Kinetics and Nanoparticle Catalytic Enhancement of Biogas Production from Wastewater Using a Magnetized Biochemical Methane Potential (MBMP) System
Abstract
:1. Introduction
2. Results
2.1. Characterised Ms
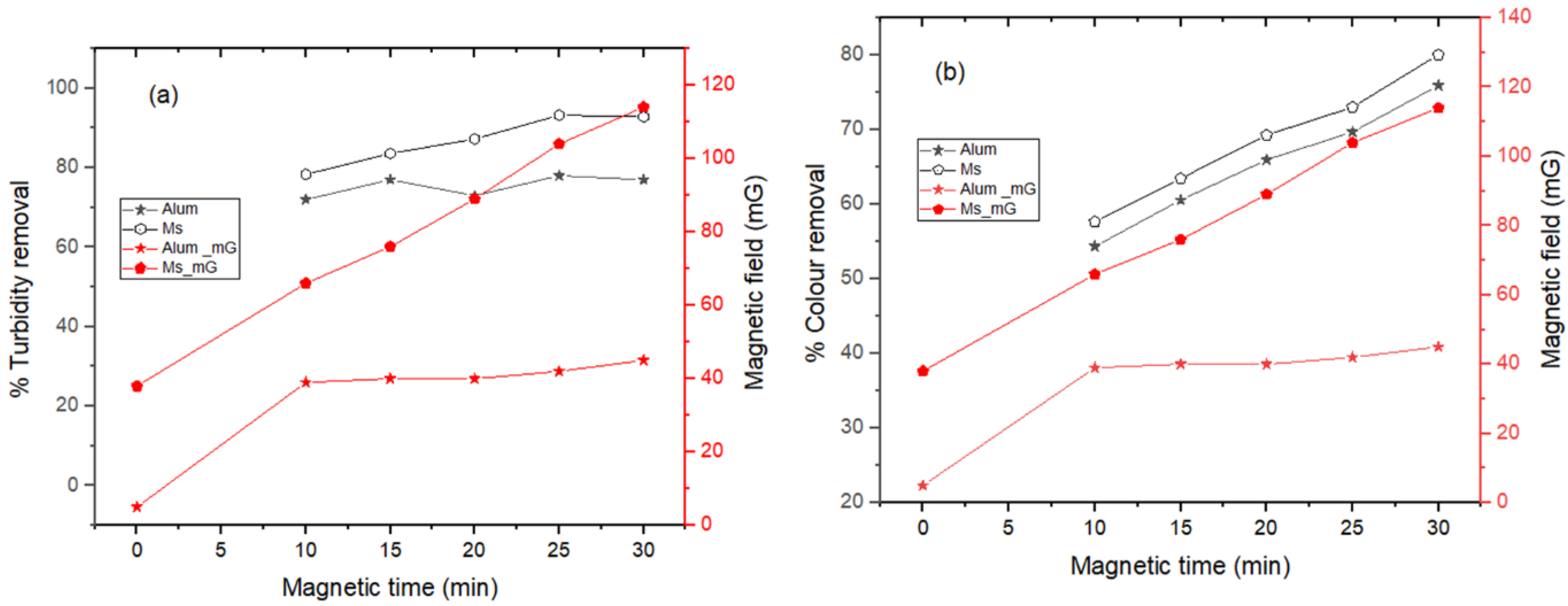
2.2. Coagulated Sludge Production
2.3. Biochemical Methane Potential (BMP)
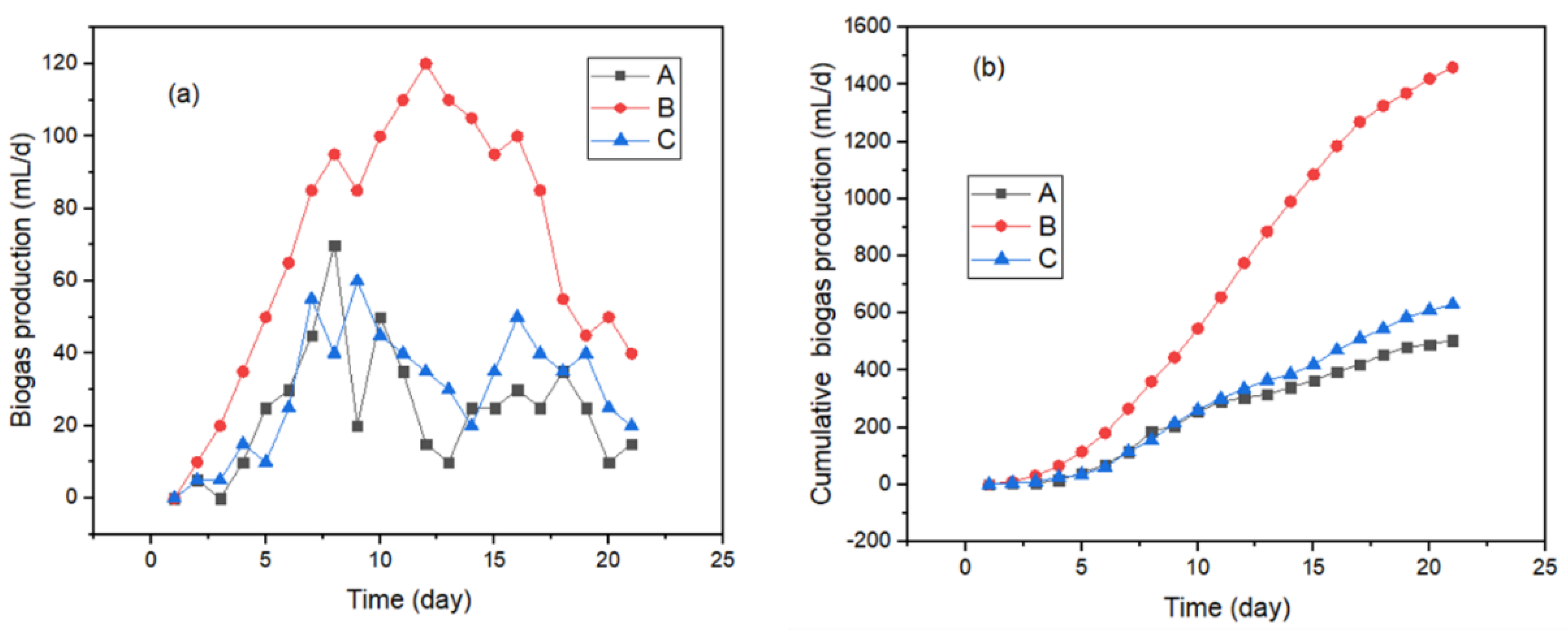
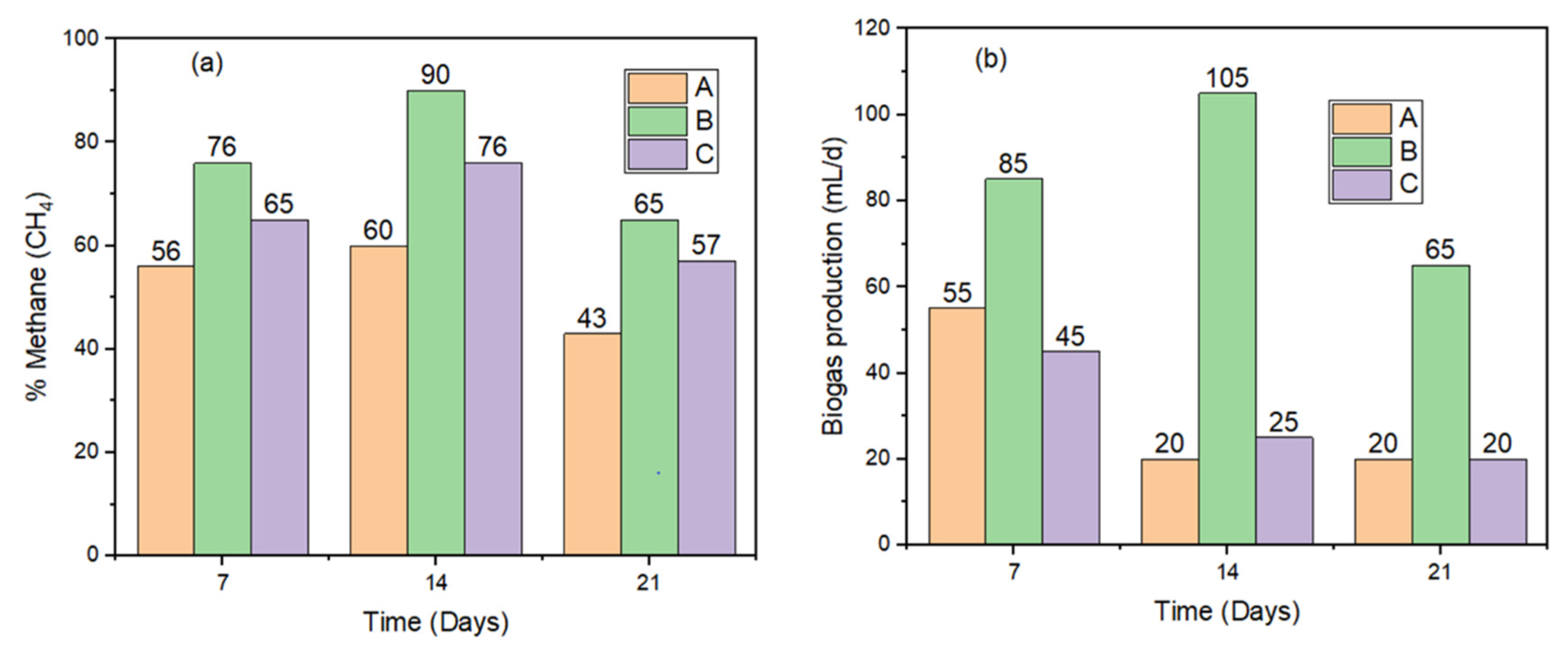
2.3.1. Biogas Production
2.3.2. SEM/EDS Results of Digester Sludge
2.3.3. Biogas Kinetics
2.3.4. Summary of Results
3. Discussions
3.1. Characteristics of Ms
3.2. Effects of Coagulant Dosage and Magnetic Field on Coagulation
3.3. Effects of Nanomaterials and Magnetic Field on Biogas and Methane Production
3.3.1. Sludge Surface Complexity and Traced Nanoparticles after Digestion
3.3.2. Biogas Production Kinetics Using First-Order and Modified Gompertz Models
4. Materials and Methods
4.1. Chemicals
4.1.1. Synthesis of Ferromagnetite (Ms)
4.1.2. Physiochemical Analysis
4.2. Wastewater and Substrate
4.3. Experimental Procedure
4.3.1. Coagulation Process
4.3.2. Biochemical Methane Potential (BMP)
5. Conclusions
- The future prospects of magnetized NPs (Fe NPs) to be used in bioenergy systems for energy conversion are economically viable. This is due to their high surface area, porous structure and supermagnetic properties, which can act as an activator for a degradation catalyst, such as a photocatalyst (Fe/TiO2).
- The development of magnetized NPs to be used as a coagulant or catalyst should be given research attention for the removal of contaminants and reduction of landfills complexity. This includes methods of recovery of the NPs after the application in the AD process to reduce environmental impact on soil and agricultural crops.
- Kinetically evaluate the effects of magnetic field on AD biogas production by considering different approaches such as substrate pre-treatment, nanoparticles dosage and inoculum mixture with respect to time should be considered.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Werner, C.M.; Logan, B.E.; Saikaly, P.E.; Amy, G.L. Wastewater treatment, energy recovery and desalination using a forward osmosis membrane in an air-cathode microbial osmotic fuel cell. J. Membr. Sci. 2013, 428, 116–122. [Google Scholar] [CrossRef]
- Goswami, L.; Kumar, R.V.; Pakshirajan, K.; Pugazhenthi, G. A novel integrated biodegradation—Microfiltration system for sustainable wastewater treatment and energy recovery. J. Hazard. Mater. 2019, 365, 707–715. [Google Scholar] [CrossRef]
- Maaz, M.; Yasin, M.; Aslam, M.; Kumar, G.; Atabani, A.E.; Idrees, M.; Anjum, F.; Jamil, F.; Ahmad, R.; Khan, A.L.; et al. Anaerobic membrane bioreactors for wastewater treatment: Novel configurations, fouling control and energy considerations. Bioresour. Technol. 2019, 283, 358–372. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Rathilal, S.; Chetty, M.; Armah, E.K.; Asante-Sackey, D. Treatment of Water and Wastewater for Reuse and Energy Generation-Emerging Technologies. In Water and Wastewater Treatment; Eyvaz, M., Ed.; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Wu, Y.; Lin, H.; Yin, W.; Shao, S.; Lv, S.; Hu, Y. Water quality and microbial community changes in an urban river after micro-nano bubble technology in situ treatment. Water 2019, 11, 66. [Google Scholar] [CrossRef] [Green Version]
- Tetteh, E.K.; Rathilal, S. Application of magnetized nanomaterial for textile effluent remediation using response surface methodology. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Zekić, E.; Vuković, Ž.; Halkijević, I. Application of nanotechnology in wastewater treatment. Gradjevinar 2018. [Google Scholar] [CrossRef]
- Zieliński, M.; Rusanowska, P.; Dębowski, M.; Hajduk, A. Influence of static magnetic field on sludge properties. Sci. Total Environ. 2018. [Google Scholar] [CrossRef] [PubMed]
- United Nations. The Sustainable Development Goals Report; United Nations Publications: New York, NY, USA, 2017. [Google Scholar] [CrossRef] [Green Version]
- Okoli, C.; Boutonnet, M.; Järas, S.; Rajarao-Kuttuva, G. Protein-functionalized magnetic iron oxide nanoparticles: Time efficient potential-water treatment. J. Nanopart. Res. 2012. [Google Scholar] [CrossRef]
- Hatamie, A.; Parham, H.; Zargar, B.; Heidari, Z. Evaluating magnetic nano-ferrofluid as a novel coagulant for surface water treatment. J. Mol. Liq. 2016. [Google Scholar] [CrossRef]
- Niemann, M.U.; Srinivasan, S.S.; Phani, A.R.; Kumar, A.; Goswami, D.Y.; Stefanakos, E.K. Nanomaterials for hydrogen storage applications: A review. J. Nanomater. 2008. [Google Scholar] [CrossRef] [Green Version]
- Marambio-Jones, C.; Hoek, E.M. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015. [Google Scholar] [CrossRef]
- Martins, F.; Felgueiras, C.; Smitková, M. Fossil fuel energy consumption in European countries. Energy Procedia 2018. [Google Scholar] [CrossRef]
- Muñoz, R.; Meier, L.; Diaz, I.; Jeison, D. A review on the state-of-the-art of physical/chemical and biological technologies for biogas upgrading. Rev. Environ. Sci. Bio/Technol. 2015, 14, 727–759. [Google Scholar] [CrossRef] [Green Version]
- Armah, E.K.; Chetty, M.; Deenadayalu, N. Effect of particle size on biogas generation from sugarcane bagasse and corn silage. Chem. Eng. Trans. 2019, 76, 1471–1476. [Google Scholar] [CrossRef]
- Laiq Ur Rehman, M.; Iqbal, A.; Chang, C.C.; Li, W.; Ju, M. Anaerobic digestion. Water Environ. Res. 2019. [Google Scholar] [CrossRef] [Green Version]
- Tetteh, E.; Amano, K.O.A.; Asante-Sackey, D.; Armah, E. Response Surface Optimisation of Biogas Potential in Co-Digestion of Miscanthus Fuscus and Cow Dung. Int. J. Technol. 2018. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, T.; Ahmad, K.; Ahad, A.; Alam, M. Characterization of water treatment sludge and its reuse as coagulant. J. Environ. Manag. 2016. [Google Scholar] [CrossRef]
- Dave, P.N.; Chopda, L.V. Application of iron oxide nanomaterials for the removal of heavy metals. J. Nanotechnol. 2014. [Google Scholar] [CrossRef] [Green Version]
- Seyedi, S.; Venkiteshwaran, K.; Benn, N.; Zitomer, D. Inhibition during Anaerobic Co-Digestion of Aqueous Pyrolysis Liquid from Wastewater Solids and Synthetic Primary Sludge. Sustainability 2020, 12, 3441. [Google Scholar] [CrossRef] [Green Version]
- Brar, S.K.; Verma, M.; Tyagi, R.D.; Surampalli, R.Y. Engineered nanoparticles in wastewater and wastewater sludge—Evidence and impacts. Waste Manag. 2010. [Google Scholar] [CrossRef] [PubMed]
- Ghernaout, D. Magnetic field generation in the water treatment perspectives: An overview. Int. J. Adv. Appl. Sci. 2017, 5, 193–203. [Google Scholar] [CrossRef]
- Santos, T.R.T.; Silva, M.F.; Nishi, L.; Vieira, A.M.S.; Klein, M.R.F.; Andrade, M.B.; Bergamasco, R. Development of a magnetic coagulant based on Moringa oleifera seed extract for water treatment. Environ. Sci. Pollut. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Sousa, J.; Pais, A.; Vitorino, C. The role of magnetic nanoparticles in cancer nanotheranostics. Materials 2020, 13, 266. [Google Scholar] [CrossRef] [Green Version]
- dos Santos, L.A.; Valença, R.B.; da Silva, L.C.S.; de Barros Holanda, S.H.; da Silva, A.F.V.; Jucá, J.F.T.; Santos, A.F.M.S. Methane generation potential through anaerobic digestion of fruit waste. J. Clean. Prod. 2020, 256, 120389. [Google Scholar] [CrossRef]
- Zhao, B.; Sha, H.; Li, J.; Cao, S.; Wang, G.; Yang, Y. Static magnetic field enhanced methane production via stimulating the growth and composition of microbial community. J. Clean. Prod. 2020, 271, 122664. [Google Scholar] [CrossRef]
- Deepanraj, B.; Sivasubramanian, V.; Jayaraj, S. Effect of substrate pretreatment on biogas production through anaerobic digestion of food waste. Int. J. Hydrog. Energy. 2017, 42, 26522–26528. [Google Scholar] [CrossRef]
- Zaidi, A.A.; RuiZhe, F.; Shi, Y.; Khan, S.Z.; Mushtaq, K. Nanoparticles augmentation on biogas yield from microalgal biomass anaerobic digestion. Int. J. Hydrog. Energy. 2018, 43, 14202–14213. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Rathilal, S. Application of organic coagulants in water and wastewater treatment. In Organic Polymers (Sand anZaki); IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Iwuozor, K.O. Prospects and Challenges of Using Coagulation-Flocculation method in the treatment of Effluents. Adv. J. Chem. Sect. A Theor. Eng. Appl. Chem. 2019, 2, 105–127. [Google Scholar] [CrossRef] [Green Version]
- Biswas, A. Nanotechnology in Biofuels Production: A Novel Approach for Processing and Production of Bioenergy. In Sustainable Approaches for Biofuels Production Technologies; Springer: Cham, Switzerland, 2019; pp. 183–193. [Google Scholar]
- Sreekanth, K.M.; Sahu, D. Effect of iron oxide nanoparticle in bio digestion of a portable food-waste digester. J. Chem. Pharm. Res. 2015, 7, 353–359. [Google Scholar]
- Ojo, P.; Ifelebuegu, A.O. The Effects of Aluminium-and Ferric-Based Chemical Phosphorus Removal on Activated Sludge Digestibility and Dewaterability. Processes 2019, 7, 228. [Google Scholar] [CrossRef] [Green Version]
- Casals, E.; Barrena, R.; García, A.; González, E.; Delgado, L.; Busquets-Fité, M.; Font, X.; Arbiol, J.; Glatzel, P.; Kvashnina, K.; et al. Programmed iron oxide nanoparticles disintegration in anaerobic digesters boosts biogas production. Small 2014, 10, 2801–2808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Water Works Association/American Public Works Association/Water Environment Federation: Washington, DC, USA, 2012. [Google Scholar]








| 2θ (°) | d (Å) | Interlinear Spacing Values (hkl) | Crystal Structure | Iron Oxides Phase (JCPDS File No. 19-0629) |
|---|---|---|---|---|
| 21.4 | 6.32 | (200) | Orthorhombic | W wustite (FeO) |
| 24.2 | 4.53 | (111) | Cubic | Ms magnetite (Fe3O4) |
| 25.3 | 3.96 | (222) | Hexagonal | H: hematite (α-Fe2O3) |
| (440) | Cubic | Ms | ||
| 26.5 | 3.76 | (104) | Hexagonal | H |
| 36.5 | 3.16 | (024) | Hexagonal | H |
| 37.8 | 2.96 | (220) | Cubic | Ms |
| 38.4 | 2.70 | (311) | Cubic | Ms |
| 42.6 | 2.52 | (200) | Orthorhombic | W |
| 48.2 | 2.41 | (116) | Hexagonal | H |
| (400) | Cubic | Ms | ||
| 53.6 | 1.96 | (422) | Cubic | Ms |
| 55.1 | 1. 74 | (511) | Cubic | Ms |
| 60.1 | 1.69 | (220) | Orthorhombic | W |
| 62.8 | 1.52 | (214) | Cubic | Ms |
| 68.7 | 1.38 | (533) | Cubic | Ms |
| Parameters | N* Ms | W* Ms | N* Alum | W* Alum |
|---|---|---|---|---|
| Dosage (mg/L) | 50 | 50 | 50 | 50 |
| Magnetic time (min) | N/A | 30 | N/A | 30 |
| Magnetic field (mG) | N/A | 111.69 | N/A | 81.05 |
| Turbidity (%) | 85 | 95 | 70 | 80 |
| Colour (%) | 70 | 80 | 60 | 75 |
| Set-Up | A—Alum | B—Ms | C—Control |
|---|---|---|---|
| Modified Gompertz Model | |||
| y | 535.28 ± 09 | 1731.3 ± 58 | 736.3 ± 67 |
| k (1/day) | 0.195 | 0.127 | 0.285 |
| c | 9.13 | 10.69 | 10.97 |
| Actual coefficient of determination (R2) | 0.977 | 0.999 | 0.965 |
| Adjustable coefficient of determination (Adj R2) | 0.969 | 0.996 | 0.954 |
| Sum of squares residual (SSR) | 6075.73 ± 35 | 2113.63 ± 92 | 4915.56 ± 41 |
| First-order model | |||
| y | 283.65 ± 25 | 827.33 ± 66 | 339.44 ± 52 |
| k (1/day) | 0.315 | 0.221 | 0.329 |
| Actual coefficient of determination (R2) | 0.985 | 0.983 | 0.976 |
| Adjustable coefficient of determination (Adj R2) | 0.983 | 0.981 | 0.966 |
| Sum of squares residual (SSR) | 1170.07 ± 75 | 932.38 ± 36 | 1292.89 ± 18 |
| Parameter | A—Alum | B—Ms | C—Control |
|---|---|---|---|
| pH | 6.4 | 6.6 | 6.8 |
| COD (mg/L) | 304 ± 92 | 157 ± 8 | 258 ± 72 |
| Turbidity (NTU) | 66 ± 44 | 24 ± 16 | 105 ± 7 |
| Color (PtCo) | 75 ± 2 | 52 ± 8 | 112 ± 24 |
| TSS (mg/L) | 38 ± 8 | 22 ± 7 | 60 ± 8 |
| Al (mg/L) | 36 ± 8 | 8 ± 4 | 30 ± 4 |
| Fe (mg/L) | 60 ± 16 | 6 ± 7 | 32 ± 3 |
| Average daily biogas (mL/d) | 25 ± 42 | 70 ± 14 | 30 ± 5 |
| Cumulative biogas (mL/d) | 505 | 1460 | 630 |
| % Methane (CH3) | 51.56 | 79.8 | 60.74 |
| % Carbon dioxide (CO2) | 48.44 | 20.2 | 39.26 |
| Substrate | Cumulative Biogas Production (mL/d) without Nanoparticles | Cumulative Biogas Production (mL/day) with Nanoparticles | Ref |
|---|---|---|---|
| Algae wastewater + Ni NPs | 488 | 618 | [30] |
| Algae wastewater + Co NPs | 488 | 535 | [30] |
| Algae wastewater + Fe NPs | 488 | 624 | [30] |
| Algae wastewater + Mg NPs | 488 | 529 | [30] |
| Sewage + Al salt | 34.91 ± 1.3 | 24.85 ± 1.3 | [35] |
| Sewage + Fe salt | 34.91 ± 1.3 | 22.46 ± 1.5 | [35] |
| Sugar refinery wastewater + Alum | 630 | 505 | * This study |
| Sugar refinery wastewater + Ms | 630 | 1460 | * This study |
| Parameter | Units | Value |
|---|---|---|
| pH | - | 6.6–7.2 |
| Chemical oxygen demand (COD) | (mg/L) | 942 ± 180 |
| Turbidity | NTU | 302 ± 18 |
| Color | Pt.Co | 250 ± 24 |
| Total suspended solids (TSS) | (mg/L) | 152 ± 13 |
| * Aluminium ion (Al) | (mg/L) | 56 ± 12 |
| * Iron (Fe) | (mg/L) | 134 ± 19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kweinor Tetteh, E.; Rathilal, S. Kinetics and Nanoparticle Catalytic Enhancement of Biogas Production from Wastewater Using a Magnetized Biochemical Methane Potential (MBMP) System. Catalysts 2020, 10, 1200. https://doi.org/10.3390/catal10101200
Kweinor Tetteh E, Rathilal S. Kinetics and Nanoparticle Catalytic Enhancement of Biogas Production from Wastewater Using a Magnetized Biochemical Methane Potential (MBMP) System. Catalysts. 2020; 10(10):1200. https://doi.org/10.3390/catal10101200
Chicago/Turabian StyleKweinor Tetteh, Emmanuel, and Sudesh Rathilal. 2020. "Kinetics and Nanoparticle Catalytic Enhancement of Biogas Production from Wastewater Using a Magnetized Biochemical Methane Potential (MBMP) System" Catalysts 10, no. 10: 1200. https://doi.org/10.3390/catal10101200
APA StyleKweinor Tetteh, E., & Rathilal, S. (2020). Kinetics and Nanoparticle Catalytic Enhancement of Biogas Production from Wastewater Using a Magnetized Biochemical Methane Potential (MBMP) System. Catalysts, 10(10), 1200. https://doi.org/10.3390/catal10101200







