A Multi-Enzyme Cascade for the Production of High-Value Aromatic Compounds
Abstract
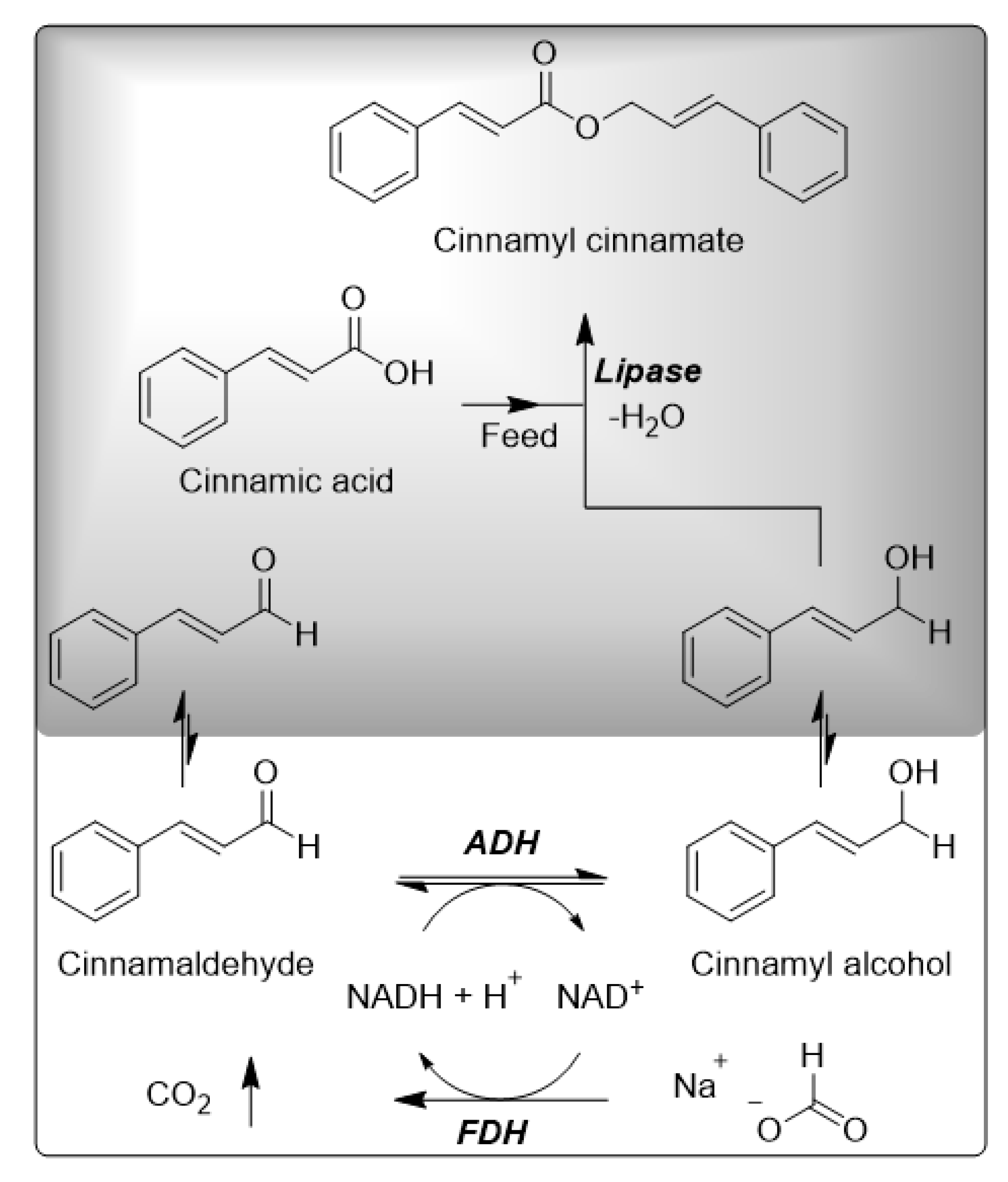
:1. Introduction
2. Results
2.1. Enzyme Characteristics
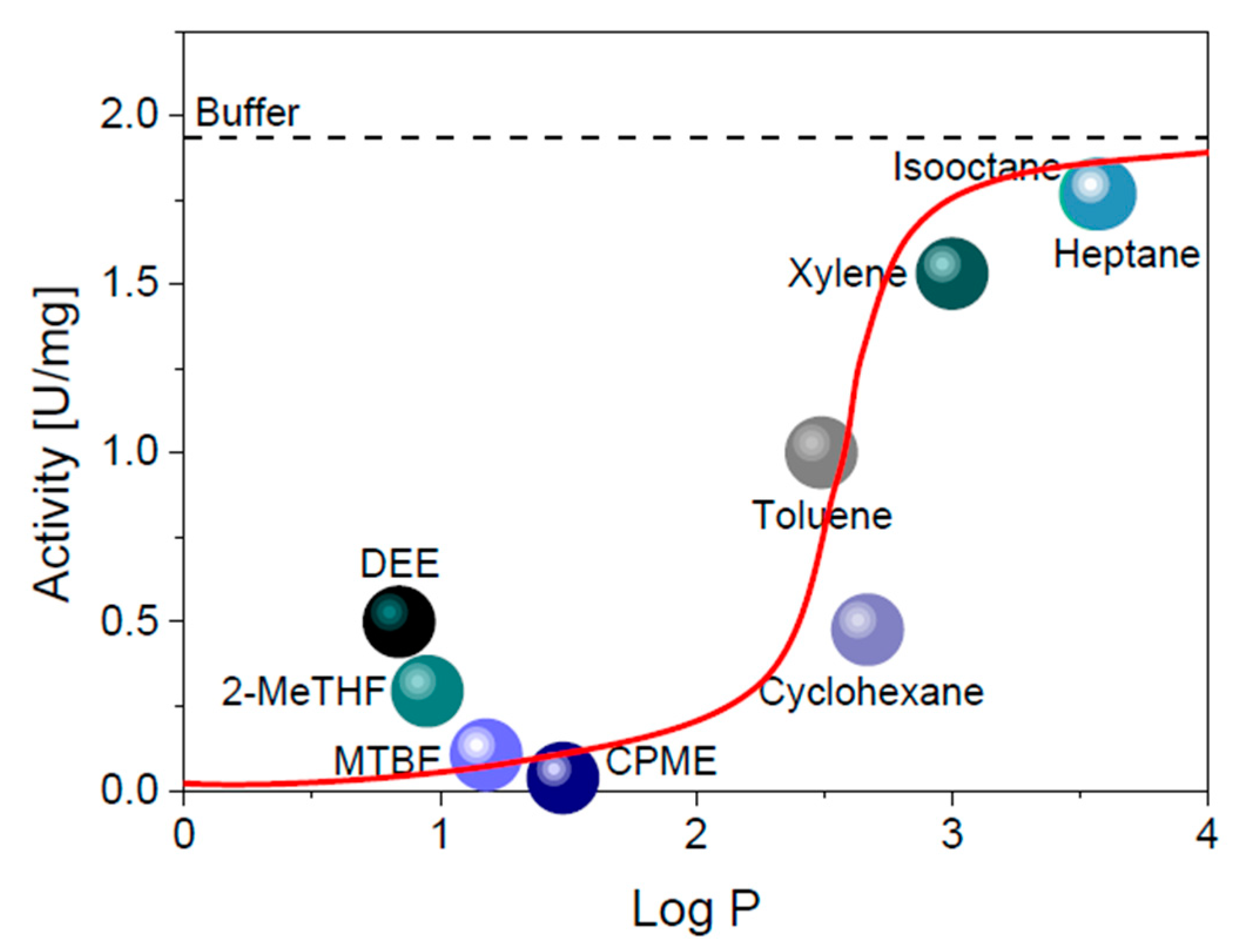
2.2. Solvent Screening
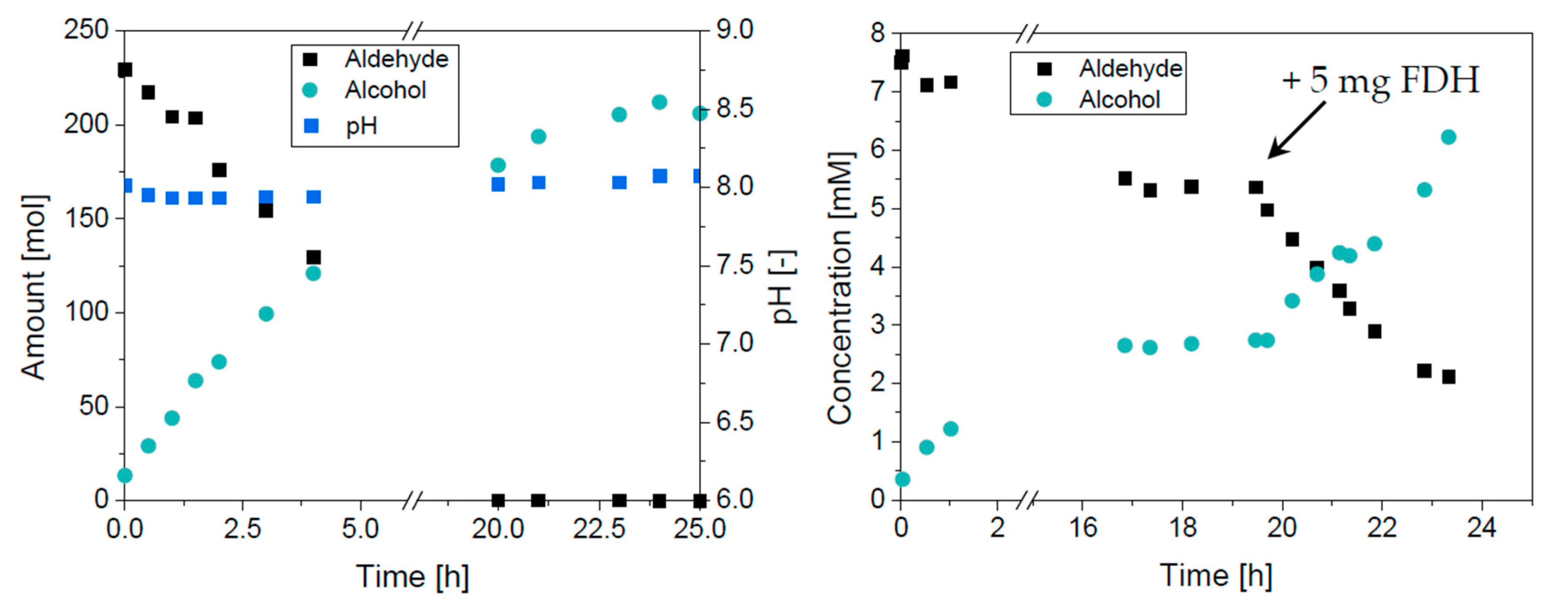
2.3. Reaction in the Aqueous Phase
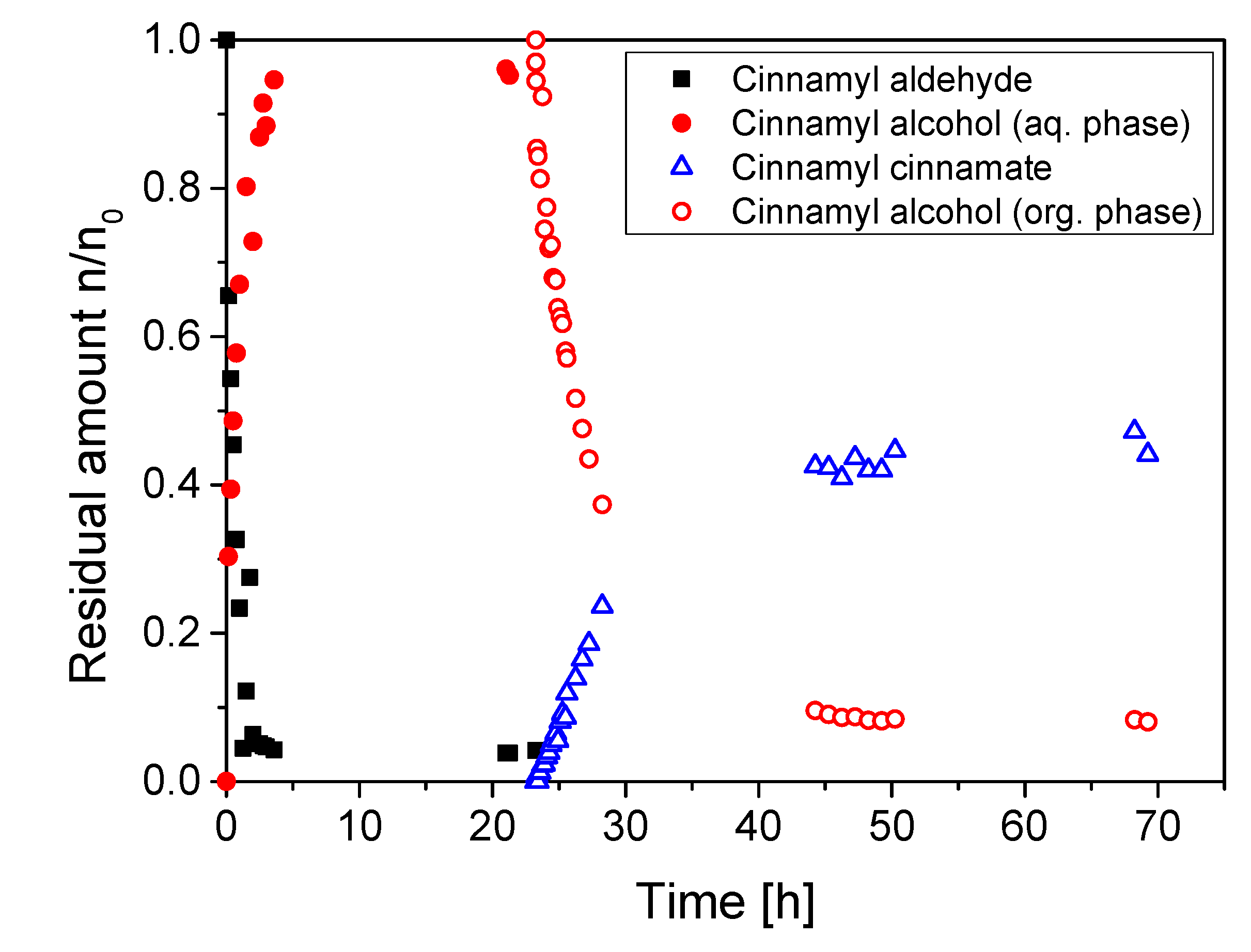
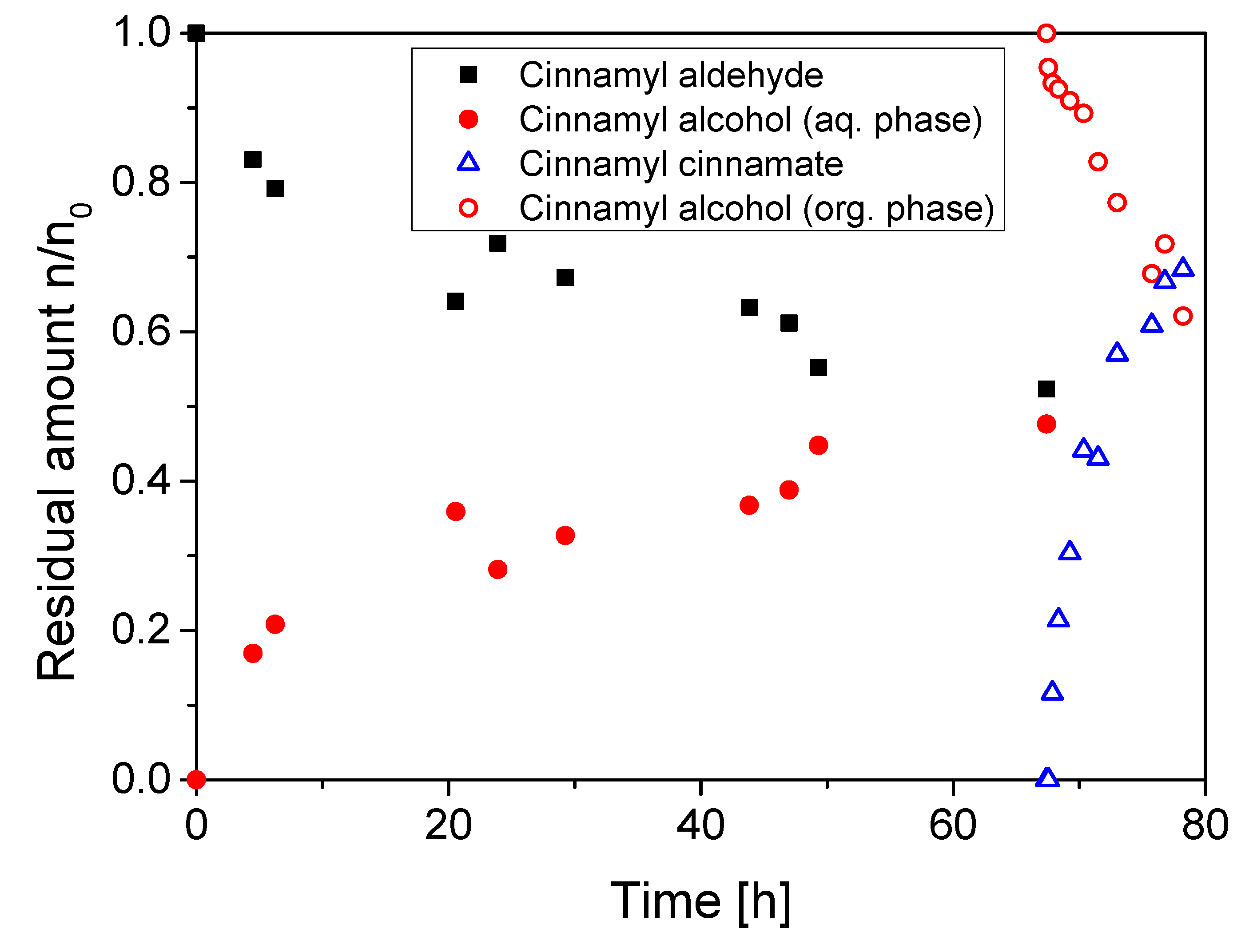
2.4. Application of the Enzyme Cascade
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Kinetics
4.3. Solvent Screening
4.4. Batch Reactions in the Aqueous Phase
4.5. Investigations via Differential Scanning Fluorimetry
4.6. Lab-Scale Cascade
4.7. Preparation of Silica Particles for Immobilization
4.8. Reaction in the Miniplant
4.9. GC Measurements
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goldberg, K.; Schroer, K.; Lütz, S.; Liese, A. Biocatalytic ketone reduction—A powerful tool for the production of chiral alcohols—Part I: Processes with isolated enzymes. Appl. Microbiol. Biotechnol. 2007, 76, 237. [Google Scholar] [CrossRef]
- Scherkus, C.; Schmidt, S.; Bornscheuer, U.T.; Gröger, H.; Kara, S.; Liese, A. A fed-batch synthetic strategy for a three-step enzymatic synthesis of poly-ε-caprolactone. ChemCatChem 2016, 8, 3446–3452. [Google Scholar] [CrossRef]
- Wu, S.; Snajdrova, R.; Moore, J.C.; Baldenius, K.; Bornscheuer, U. Biocatalysis: Enzymatic Synthesis for Industrial Applications. Angew. Chem. Int. Ed. 2020. [Google Scholar] [CrossRef] [PubMed]
- Mali, R.S.; Papalkar, A.S. Synthesis of naturally occurring cinnamyl cinnamates. J. Chem. Res. 2001, 2001, 433–435. [Google Scholar] [CrossRef]
- Demir, A.S.; Talpur, F.N.; Sopaci, S.B.; Kohring, G.-W.; Celik, A. Selective oxidation and reduction reactions with cofactor regeneration mediated by galactitol-, lactate-, and formate dehydrogenases immobilized on magnetic nanoparticles. J. Biotechnol. 2011, 152, 176–183. [Google Scholar] [CrossRef]
- Van Der Donk, W.A.; Zhao, H. Recent developments in pyridine nucleotide regeneration. Curr. Opin. Biotechnol. 2003, 14, 421–426. [Google Scholar] [CrossRef]
- Seelbach, K.; Riebel, B.; Hummel, W.; Kula, M.-R.; Tishkov, V.I.; Egorov, A.M.; Wandrey, C.; Kragl, U. A novel, efficient regenerating method of NADPH using a new formate dehydrogenase. Tetrahedron Lett. 1996, 37, 1377–1380. [Google Scholar] [CrossRef]
- Song, H.; Ma, C.; Liu, P.; You, C.; Lin, J.; Zhu, Z. A hybrid CO2 electroreduction system mediated by enzyme-cofactor conjugates coupled with Cu nanoparticle-catalyzed cofactor regeneration. J. CO2 Util. 2019, 34, 568–575. [Google Scholar] [CrossRef]
- Han, L.; Liang, B. New approaches to NAD(P)H regeneration in the biosynthesis systems. World J. Microbiol. Biotechnol. 2018, 34, 141. [Google Scholar] [CrossRef]
- Wang, X.; Saba, T.; Yiu, H.H.; Howe, R.F.; Anderson, J.A.; Shi, J. Cofactor NAD (P) H regeneration inspired by heterogeneous pathways. Chem 2017, 2, 621–654. [Google Scholar] [CrossRef] [Green Version]
- Aslan, A.S.; Valjakka, J.; Ruupunen, J.; Yildirim, D.; Turner, N.J.; Turunen, O.; Binay, B. Chaetomium thermophilum formate dehydrogenase has high activity in the reduction of hydrogen carbonate (HCO3-) to formate. Protein Eng. Des. Select. PEDS 2017, 30, 47–55. [Google Scholar]
- Pennacchio, A.; Rossi, M.; Raia, C.A. Synthesis of cinnamyl alcohol from cinnamaldehyde with Bacillus stearothermophilus alcohol dehydrogenase as the isolated enzyme and in recombinant E. coli cells. Appl. Biochem. Biotechnol. 2013, 170, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Cocchiara, J.; Letizia, C.; Lalko, J.; Lapczynski, A.; Api, A. Fragrance material review on cinnamaldehyde. Food Chem. Toxicol. 2005, 43, 867–923. [Google Scholar] [CrossRef] [PubMed]
- Vitolo, M.; Yoriyaz, E.J. Reduction of prochiralic ketones by NAD (H)-dependent alcohol dehydrogenase in membrane reactor. Athens J. Sci. 2015, 2, 161–175. [Google Scholar] [CrossRef]
- Marpani, F.; Sárossy, Z.; Pinelo, M.; Meyer, A.S. Kinetics based reaction optimization of enzyme catalyzed reduction of formaldehyde to methanol with synchronous cofactor regeneration. Biotechnol. Bioeng. 2017, 114, 2762–2770. [Google Scholar] [CrossRef]
- Mee, B.; Kelleher, D.; Frias, J.; Malone, R.; Tipton, K.F.; Henehan, G.T.; Windle, H.J. Characterization of cinnamyl alcohol dehydrogenase of Helicobacter pylori: An aldehyde dismutating enzyme. FEBS J. 2005, 272, 1255–1264. [Google Scholar] [CrossRef]
- Kroutil, W.; Mang, H.; Edegger, K.; Faber, K. Biocatalytic oxidation of primary and secondary alcohols. Adv. Synth. Catal. 2004, 346, 125–142. [Google Scholar] [CrossRef]
- Liese, A.; Seelbach, K.; Wandrey, C. Industrial Biotransformations; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Ansorge-Schumacher, M.B.; Slusarczyk, H.; Schümers, J.; Hirtz, D. Directed evolution of formate dehydrogenase from Candida boidinii for improved stability during entrapment in polyacrylamide. FEBS J. 2006, 273, 3938–3945. [Google Scholar] [CrossRef]
- Weckbecker, A.; Hummel, W. Improved synthesis of chiral alcohols with Escherichia coli cells co-expressing pyridine nucleotide transhydrogenase, NADP+-dependent alcohol dehydrogenase and NAD+-dependent formate dehydrogenase. Biotechnol. Lett. 2004, 26, 1739–1744. [Google Scholar] [CrossRef]
- Schirwitz, K.; Schmidt, A.; Lamzin, V.S. High-resolution structures of formate dehydrogenase from Candida boidinii. Protein Sci. 2007, 16, 1146–1156. [Google Scholar] [CrossRef] [Green Version]
- Garcia, T.; Coteron, A.; Martinez, M.; Aracil, J. Kinetic modelling of esterification reactions catalysed by immobilized lipases. Chem. Eng. Sci. 1996, 51, 2841–2846. [Google Scholar] [CrossRef]
- Fjerbaek, L.; Christensen, K.V.; Norddahl, B. A review of the current state of biodiesel production using enzymatic transesterification. Biotechnol. Bioeng. 2009, 102, 1298–1315. [Google Scholar] [CrossRef] [PubMed]
- Wierschem, M.; Schlimper, S.; Heils, R.; Smirnova, I.; Kiss, A.A.; Skiborowski, M.; Lutze, P. Pilot-scale validation of enzymatic reactive distillation for butyl butyrate production. Chem. Eng. J. 2017, 312, 106–117. [Google Scholar] [CrossRef] [Green Version]
- Bansode, S.R.; Hardikar, M.A.; Rathod, V.K. Evaluation of reaction parameters and kinetic modelling for Novozym 435 catalysed synthesis of isoamyl butyrate. J. Chem. Technol. Biotechnol. 2017, 92, 1306–1314. [Google Scholar] [CrossRef]
- Heinsman, N.W.; Valente, A.M.; Smienk, H.G.; van der Padt, A.; Franssen, M.C.; de Groot, A.; van ‘t Riet, K. The effect of ethanol on the kinetics of lipase-mediated enantioselective esterification of 4-methyloctanoic acid and the hydrolysis of its ethyl ester. Biotechnol. Bioeng. 2001, 76, 193–199. [Google Scholar] [CrossRef]
- Yadav, G.D.; Borkar, I.V. Synthesis of n-butyl acetamide over immobilized lipase. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2009, 84, 420–426. [Google Scholar] [CrossRef]
- Yadav, G.D.; Dhoot, S.B. Immobilized lipase-catalysed synthesis of cinnamyl laurate in non-aqueous media. J. Mol. Catal. B Enzym. 2009, 57, 34–39. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Y.; Zhou, L.; Gao, J. Enzymatic esterification of ammonium lactate with ethanol in organic solvent: Kinetic study. In Proceedings of the 2010 4th International Conference on Bioinformatics and Biomedical Engineering, Chengdu, China, 18–20 June 2010; IEEE: Piscataway, NJ, USA, 2010; pp. 1–4. [Google Scholar]
- Miguel, V.; Trubiano, G.; Perez, G.; Borio, D.; Errazu, A. Kinetic analysis of enzymatic esterification of fatty acids and ethanol. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2001; Volume 133, pp. 619–624. [Google Scholar]
- Ganapati, D.; Yadav, K.; Manjula, D. Immobilization lipase-catalysed esterfication and transesterification reactions in non-aqueous media for the synthesis of tetrahydrofurfuryl butyrate: Comparison and kinetic modeling. Chem. Eng. Sci. 2004, 59, 373–383. [Google Scholar]
- Straathof, A. Development of a computer program for analysis of enzyme kinetics by progress curve fitting. J. Mol. Catal. B Enzym. 2001, 11, 991–998. [Google Scholar] [CrossRef]
- Duggleby, R.G.; Morrison, J.F. Progress curve analysis in enzyme kinetics. Model discrimination and parameter estimation. Biochim. Biophys. Acta (BBA) Enzymol. 1978, 526, 398–409. [Google Scholar] [CrossRef]
- Shinde, S.D.; Yadav, G.D. Insight into microwave-assisted lipase catalyzed synthesis of geranyl cinnamate: Optimization and kinetic modeling. Appl. Biochem. Biotechnol. 2015, 175, 2035–2049. [Google Scholar] [CrossRef] [PubMed]
- Geng, B.; Wang, M.; Qi, W.; Su, R.; He, Z. Cinnamyl acetate synthesis by lipase-catalyzed transesterification in a solvent-free system. Biotechnol. Appl. Biochem. 2012, 59, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Alfonsi, K.; Colberg, J.; Dunn, P.J.; Fevig, T.; Jennings, S.; Johnson, T.A.; Kleine, H.P.; Knight, C.; Nagy, M.A.; Perry, D.A. Green chemistry tools to influence a medicinal chemistry and research chemistry based organisation. Green Chem. 2008, 10, 31–36. [Google Scholar] [CrossRef]
- Bes, M.T. Gomez-Moreno, C.; Guisan, J.M. Fernandez-Lafuente, R. Selective oxidation: Stabilisation by multipoint attachment of ferredoxin NADP+ reductase, an interesting cofactor recycling enzyme. J. Mol. Catal. A Chem. 1995, 98, 161–169. [Google Scholar] [CrossRef]
- Stepankova, V.; Bidmanova, S.; Koudelakova, T.; Prokop, Z.; Chaloupkova, R.; Damborsky, J. Strategies for stabilization of enzymes in organic solvents. Acs Catal. 2013, 3, 2823–2836. [Google Scholar] [CrossRef]
- Straathof, A. Auxiliary phase guidelines for microbial biotransformations of toxic substrate into toxic product. Biotechnol. Prog. 2003, 19, 755–762. [Google Scholar] [CrossRef]
- Grundtvig, I.P.R.; Heintz, S.; Krühne, U.; Gernaey, K.V.; Adlercreutz, P.; Hayler, J.D.; Wells, A.S.; Woodley, J.M. Screening of organic solvents for bioprocesses using aqueous-organic two-phase systems. Biotechnol. Adv. 2018, 36, 1801–1814. [Google Scholar] [CrossRef] [Green Version]
- Laane, C.; Boeren, S.; Vos, K.; Veeger, C. Rules for optimization of biocatalysis in organic solvents. Biotechnol. Bioeng. 1987, 30, 81–87. [Google Scholar] [CrossRef]
- Cantarella, L.; Alfani, F.; Cantarella, M. Stability and activity of immobilized hydrolytic enzymes in two-liquid-phase systems: Acid phosphatase, β-glucosidase, and β-fructofuranosidase entrapped in poly (2-hydroxyethyl methacrylate) matrices. Enzym. Microb. Technol. 1993, 15, 861–867. [Google Scholar] [CrossRef]
- Ansorge-Schumacher, M.B.; Steinsiek, S.; Eberhard, W.; Keramidas, N.; Erkens, K.; Hartmeier, W.; Büchs, J. Assaying CO2 release for determination of formate dehydrogenase activity in entrapment matrices and aqueous-organic two-phase systems. Biotechnol. Bioeng. 2006, 95, 199–203. [Google Scholar] [CrossRef]
- Duan, Z.; Sun, R. An improved model calculating CO2 solubility in pure water and aqueous NaCl solutions from 273 to 533 K and from 0 to 2000 bar. Chem. Geol. 2003, 193, 257–271. [Google Scholar] [CrossRef]
- Bommarius, A.S.; Paye, M.F. Stabilizing biocatalysts. Chem. Soc. Rev. 2013, 42, 6534–6565. [Google Scholar] [CrossRef] [PubMed]
- Bommarius, A.S.; Karau, A. Deactivation of formate dehydrogenase (FDH) in solution and at gas-liquid interfaces. Biotechnol. Prog. 2005, 21, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Hartlef, N. Experimentelle Charakterisierung und Computergestützte Modellierung Enzymatisch Katalysierter RedOx-Reaktionen; Hamburg University of Technology: Hamburg, Germany, 2019. [Google Scholar]
- Orlich, B.; Schomaecker, R. Enzymatic reduction of a less water-soluble ketone in reverse micelles with nadh regeneration. Biotechnol. Bioeng. 1999, 65, 357–362. [Google Scholar] [CrossRef]
- Gröger, H.; Hummel, W.; Rollmann, C.; Chamouleau, F.; Hüsken, H.; Werner, H.; Wunderlich, C.; Abokitse, K.; Drauz, K.; Buchholz, S. Preparative asymmetric reduction of ketones in a biphasic medium with an (S)-alcohol dehydrogenase under in situ-cofactor-recycling with a formate dehydrogenase. Tetrahedron 2004, 60, 633–640. [Google Scholar] [CrossRef]
- Johannsen, J.; Engelmann, C.; Liese, A.; Fieg, G.; Bubenheim, P.; Waluga, T. Pilot-scale Operation of a Multi-enzymatic Cascade Reaction in a Multiphase System. Chem. Eng. Trans. 2020, 79, 25–30. [Google Scholar]
- Engelmann, C.; Ekambaram, N.; Johannsen, J.; Fellechner, O.; Waluga, T.; Fieg, G.; Liese, A.; Bubenheim, P. Enzyme Immobilization on Synthesized Nanoporous Silica Particles and their Application in a Bi-enzymatic Reaction. ChemCatChem 2020, 12, 2245–2252. [Google Scholar] [CrossRef]

| Enzyme | vmax,f | vmax,b | KM,S1[a] | KM,S2[a] | KM,P1[b] | KM,P2[b] | KI[c] | kcat[d] |
|---|---|---|---|---|---|---|---|---|
| ADH [e] | 3.49 U/mg ± 1.53 | n.e.[g] | CA 0.39 mM ± 0.20 | NADH 0.08 mM ± 0.07 | n.e. [g] | n.e. [g] | CAl 0.04 mM ± 0.3 | 1.6 s−1 |
| FDH [e] | 0.46 U/mg ± 0.07 | n.e.[g] | NF 10.14 mM ± 6.67 | NAD+ 0.04 mM ± 0.02 | n.e. [g] | n.e. [g] | NADH 0.27 mM ± 0.42 | 8.1 s−1 |
| Lipase [f] | 6.31 U/g ± 1.19 | 14.75 U/g ± 2.79 | CAl 2.77 mM ± 0.52 | CAc 1.40 mM ± 0.27 | CC 0.78 mM ± 0.15 | H2O 252.89 mM ± 47.86 | CAc 13.72 mM ± 2.60 | 3500 s−1 |
| Solvent | logP 1 | Boiling Point 2 | Hazardous Nature (Pfizer) 3 | Miniplant Suitability 4 |
|---|---|---|---|---|
| Heptane | 3.58 | 98 °C | 2 | c |
| Isooctane | 3.56 | 99 °C | 2 | c |
| p-Xylene | 3.00 | 138 °C | 2 | a |
| Cyclohexane | 2.67 | 84 °C | 2 | c |
| Toluene | 2.49 | 111 °C | 2 | d |
| Cyclopentylmethyl ether | 1.48 | 106 °C | - 5 | a |
| Methyl-tert-butyl ether | 1.18 | 55 °C | 2 | a |
| 2-Methyltetrahydrofurane | 0.95 | 80 °C | 2 | b |
| Diethyl ether | 0.84 | 35 °C | 3 | d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engelmann, C.; Johannsen, J.; Waluga, T.; Fieg, G.; Liese, A.; Bubenheim, P. A Multi-Enzyme Cascade for the Production of High-Value Aromatic Compounds. Catalysts 2020, 10, 1216. https://doi.org/10.3390/catal10101216
Engelmann C, Johannsen J, Waluga T, Fieg G, Liese A, Bubenheim P. A Multi-Enzyme Cascade for the Production of High-Value Aromatic Compounds. Catalysts. 2020; 10(10):1216. https://doi.org/10.3390/catal10101216
Chicago/Turabian StyleEngelmann, Claudia, Jens Johannsen, Thomas Waluga, Georg Fieg, Andreas Liese, and Paul Bubenheim. 2020. "A Multi-Enzyme Cascade for the Production of High-Value Aromatic Compounds" Catalysts 10, no. 10: 1216. https://doi.org/10.3390/catal10101216
APA StyleEngelmann, C., Johannsen, J., Waluga, T., Fieg, G., Liese, A., & Bubenheim, P. (2020). A Multi-Enzyme Cascade for the Production of High-Value Aromatic Compounds. Catalysts, 10(10), 1216. https://doi.org/10.3390/catal10101216






