Synthetic Perspective. Eur. J. Org. Chem. 2016, 2016, 2356–2368. [Google Scholar] [CrossRef]
- Ricker, J.D.; Geary, L.M. Recent Advances in the Pauson–Khand Reaction. Top. Catal. 2017, 60, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Jeong, N.; Hwang, S.H.; Lee, Y.S.; Chung, Y.K. Catalytic Version of the Intramolecular Pauson-Khand Reaction. J. Am. Chem. Soc. 1994, 116, 3159–3160. [Google Scholar] [CrossRef]
- Hayashi, M.; Hashimoto, Y.; Yamamoto, Y.; Usuki, J.; Saigo, K. Phosphane Sulfide/Octacarbonyldicobalt-Catalyzed Pauson-Khand Reaction under an Atmospheric Pressure of Carbon Monoxide. Angew. Chem. Int. Ed. 2000, 39, 631–632. [Google Scholar] [CrossRef]
- Krafft, M.E.; Boñaga, L.V.R.; Wright, J.A.; Hirosawa, C. Cobalt Carbonyl-Mediated Carbocyclizations of Enynes: Generation of Bicyclooctanones or Monocyclic Alkenes. J. Org. Chem. 2002, 67, 1233–1246. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, L.; Yu, R.; Chen, J.; Yang, Z. CoBr2–TMTU–zinc catalysed-Pauson–Khand reaction. Chem. Commun. 2012, 48, 8183–8185. [Google Scholar] [CrossRef]
- Orgue, S.; Leon, T.; Riera, A.; Verdaguer, X. Asymmetric Intermolecular Cobalt Catalyzed Pauson-Khand Reaction Using a Pstereogenic BisPhosphane. Org. Lett. 2015, 17, 250–253. [Google Scholar] [CrossRef] [Green Version]
- Koga, Y.; Kobayashi, T.; Narasaka, K. Rhodium-Catalyzed Intramolecular Pauson-Khand Reaction. Chem. Lett. 1998, 3, 249–250. [Google Scholar] [CrossRef]
- Jeong, N.; Lee, S.; Sung, B.K. Rhodium(I)-Catalyzed Intramolecular Pauson−Khand Reaction. Organometallics 1998, 17, 3642–3644. [Google Scholar] [CrossRef]
- Fan, B.M.; Xie, J.H.; Li, S.; Tu, Y.Q.; Zhou, Q.L. Rhodium-Catalyzed Asymmetric Pauson–Khand Reaction Using Monophosphoramidite Ligand SIPHOS. Adv. Synth. Catal. 2005, 347, 759–762. [Google Scholar] [CrossRef]
- Jeong, N.; Sung, B.K.; Choi, Y.K. Rhodium(I)-Catalyzed Asymmetric Intramolecular Pauson-Khand-Type Reaction. J. Am. Chem. Soc. 2000, 122, 6771–6772. [Google Scholar] [CrossRef]
- Jeong, N.; Kim, D.H.; Choi, J.H. Desymmetrization of meso-dienyne by asymmetric Pauson–Khand type reaction catalysts. Chem. Commun. 2004, 1134–1135. [Google Scholar] [CrossRef] [PubMed]
- Kwong, F.Y.; Li, Y.M.; Lam, W.H.; Qiu, L.Q.; Lee, H.W.; Yeung, C.H.; Chan, K.S.; Chan, A.S.C. Rhodium-Catalyzed Asymmetric Aqueous Pauson–Khand-Type Reaction. Chem. Eur. J. 2005, 11, 3872–3880. [Google Scholar] [CrossRef]
- Chen, G.Q.; Shi, M. Rhodium-catalyzed tandem Pauson–Khand type reactions of 1,4-enynes tethered by a cyclopropyl group. Chem. Commun. 2013, 49, 698–700. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Sawyer, J.R.; Evans, P.A.; Baik, M.H. Mechanistic Insight into the Diastereoselective Rhodium-Catalyzed Pauson–Khand Reaction: Role of Coordination Number in Stereocontrol. Angew. Chem. Int. Ed. 2008, 47, 342–345. [Google Scholar] [CrossRef]
- Kondo, T.; Suzuki, N.; Okada, T.; Mitsudo, T. First Ruthenium-Catalyzed Intramolecular Pauson-Khand Reaction. J. Am. Chem. Soc. 1997, 119, 6187–6188. [Google Scholar] [CrossRef]
- Morimoto, T.; Chatani, N.; Fukumoto, Y.; Murai, S. Ru3(CO)12-Catalyzed Cyclocarbonylation of 1,6-Enynes to Bicyclo[3.3.0]Octenones. J. Org. Chem. 1997, 62, 3762–3765. [Google Scholar] [CrossRef]
- Chatani, N.; Morimoto, T.; Fukumoto, Y.; Murai, S. Ru3(CO)12-Catalyzed Cyclocarbonylation of Yne-Aldehydes to Bicyclic α, β-Unsaturated γ-Butyrolactones. J. Am. Chem. Soc. 1998, 120, 5335–5336. [Google Scholar] [CrossRef]
- Miura, H.; Takeuchi, K.; Shishido, T. Intermolecular [2+2+1] Carbonylative Cycloaddition of Aldehydes with Alkynes, and Subsequent Oxidation to γ-Hydroxybutenolides by a Supported Ruthenium Catalyst. Angew. Chem. Int. Ed. 2016, 55, 278–282. [Google Scholar] [CrossRef]
- Kondo, T.; Nomura, M.; Ura, Y.; Wada, K.; Mitsudo, T. Ruthenium-catalyzed [2 + 2 + 1] Cocyclization of Isocyanates, Alkynes, and CO Enables the Rapid Synthesis of Polysubstituted Maleimides. J. Am. Chem. Soc. 2006, 128, 14816–14817. [Google Scholar] [CrossRef]
- Hicks, F.A.; Kablaoui, N.M.; Buchwald, S.L. Scope of the Intramolecular Titanocene-Catalyzed Pauson-Khand Type Reaction. J. Am. Chem. Soc. 1999, 121, 5881–5898. [Google Scholar] [CrossRef]
- Hicks, F.A.; Buchwald, S.L. An intramolecular Titanium-Catalyzed Asymmetric Pauson-Khand Type Reaction. J. Am. Chem. Soc. 1999, 121, 7026–7033. [Google Scholar] [CrossRef]
- Zhao, Z.B.; Ding, Y.; Zhao, G. Bicyclization of Enynes Using the Cp2TiCl2−Mg−BTC System: A Practical Method to Bicyclic Cyclopentenones. J. Org. Chem. 1998, 63, 9285–9291. [Google Scholar] [CrossRef]
- Hicks, F.A.; Kablaoui, N.M.; Buchwald, S.L. Titanocene-Catalyzed Cyclocarbonylation of Enynes to Cyclopentenones. J. Am. Chem. Soc. 1996, 118, 9450–9451. [Google Scholar] [CrossRef]
- Shibata, T.; Takagi, K. Iridium-Chiral Diphosphine Complex Catalyzed Highly Enantioselective Pauson-Khand-Type Reaction. J. Am. Chem. Soc. 2000, 122, 9852–9853. [Google Scholar] [CrossRef]
- Shibata, T.; Toshida, N.; Yamasaki, M.; Maekawa, S.; Takagi, K. Iridium-catalyzed enantioselective Pauson–Khand-type reaction of 1,6-enynes. Tetrahedron 2005, 61, 9974–9979. [Google Scholar] [CrossRef]
- Kwong, F.Y.; Lee, H.W.; Lam, W.H.; Qiu, L.Q.; Chan, A.S.C. Iridium-catalyzed cascade decarbonylation/highly enantioselective Pauson–Khand-type cyclization reactions. Tetrahedron Asymm. 2006, 17, 1238–1252. [Google Scholar] [CrossRef]
- Zhang, M.H.; Buchwald, S.L. A Nickel(0)-Catalyzed Process for the Transformation of Enynes to Bicyclic Cyclopentenones. J. Org. Chem. 1996, 61, 4498–4499. [Google Scholar] [CrossRef]
- Kent, J.L.; Wan, H.H.; Brummond, K.M. A new allenic Pauson-Khand cycloaddition for the preparation of α-methylene cyclopentenones. Tetrahedron Lett. 1995, 36, 2407–2410. [Google Scholar] [CrossRef]
- Adrio, J.; Rivero, M.R.; Carretero, J.C. Mild and Efficient Molybdenum-Mediated Pauson−Khand-Type Reaction. Org. Lett. 2005, 7, 431–434. [Google Scholar] [CrossRef]
- Shibata, T.; Koga, Y.; Narasaka, K. Intra- and Intermolecular Allene–Alkyne Coupling Reactions by the Use of Fe(CO)4(NMe3). Bull. Chem. Soc. Jpn. 1995, 68, 911–919. [Google Scholar] [CrossRef]
- Rutherford, D.T.; Christie, S.D.R. Soluble polymer-supported synthesis of arylpiperazines. Tetrahedron Lett. 1998, 39, 9505–9508. [Google Scholar]
- Park, K.H.; Son, S.U.; Chung, Y.K. Soluble polymer-supported synthesis of arylpiperazines. Chem. Commun. 2003, 1898–1899. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Son, S.U.; Chung, Y.K. Immobilized heterobimetallic Ru/Co nanoparticle-catalyzed Pauson–Khand-type reactions in the presence of pyridylmethyl formate. Chem. Commun. 2008, 2388–2390. [Google Scholar] [CrossRef] [PubMed]
- Khand, I.U.; Pauson, P.L. Organometallic Route to 2,7-Dihydrothiepin-1,1-Dioxides. Heterocycles 1978, 11, 59–67. [Google Scholar]
- Magnus, P.; Principe, L.M. Origins of 1,2-Stereoselectivity and 1,3-Stereoselectivity in Dicobaltoctacarbonyl Alkene Alkyne Cyclizations for the Synthesis of Substituted Bicyclo[3.3.0]Octenones. Tetrahedron Lett. 1985, 26, 4851–4854. [Google Scholar] [CrossRef]
- Magnus, P.; Exon, C.; Albaughrobertson, P. Dicobaltoctacarbonyl Alkyne Complexes as Intermediates in the Synthesis of Bicyclo[3.3.0]Octenones for the Synthesis of Coriolin and Hirsutic Acid. Tetrahedron 1985, 41, 5861–5869. [Google Scholar] [CrossRef]
- Magnus, P.; Principe, L.M.; Slater, M.J. Stereospecific Dicobalt Octacarbonyl Mediated Enyne Cyclization for the Synthesis of the Cytotoxic Sesquiterpene (+/−)-Quadrone. J. Org. Chem. 1987, 52, 1483–1486. [Google Scholar] [CrossRef]
- Labelle, B.E.; Knudsen, M.J.; Olmstead, M.M.; Hope, H.; Yanuck, M.D.; Schore, N.E. Synthesis of 11-Oxatricyclo [5.3.1.02,6] Undecane Derivatives Via Organometallic Cyclizations. J. Org. Chem. 1985, 50, 5215–5222. [Google Scholar] [CrossRef]
- Krafft, M.E.; Romero, R.H.; Scott, I.L. Pauson-Khand Reaction with Electron-Deficient Alkynes. J. Org. Chem. 1992, 57, 5277–5278. [Google Scholar] [CrossRef]
- Robert, F.; Milet, A.; Gimbert, Y.; Konya, D.; Greene, A.E. Regiochemistry in the Pauson-Khand Reaction: Has a Trans Effect Been Overlooked? J. Am. Chem. Soc. 2001, 123, 5396–5400. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kuwabara, S.; Ando, Y.; Nagata, H.; Nishiyama, H.; Itoh, K. Palladium(0)-Catalyzed Cyclization of Electron-Deficient Enynes and Enediynes. J. Org. Chem. 2004, 69, 6697–6705. [Google Scholar] [CrossRef] [PubMed]
- Pauson, P.L. The Khand Reaction—A Convenient and General-Route to a Wide-Range of Cyclopentenone Derivatives. Tetrahedron 1985, 41, 5855–5860. [Google Scholar] [CrossRef]
- De Bruin, T.J.M.; Milet, A.; Greene, A.E.; Gimbert, Y. Insight into the Reactivity of Olefins in the Pauson-Khand Reaction. J. Org. Chem. 2004, 69, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Krafft, M.E. Regiocontrol in the Intermolecular Cobalt-Catalyzed Olefin Acetylene Cyclo-Addition. J. Am. Chem. Soc. 1988, 110, 968–970. [Google Scholar] [CrossRef]
- Krafft, M.E. Steric Control in the Pauson Cyclo-Addition—Further Support for the Proposed Mechanism. Tetrahedron Lett. 1988, 29, 999–1002. [Google Scholar] [CrossRef]
- Sola, J.; Riera, A.; Verdaguer, X.; Maestro, M.A. Phosphine-Substrate Recognition through the C-H⋯O Hydrogen Bond: Application to the Asymmetric Pauson-Khand Reaction. J. Am. Chem. Soc. 2005, 127, 13629–13633. [Google Scholar] [CrossRef]
- Ahmar, M.; Antras, F.; Cazes, B. Pauson-Khand Reaction with Allenic Compounds.1. Synthesis of 4-Alkylidene-2-Cyclopentenones. Tetrahedron Lett. 1995, 36, 4417–4420. [Google Scholar] [CrossRef]
- Ahmar, M.; Chabanis, O.; Gauthier, J.; Cazes, B. Pauson-Khand Reaction with Allenic Compounds II: Reactivity of Functionalized Allenes. Tetrahedron Lett. 1997, 38, 5277–5280. [Google Scholar] [CrossRef]
- Wu, N.; Deng, L.J.; Liu, L.Z.; Liu, Q.; Li, C.C.; Yang, Z. Reverse Regioselectivity in the Palladium(II) Thiourea Catalyzed Intermolecular Pauson-Khand Reaction. Chem. Asian J. 2013, 8, 65–68. [Google Scholar] [CrossRef]
- Smit, W.A.; Gybin, A.S.; Shashkov, A.S.; Strychkov, Y.T.; Kyzmina, L.G.; Mikaelian, G.S.; Caple, R.; Swanson, E.D. New Route to the Synthesis of Polycyclic Compounds Based on a Stepwise Ade-Reaction of Dicobalt Hexacarbonyl Complexes of Conjugated Enynes with a Subsequent Intramolecular Khand-Pauson Type Reaction. Tetrahedron Lett. 1986, 27, 1241–1244. [Google Scholar] [CrossRef]
- Shen, J.K.; Gao, Y.C.; Shi, Q.Z.; Basolo, F. Oxygen Atom Transfer-Reactions to Metal-Carbonyls—Kinetics and Mechanism of Co Substitution of Fe(Co)5, Ru(Co)5, Os(Co)5 in the Presence of (CH3)3NO. Organometallics 1989, 8, 2144–2147. [Google Scholar] [CrossRef]
- Shambayati, S.; Crowe, W.E.; Schreiber, S.L. N-Oxide Promoted Pauson-Khand Cyclizations at Room-Temperature. Tetrahedron Lett. 1990, 31, 5289–5292. [Google Scholar] [CrossRef]
- Gallagher, A.G.; Tian, H.; Torres-Herrera, O.A.; Yin, S.; Xie, A.; Lange, D.M.; Wilson, J.K.; Mueller, L.G.; Gau, M.R.; Carroll, P.J.; et al. Access to Highly Functionalized Cyclopentenones via Diastereoselective Pauson-Khand Reaction of Siloxy-Tethered 1,7-Enynes. Org. Lett. 2019, 21, 8646–8651. [Google Scholar] [CrossRef]
- Hiroi, K.; Watanabe, T.; Kawagishi, R.; Abe, I. Asymmetric Catalytic Pauson-Khand Reactions with Chiral Phosphine Ligands: Dramatic Effects of Substituents in 1,6-Enyne Systems. Tetrahedron Lett. 2000, 41, 891–895. [Google Scholar] [CrossRef]
- Tang, Y.F.; Deng, L.J.; Zhang, Y.D.; Dong, G.B.; Chen, J.H.; Yang, Z. Tetramethyl thiourea/Co2(CO)8-catalyzed Pauson-Khand Reaction under Balloon Pressure of CO. Org. Lett. 2005, 7, 593–595. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, T.; Yamada, M.; Ban, H.; Yamaguchi, M.; Kaneko, C. Rate Enhancement of the Pauson-Khand Reaction by Primary Amines. Angew. Chem. Int. Ed. 1997, 36, 2801–2804. [Google Scholar] [CrossRef]
- Del Valle, C.P.; Milet, A.; Gimbert, Y.; Greene, A.E. Lewis Base Promoters in the Pauson-Khand Reaction: A Different Scenario. Angew. Chem. Int. Ed. 2005, 44, 5717–5719. [Google Scholar] [CrossRef]
- Kobayashi, T.; Koga, Y.; Narasaka, K. The Rhodium-Catalyzed Pauson-Khand Reaction. J. Organomet. Chem. 2001, 624, 73–87. [Google Scholar] [CrossRef]
- Schmid, T.M.; Consiglio, G. Mechanistic and Stereochemical Aspects of the Asymmetric Cyclocarbonylation of 1,6-Enynes with Rhodium Catalysts. Chem. Commun. 2004, 20, 2318–2319. [Google Scholar] [CrossRef]
- Wender, P.A.; Deschamps, N.M.; Williams, T.J. Intermolecular Dienyl Pauson–Khand Reaction. Angew. Chem. Int. Ed. 2004, 43, 3076–3079. [Google Scholar] [CrossRef] [PubMed]
- Wender, P.A.; Croatt, M.P.; Deschamps, N.M. Metal-Catalyzed [2+2+1] Cycloadditions of 1,3-Dienes, Allenes, and CO. Angew. Chem. Int. Ed. 2006, 45, 2459–2462. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Toshida, N.; Takagi, K. Catalytic Pauson-Khand-Type Reaction Using Aldehydes as a CO Source. Org. Lett. 2002, 4, 1619–1621. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Cho, Y.; Chung, Y.K. Rhodium-Catalyzed Pauson-Khand-Type Reaction Using Alcohol as a Source of Carbon Monoxide. Angew. Chem. Int. Ed. 2010, 49, 5138–5141. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Chan, A.S.C.; Kwong, F.Y. Formate as a CO surrogate for cascade processes: Rh-catalyzed cooperative decarbonylation and asymmetric Pauson–Khand-type cyclization reactions. Chem. Commun. 2007, 25, 2633–2635. [Google Scholar] [CrossRef] [PubMed]
- Lang, X.-D.; You, F.; He, X.; Yu, Y.-C.; He, L.-N. Rhodium(I)-catalyzed Pauson Khand-type Reaction Using Formic Acid as a CO Surrogate: An Alternative Approach for Indirect CO2 Utilization. Green Chem. 2019, 21, 509–514. [Google Scholar] [CrossRef]
- Jorgensen, L.; McKerrall, S.J.; Kuttruff, C.A.; Ungeheuer, F.; Felding, J.; Baran, P.S. 14-Step Synthesis of (+)-Ingenol from (+)-3-Carene. Science 2013, 341, 878–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, M.J.; Liang, B.; Wang, C.H.; Chen, J.H.; Yang, Z. Synthesis of a Novel C-2-Symmetric Thiourea and Its Application in the Pd-Catalyzed Cross-Coupling Reactions with Arenediazonium Salts under Aerobic Conditions. Org. Lett. 2004, 6, 221–224. [Google Scholar] [CrossRef]
- Xiong, Z.C.; Wang, N.D.; Dai, M.J.; Li, A.; Chen, J.H.; Yang, Z. Synthesis of Novel Palladacycles and Their Application in Heck and Suzuki Reactions under Aerobic Conditions. Org. Lett. 2004, 6, 3337–3340. [Google Scholar] [CrossRef]
- Liao, Y.; Smith, J.; Fathi, R.; Yang, Z. Novel Pd(II)-Mediated Cascade Carboxylative Annulation to Construct BenzoFuran-3-Carboxylic Acids. Org. Lett. 2005, 7, 2707–2709. [Google Scholar] [CrossRef]
- Deng, L.J.; Liu, J.; Huang, J.Q.; Hu, Y.H.; Chen, M.; Lan, Y.; Chen, J.H.; Lei, A.W.; Yang, Z. Effect of Lithium Chloride on Tuning the Reactivity of Pauson-Khand Reactions Catalyzed by Palladium-Tetramethylthiourea. Synthesis 2007, 2007, 2565–2570. [Google Scholar] [CrossRef]
- Tang, Y.; Deng, L.; Zhang, Y.; Dong, G.; Chen, J.; Yang, Z. Thioureas as Ligands in the Pd-Catalyzed Intramolecular Pauson−Khand Reaction. Org. Lett. 2005, 7, 1657–1659. [Google Scholar] [CrossRef]
- Lan, Y.; Deng, L.J.; Liu, J.; Wang, C.; Wiest, O.; Yang, Z.; Wu, Y.D. On the Mechanism of the Palladium Catalyzed Intramolecular Pauson-Khand-Type Reaction. J. Org. Chem. 2009, 74, 5049–5058. [Google Scholar] [CrossRef] [PubMed]
- Crowe, W.E.; Vu, A.T. Direct Synthesis of Fused, Bicyclic γ-Butyrolactones via Tandem Reductive Cyclization−Carbonylation of Tethered Enals and Enones. J. Am. Chem. Soc. 1996, 118, 1557–1558. [Google Scholar] [CrossRef]
- Mandal, S.K.; Amin, S.R.; Crowe, W.E. γ-Butyrolactone Synthesis via Catalytic Asymmetric Cyclocarbonylation. J. Am. Chem. Soc. 2001, 123, 6457–6458. [Google Scholar] [CrossRef] [PubMed]
- Kablaoui, N.M.; Hicks, F.A.; Buchwald, S.L. Diastereoselective Synthesis of γ-Butyrolactones from Enones Mediated or Catalyzed by a Titanocene Complex. J. Am. Chem. Soc. 1996, 118, 5818–5819. [Google Scholar] [CrossRef]
- Kablaoui, N.M.; Hicks, F.A.; Buchwald, S.L. Titanocene-Catalyzed Cyclocarbonylation of o-Allyl Aryl Ketones to γ-Butyrolactones. J. Am. Chem. Soc. 1997, 119, 4424–4431. [Google Scholar] [CrossRef]
- Chatani, N.; Morimoto, T.; Kamitani, A.; Fukumoto, Y.; Murai, S.J. Ru3(CO)12-catalyzed reaction of yne–imines with carbon monoxide leading to bicyclic α, β-unsaturated lactams. Organomet. Chem. 1999, 579, 177–181. [Google Scholar] [CrossRef]
- Adrio, J.; Carretero, J.C. Butenolide Synthesis by Molybdenum-Mediated Hetero-Pauson−Khand Reaction of Alkynyl Aldehydes. J. Am. Chem. Soc. 2007, 129, 778–779. [Google Scholar] [CrossRef]
- Saito, T.; Sugizaki, K.; Osada, H.; Kutsumura, N.; Otani, T. A Hetero Pauson-Khand Reaction of Ketenimines: A New Synthetic Method for γ-Exomethylene-α, β-unsaturated γ-Lactams. Heterocycles 2010, 80, 207–211. [Google Scholar] [CrossRef]
- Finnegan, D.F.; Snapper, M.L. Formation of Polycyclic Lactones through a Ruthenium-Catalyzed Ring-Closing Metathesis/Hetero-Pauson–Khand Reaction Sequence. J. Org. Chem. 2011, 76, 3644–3653. [Google Scholar] [CrossRef]
- Gao, P.; Xu, P.F.; Zhai, H. Expeditious Construction of (+)-Mintlactone via Intramolecular Hetero-Pauson−Khand Reaction. J. Org. Chem. 2009, 74, 2592–2593. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gao, P.; Yu, F.; Yang, Y.; Zhu, S.; Zhai, H. Total Synthesis of (±)-Merrilactone A. Angew. Chem. Int. Ed. 2012, 51, 5897–5899. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-H.; Martinez, M.D.; Shenvi, R.A. An eight-step gram-scale synthesis of (−)-jiadifenolide. Nat. Chem. 2015, 7, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Chirkin, E.; Michel, S.; Porée, F.H. Viability of a [2 + 2 + 1] Hetero-Pauson–Khand Cycloaddition Strategy toward Securinega Alkaloids: Synthesis of the BCD-Ring Core of Securinine and Related Alkaloids. J. Org. Chem. 2015, 80, 6525–6528. [Google Scholar] [CrossRef] [PubMed]
- Mukai, C.; Yoshida, T.; Sorimachi, M.; Odani, A. Co2(CO)8-Catalyzed Intramolecular Hetero-Pauson−Khand Reaction of Alkynecarbodiimide: Synthesis of (±)-Physostigmine. Org. Lett. 2006, 8, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Huang, J.; Qu, Y.; Zhang, W.; Gong, J.; Yang, Z. Total Syntheses of Crinipellins Enabled by Cobalt-Mediated and Palladium-Catalyzed Intramolecular Pauson-Khand Reactions. Angew. Chem. Int. Ed. 2018, 57, 8744–8748. [Google Scholar] [CrossRef]
- Peng, C.; Arya, P.; Zhou, Z.; Snyder, S.A. A Concise Total Synthesis of (+)-Waihoensene Guided by Quaternary Center Analysis. Angew. Chem. Int. Ed. 2020, 59, 13521–13525. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, Z.; Zhang, Z.; Zhang, W.; Huang, J.; Yang, Z. Asymmetric Total Synthesis of (+)-Waihoensene. J. Am. Chem. Soc. 2020, 142, 6511–6515. [Google Scholar] [CrossRef]
- Knudsen, M.J.; Schore, N.E. Synthesis of the Angularly Fused Triquinane Skeleton Via Intramolecular Organometallic Cyclization. J. Org. Chem. 1984, 49, 5025–5026. [Google Scholar] [CrossRef]
- Pallerla, M.K.; Fox, J.M. Enantioselective Synthesis of (−)-Pentalenene. Org. Lett. 2007, 9, 5625–5628. [Google Scholar] [CrossRef] [PubMed]
- Millham, A.B.; Kier, M.J.; Leon, R.M.; Karmakar, R.; Stempel, Z.D.; Micalizio, G.C. A Complementary Process to PausonKhand-Type Annulation Reactions for the Construction of Fully Substituted Cyclopentenones. Org. Lett. 2019, 21, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Bird, R.; Knipe, A.C.; Stirling, C.J.M.J. Intramolecular Reactions. Part X. Transition States in the Cyclisation of N-ω-Halogeno-alkylamines and –sulphonamides. Chem. Soc. Perkin Trans. 2 1973, 9, 1215–1220. [Google Scholar] [CrossRef]
- Wiesner, K.; Valenta, Z.; Findlay, J.A. The structure of ryanodine. Tetrahedron Lett. 1967, 8, 221–223. [Google Scholar] [CrossRef]
- Srivastava, S.N.; Przybylska, M. The molecular structure of ryanodol-p-bromo benzyl ether. Can. J. Chem. 1968, 46, 795–797. [Google Scholar] [CrossRef] [Green Version]
- Chuang, K.V.; Xu, C.; Reisman, S.E. A 15-step synthesis of (+)-ryanodol. Science 2016, 353, 912–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Li, Y.; Zhao, D.; He, Y.; Gong, J.; Yang, Z. A Concise Synthesis of Presilphiperfolane Corethrough a Tandem TMTU-Co-Catalyzed Pauson-Khand Reaction and a 6pi Electrocyclization Reaction (TMTU = Tetramethyl Thiourea). Chem. Eur. J. 2017, 23, 1258–1262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, D.; He, Y.; Yang, Z.; Gong, J. Total syntheses of dehydrobotrydienal, dehydrobotrydienol and 10-oxodehydro- dihydrobotrydial. Chin. Chem. Lett. 2019, 30, 1503–1505. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, D.; Zhang, Z.; Tan, X.; Gong, J.; Yang, Z. Synthesis of 4-Desmethyl-rippertenol and 7-Epi-rippertenol via Photo-induced Cyclization of Dienones. CCS Chem. 2020. [Google Scholar] [CrossRef]
- Sun, T.W.; Liu, D.D.; Wang, K.Y.; Tong, B.Q.; Xie, J.X.; Jiang, Y.L.; Li, Y.; Zhang, B.; Liu, Y.F.; Wang, Y.X.; et al. Asymmetric Total Synthesis of Lancifodilactone G Acetate. 1. Diastereoselective Synthesis of CDEFGH Ring System. J. Org. Chem. 2018, 83, 6893–6906. [Google Scholar] [CrossRef]
- Wang, K.Y.; Liu, D.D.; Sun, T.W.; Lu, Y.; Zhang, S.L.; Li, Y.H.; Han, Y.X.; Liu, H.Y.; Peng, C.; Wang, Q.Y.; et al. Asymmetric Total Synthesis of Lancifodilactone G Acetate. 2. Final Phase and Completion of the Total Synthesis. J. Org. Chem. 2018, 83, 6907–6923. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ding, M.; Li, J.; Guo, Z.; Lu, M.; Chen, Y.; Liu, L.; Shen, Y.H.; Li, A. Total Synthesis of Hybridaphniphylline B. J. Am. Chem. Soc. 2018, 140, 4227–4231. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.K.; Lee, B.Y.; Jeong, N.; Hudecek, M.; Pauson, P.L. Promoters for the (alkyne) hexacarbonyldicobalt-based cyclopentenone synthesis. Organometallics 1993, 12, 220–223. [Google Scholar] [CrossRef]
- Hu, N.; Dong, C.; Zhang, C.; Liang, G. Total Synthesis of (−)-Indoxamycins A and B. Angew. Chem. Int. Ed. 2019, 58, 6659–6662. [Google Scholar] [CrossRef]
- Hu, X.; Musacchio, A.J.; Shen, X.; Tao, Y.; Maimone, T.J. Allylative Approaches to the Synthesis of Complex Guaianolide Sesquiterpenes from Apiaceae and Asteraceae. J. Am. Chem. Soc. 2019, 141, 14904–14915. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.T.; Chen, J.H.; Yang, Z. Asymmetric Total Synthesis of (−)-Spirochensilide A. J. Am. Chem. Soc. 2020, 142, 8116–8121. [Google Scholar] [CrossRef]
- Xu, B.; Xun, W.; Su, S.; Zhai, H. Total Syntheses of (−)-Conidiogenone B, (−)-Conidiogenone, and (−)-Conidiogenol. Angew. Chem. Int. Ed. 2020, 132, 16617–16621. [Google Scholar]
- Clark, J.S.; Xu, C. Total Synthesis of (−)-Nakadomarin A. Angew. Chem. Int. Ed. 2016, 55, 4332–4335. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Tu, Q.; Gong, J.; Hao, X.; Yang, Z. Asymmetric total synthesis of (−)-perforanoid A. Tetrahedron 2017, 73, 3612–3621. [Google Scholar] [CrossRef]
- Cassayre, J.; Gagosz, F.; Zard, S.Z. A Short Synthesis of (±)-13-Deoxyserratine. Angew. Chem. Int. Ed. 2002, 41, 1783–1785. [Google Scholar] [CrossRef]
- Nakayama, A.; Kogure, N.; Kitajima, M.; Takayama, H. Asymmetric Total Synthesis of a Pentacyclic Lycopodium Alkaloid: Huperzine-Q. Angew. Chem. Int. Ed. 2011, 50, 8025–8028. [Google Scholar] [CrossRef] [PubMed]
- Itoh, N.; Iwata, T.; Sugihara, H.; Inagaki, F.; Mukai, C. Total Syntheses of (±)-Fawcettimine, (±)-Fawcettidine, (±)-Lycoflexine, and (±)-Lycoposerramine-Q. Chem. Eur. J. 2013, 19, 8665–8672. [Google Scholar] [CrossRef]
- Williams, B.M.; Trauner, D. Expedient Synthesis of (+)-Lycopalhine A. Angew. Chem. Int. Ed. 2016, 55, 2191–2194. [Google Scholar] [CrossRef]
- Yamakoshi, H.; Sawayama, Y.; Akahori, Y.; Kato, M.; Nakamura, S. Total Syntheses of (+)-Marrubiin and (−)-Marrulibacetal. Org. Lett. 2016, 18, 3430–3433. [Google Scholar] [CrossRef] [PubMed]
- Sakagami, Y.; Kondo, N.; Sawayama, Y.; Yamakoshi, H.; Nakamura, S. Total syntheses of marrubiin and related labdane diterpene lactones. Molecules 2020, 25, 1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bose, S.; Yang, J.; Yu, Z.-X. Formal Synthesis of Gracilamine Using Rh(I)- Catalyzed [3 + 2 + 1] Cycloaddition of 1-Yne-Vinylcyclopropanes and CO. J. Org. Chem. 2016, 81, 6757–6765. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yang, B.; Cai, S.; Gao, S. Total Synthesis of Gracilamine. Angew. Chem. Int. Ed. 2014, 53, 9539–9543. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Perez, B.; Maestro, M.A.; Mourino, A. Total synthesis of 1α,25-dihydroxyvitamin D3 (calcitriol) through a Si-assisted allylic substitution. Chem. Commun. 2017, 53, 8144–8147. [Google Scholar] [CrossRef] [PubMed]
- Salam, A.; Ray, S.; Zaid, M.A.; Kumar, D.; Khan, T. Total syntheses of several iridolactones and the putative structure of noriridoid scholarein A: An intramolecular Pauson–Khand reaction based one-stop synthetic solution. Org. Biomol. Chem. 2019, 17, 6831–6842. [Google Scholar] [CrossRef]
- Kourav, M.S.; Kumar, V.; Kumar, D.; Khan, T. A De Novo Approach for the Stereoselective Synthesis of Cyclopenta[c]pyranone Scaffold Present in Iridoids: Formal Syntheses of Isoboonein, Iridomyrmecin and Isoiridomyrmecin. ChemistrySelect 2018, 3, 5566–5570. [Google Scholar] [CrossRef]
- Hirasawa, Y.; Morita, H.; Shiro, M.; Kobayashi, J. Sieboldine A, a Novel Tetracyclic Alkaloid from Lycopodium sieboldii, Inhibiting Acetylcholinesterase. Org. Lett. 2003, 5, 3991–3993. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Gaber, M.K.; Yasuda, S.; Iida, E.; Mukai, C. Enantioselective Total Synthesis of (+)-Sieboldine A. Org. Lett. 2017, 19, 320–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, C.; Zhang, J.; Chen, X.; Wang, H.; Li, Y.; Cheng, B.; Zhai, H. Formal Synthesis of (+/−)-Aplykurodinone-1 through a Hetero-Pauson-Khand Cycloaddition Approach. Org. Lett. 2017, 19, 1056–1059. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Deng, L.-L.; Mu, S.-Z.; Sun, Q.-Y.; Hao, X.-J.; Zhang, Y.-H. Sinoraculine, the Precursor of the Novel Alkaloid Sinoracutine from Stephania cepharantha Hayata. Helvetica Chimica Acta 2012, 95, 1198–1201. [Google Scholar] [CrossRef]
- Bao, G.-H.; Wang, X.-L.; Tang, X.-C.; Chiu, P.; Qin, G.-W. Sinoracutine, a novel skeletal alkaloid with cell-protective effects from Sinomenium acutum. Tetrahedron Lett. 2009, 50, 4375–4377. [Google Scholar] [CrossRef]
- Volpin, G.; Veprek, N.A.; Bellan, A.B.; Trauner, D. Enantioselective Synthesis and Racemization of (−)-Sinoracutine. Angew. Chem. Int. Ed. 2017, 56, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Sennett, S.H.; Pompeni, S.A.; Wright, A.E. Diterpene Metabolites from Two Chemotypes of the Marine Sponge Myrmekioderma styx. J. Nat. Prod. 1992, 55, 1421–1429. [Google Scholar] [CrossRef]
- Chang, Y.; Shi, L.; Huang, J.; Shi, L.; Zhang, Z.; Hao, H.D.; Gong, J.; Yang, Z. Stereoselective Total Synthesis of (+/−)-5-epi-Cyanthiwigin I via an Intramolecular Pauson-Khand Reaction as the Key Step. Org. Lett. 2018, 20, 2876–2879. [Google Scholar] [CrossRef]
- Kaneko, H.; Takahashi, S.; Kogure, N.; Kitajima, M.; Takayama, H. Asymmetric Total Synthesis of Fawcettimine-Type Lycopodium Alkaloid, Lycopoclavamine-A. J. Org. Chem. 2019, 84, 5645–5654. [Google Scholar] [CrossRef]
- Sadler, I.H.; Simpson, T.J. The determination by n.m.r. methods of the structure and stereochemistry of astellatol, a new and unusual sesterterpene. J. Chem. Soc. Chem. Commun. 1989, 21, 1602–1604. [Google Scholar] [CrossRef]
- Zhao, N.; Xie, S.; Tian, P.; Tong, R.; Ning, C.; Xu, J. Asymmetric total synthesis of (+)-astellatol and (−)-astellatene. Org. Chem. Front. 2019, 6, 2014–2022. [Google Scholar] [CrossRef]
- Chirkin, E.; Bouzidi, C.; Porée, F.H. Tungsten-Promoted Hetero-Pauson–Khand Cycloaddition: Application to the Total Synthesis of (–)-Allosecurinine. Synthesis 2019, 51, 2001–2006. [Google Scholar] [CrossRef]
- Hugelshofer, C.L.; Palani, V.; Sarpong, R. Calyciphylline BType Alkaloids: Total Syntheses of (−)-Daphlongamine H and (−)-Isodaphlongamine H. J. Am. Chem. Soc. 2019, 141, 8431–8435. [Google Scholar] [CrossRef] [Green Version]
- Winther, M.; Liu, H.; Sonntag, Y.; Olesen, C.; Le Maire, M.; Soehoel, H.; Olsen, C.E.; Christensen, S.B.; Nissen, P.; MÜller, J.V. Critical Roles of Hydrophobicity and Orientation of Side Chains for Inactivation of Sarcoplasmic Reticulum Ca2+-ATPase with Thapsigargin and Thapsigargin Analogs. J. Biol. Chem. 2010, 285, 28883–28892. [Google Scholar] [CrossRef] [Green Version]
- Skytte, D.M.; MÜller, J.V.; Liu, H.; Nielsen, H.Ø.; Svenningsen, L.E.; Jensen, C.M.; Olsen, C.E.; Christensen, S.B. Elucidation of the Topography of the Thapsigargin Binding Site in the Sarco-endoplasmic Calcium ATPase. Bioorg. Med. Chem. 2010, 18, 5634–5646. [Google Scholar] [CrossRef] [PubMed]
- Denmeade, S.R.; Mhaka, A.M.; Rosen, D.M.; Brennen, W.N.; Dalrymple, S.; Dach, I.; Olesen, C.; Gurel, B.; DeMarzo, A.M.; Wilding, G.; et al. Engineering a Prostate-Specific Membrane Antigen-Activated Tumor Endothelial Cell Prodrug for Cancer Therapy. Sci. Transl. Med. 2012, 4, 140ra86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, J.K.; Hutchison, J.J.; Fu, X.; Kunnen, K. Methods of Making Cancer Compositions. WO 2014145035 A1, 18 September 2014. [Google Scholar]
- Zimmermann, T.; Christensen, S.B.; Franzyk, H. Preparation of Enzyme-Activated Thapsigargin Prodrugs by Solid-Phase Synthesis. Molecules 2018, 23, 1463. [Google Scholar] [CrossRef] [Green Version]
- Sanogo, Y.; Othman, R.B.; Dhambri, S.; Selkti, M.; Jeuken, A.; Prunet, J.; Ferezou, J.P.; Ardisson, J.; Lannou, M.I.; Sorin, G. Ti(II) and Rh(I) Complexes as Reagents toward a Thapsigargin Core. J. Org. Chem. 2019, 84, 5821–5830. [Google Scholar] [CrossRef]
- Tian, H.Y.; Ruan, L.J.; Yu, T.; Zheng, Q.F.; Chen, N.H.; Wu, R.B.; Zhang, X.Q.; Wang, L.; Jiang, R.W.; Ye, W.C. Bufospirostenin A and Bufogargarizin C, Steroids with Rearranged Skeletons from the Toad Bufo Bufo Gargarizans. J. Nat. Prod. 2017, 80, 1182–1186. [Google Scholar] [CrossRef]
- Cheng, M.J.; Zhong, L.P.; Gu, C.C.; Zhu, X.J.; Chen, B.; Liu, J.S.; Wang, L.; Ye, W.C.; Li, C.C. Asymmetric Total Synthesis of Bufospirostenin A. J. Am. Chem. Soc. 2020, 142, 12602–12607. [Google Scholar] [CrossRef]
- Closser, K.D.; Quintal, M.M.; Shea, K.M. The Scope and Limitations of Intramolecular Nicholas and Pauson−Khand Reactions for the Synthesis of Tricyclic Oxygen- and Nitrogen-Containing Heterocycles. J. Org. Chem. 2009, 74, 3680–3688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comer, E.; Rohan, E.; Deng, L.; Porco, J.A. An Approach to Skeletal Diversity Using Functional Group Pairing of Multifunctional Scaffolds. Org. Lett. 2007, 9, 2123–2126. [Google Scholar] [CrossRef]
- Nie, F.; Kunciw, D.L.; Wilcke, D.; Stokes, J.E.; Galloway, W.R.; Bartlett, S.; Sore, H.F.; Spring, D.R. A Multidimensional Diversity-Oriented Synthesis Strategy for Structurally Diverse and Complex Macrocycles. Angew. Chem. Int. Ed. 2016, 55, 11139–11143. [Google Scholar] [CrossRef] [PubMed]



































Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Jiang, C.; Zheng, N.; Yang, Z.; Shi, L. Evolution of Pauson-Khand Reaction: Strategic Applications in Total Syntheses of Architecturally Complex Natural Products (2016–2020). Catalysts 2020, 10, 1199. https://doi.org/10.3390/catal10101199
Chen S, Jiang C, Zheng N, Yang Z, Shi L. Evolution of Pauson-Khand Reaction: Strategic Applications in Total Syntheses of Architecturally Complex Natural Products (2016–2020). Catalysts. 2020; 10(10):1199. https://doi.org/10.3390/catal10101199
Chicago/Turabian StyleChen, Sijia, Chongguo Jiang, Nan Zheng, Zhen Yang, and Lili Shi. 2020. "Evolution of Pauson-Khand Reaction: Strategic Applications in Total Syntheses of Architecturally Complex Natural Products (2016–2020)" Catalysts 10, no. 10: 1199. https://doi.org/10.3390/catal10101199
APA StyleChen, S., Jiang, C., Zheng, N., Yang, Z., & Shi, L. (2020). Evolution of Pauson-Khand Reaction: Strategic Applications in Total Syntheses of Architecturally Complex Natural Products (2016–2020). Catalysts, 10(10), 1199. https://doi.org/10.3390/catal10101199