Facile Synthesis of N-Doped WS2 Nanosheets as an Efficient and Stable Electrocatalyst for Hydrogen Evolution Reaction in Acidic Media
Abstract
:1. Introduction
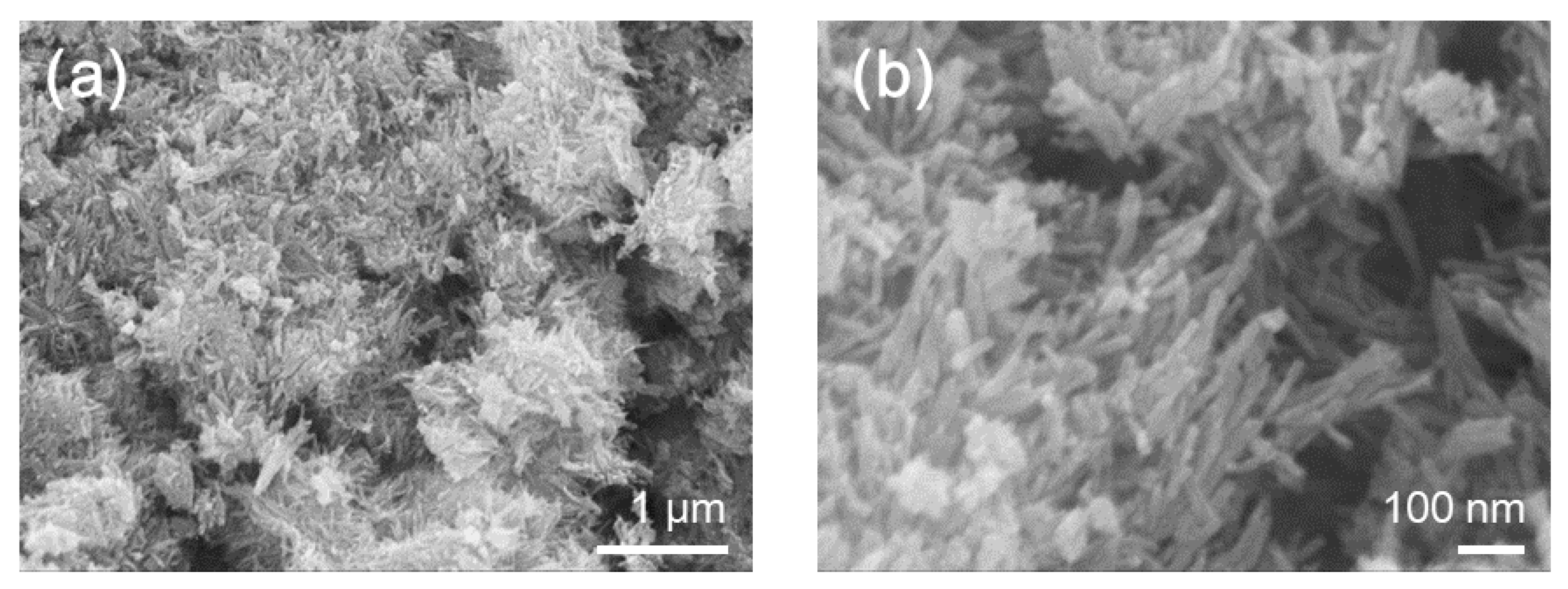
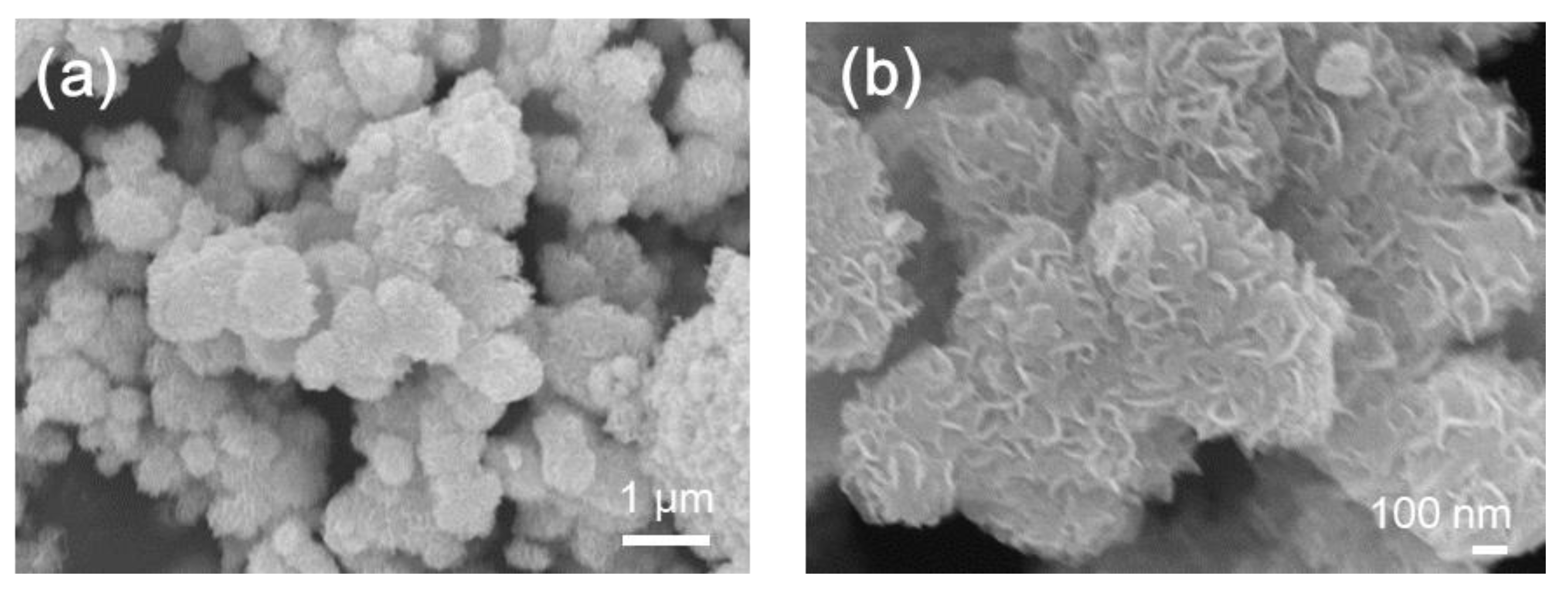
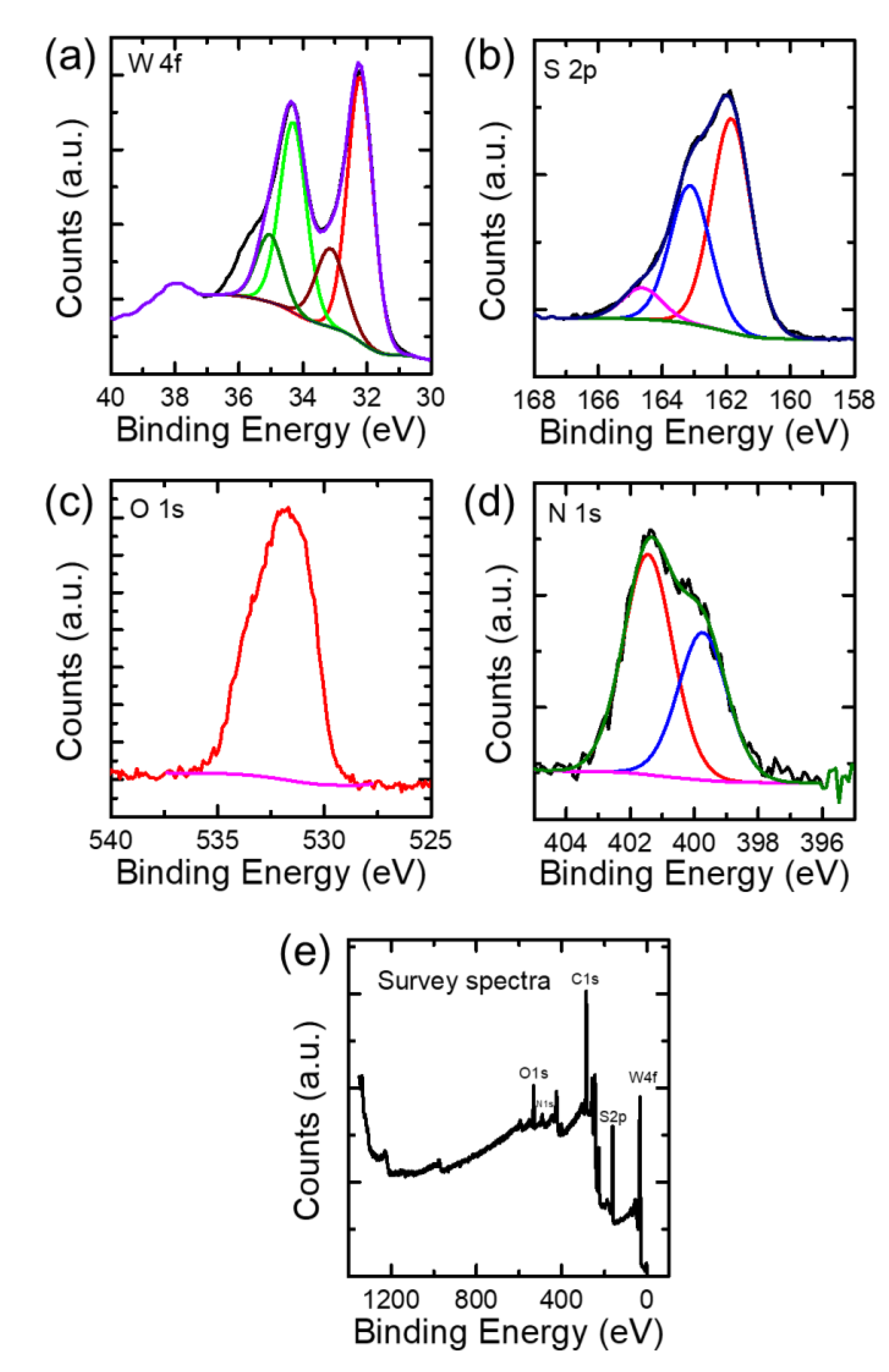
2. Results and Discussion
3. Experimental Procedure
3.1. Synthesis of W18O49 Nanowires
3.2. Synthesis of Hex-WO3 Nanowires
3.3. Synthesis of Hex-WS2 Nanosheets
3.4. Structural and Chemical Characterizations
3.5. Electrochemical Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Faisal, F.; Stumm, C.; Bertram, M.; Waidhas, F.; Lykhach, Y.; Cherevko, S.; Xiang, F.; Ammon, M.; Vorokhta, M.; Šmíd, B.; et al. Electrifying model catalysts for understanding electrocatalytic reactions in liquid electrolytes. Nat. Mater. 2018, 17, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, V.R.; Strmcnik, D.; Lopes, P.P.; Markovic, N.M. Energy and fuels from electrochemical interfaces. Nat. Mater. 2016, 16, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorso, F.; Colombo, L.; Yu, G.; Stoller, M.; Tozzini, V.; Ferrari, A.C.; Ruoff, R.S.; Pellegrini, V. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 2015, 347, 1246501. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Cui, Y.; Liu, N. The path towards sustainable energy. Nat. Mater. 2016, 16, 16–22. [Google Scholar] [CrossRef]
- Han, H.; Choi, H.; Mhin, S.; Hong, Y.-R.; Kim, K.M.; Kwon, J.; Ali, G.; Chung, K.Y.; Je, M.; Umh, H.N.; et al. Advantageous crystalline–amorphous phase boundary for enhanced electrochemical water oxidation. Energy Environ. Sci. 2019, 12, 2443–2454. [Google Scholar] [CrossRef]
- Han, H.; Nayak, A.K.; Choi, H.; Ali, G.; Kwon, J.; Choi, S.G.; Paik, U.; Song, T. Partial Dehydration in Hydrated Tungsten Oxide Nanoplates Leads to Excellent and Robust Bifunctional Oxygen Reduction and Hydrogen Evolution Reactions in Acidic Media. ACS Sustain. Chem. Eng. 2020, 8, 9507–9518. [Google Scholar] [CrossRef]
- Cheng, N.; Stambula, S.; Wang, D.; Banis, M.N.; Liu, J.; Riese, A.; Xiao, B.; Li, R.; Sham, T.-K.; Liu, L.-M.; et al. Platinum single-atom and cluster catalysis of the hydrogen evolution reaction. Nat. Commun. 2016, 7, 13638. [Google Scholar] [CrossRef]
- Song, S.; Guo, M.; Zhang, S.; Zhan, K.; Yan, Y.; Yang, J.; Zhao, B.; Xu, M. Plasma-assisted synthesis of hierarchical NiCoxPy nanosheets as robust and stable electrocatalyst for hydrogen evolution reaction in both acidic and alkaline media. Electrochim. Acta 2020, 331, 135431. [Google Scholar] [CrossRef]
- Ma, B.; Yang, Z.; Chen, Y.; Yuan, Z.-H. Nickel cobalt phosphide with three-dimensional nanostructure as a highly efficient electrocatalyst for hydrogen evolution reaction in both acidic and alkaline electrolytes. Nano Res. 2018, 12, 375–380. [Google Scholar] [CrossRef]
- Shan, S.J.; Ling, T.; Davey, K.; Zheng, Y.; Qiao, S. Transition-Metal-Doped RuIr Bifunctional Nanocrystals for Overall Water Splitting in Acidic Environments. Adv. Mater. 2019, 31, e1900510. [Google Scholar] [CrossRef]
- Xu, K.; Wang, F.; Wang, Z.; Zhan, X.; Wang, Q.; Cheng, Z.; Safdar, M.; He, J. Component-Controllable WS2(1–x)Se2x Nanotubes for Efficient Hydrogen Evolution Reaction. Acs Nano 2014, 8, 8468–8476. [Google Scholar] [CrossRef]
- Lukowski, M.A.; Daniel, A.S.; English, C.R.; Meng, F.; Forticaux, A.; Hamers, R.J.; Jin, S. Highly active hydrogen evolution catalysis from metallic WS2 nanosheets. Energy Environ. Sci. 2014, 7, 2608–2613. [Google Scholar] [CrossRef]
- Tsai, C.; Chan, K.; Abild-Pedersen, F.; Nørskov, J.K. Active edge sites in MoSe2 and WSe2 catalysts for the hydrogen evolution reaction: A density functional study. Phys. Chem. Chem. Phys. 2014, 16, 13156–13164. [Google Scholar] [CrossRef]
- Tan, S.M.; Pumera, M. Bottom-up Electrosynthesis of Highly Active Tungsten Sulfide (WS3–x) Films for Hydrogen Evolution. Acs Appl. Mater. Interfaces 2016, 8, 3948–3957. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, J.; Ma, J.; Liu, P.; Gao, D.; Tao, K.; Xue, D. N-doped WS2 nanosheets: A high-performance electrocatalyst for the hydrogen evolution reaction. J. Mater. Chem. A 2016, 4, 11234–11238. [Google Scholar] [CrossRef]
- Anurupa, M.; Suneel, K.S. Sulphur edge and vacancy assisted nitrogen–phosphorus co-doped exfoliated tungsten disulfide: A superior electrocatalyst for hydrogen evolution reaction. J. Mater. Chem A 2018, 6, 19712–19726. [Google Scholar]
- Bai, S.; Zhang, K.; Wang, L.; Sun, J.; Luo, R.; Li, D.; Chen, A. Synthesis mechanism and gas-sensing application of nanosheet-assembled tungsten oxide microspheres. J. Mater. Chem. A 2014, 2, 7927–7934. [Google Scholar] [CrossRef]
- Bin Yang, H.; Miao, J.; Hung, S.-F.; Chen, J.; Tao, H.B.; Wang, X.; Zhang, L.; Chen, R.; Gao, J.; Chen, H.M.; et al. Identification of catalytic sites for oxygen reduction and oxygen evolution in N-doped graphene materials: Development of highly efficient metal-free bifunctional electrocatalyst. Sci. Adv. 2016, 2, e1501122. [Google Scholar] [CrossRef] [Green Version]
- Van-Truong, N.; Yang, T.-Y.; Le, P.A.; Yen, P.-J.; Chueh, Y.-L.; Wei, K.-H. New Simultaneous Exfoliation and Doping Process for Generating MX2 Nanosheets for Electrocatalytic Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces. 2019, 11, 14786–14795. [Google Scholar]
- Amirohossein, H.; Nguyen, T.P.; Tekalgne, M.; Le, Q.V.; Choi, K.S.; Lee, T.H.; Park, T.J.; Kim, S.Y.; Jang, H.W. The role of metal dopants in WS2 nanoflowers in enhancing the hydrogen evolution reaction. Appl. Catal. A Gen. 2018, 567, 73–79. [Google Scholar]
- Anning, J.; Zhang, B.; Li, Z.; Jin, G.; Hao, J. Vanadium-Doped WS2 Nanosheets Grown on Carbon Cloth as a Highly Efficient Electrocatalyst for the Hydrogen Evolution Reaction. Chem. Asian J. 2018, 13, 1438–1446. [Google Scholar]
- Pan, Y.; Zheng, F.; Wang, X.; Qin, H.; Liu, E.; Sha, J.; Zhao, N.; Zhang, P.; Ma, L. Enhanced electrochemical hydrogen evolution performance of WS2 nanosheets by Te doping. J. Catal. 2020, 382, 204–211. [Google Scholar] [CrossRef]




| Materials | Overpotential (mV) | Tafel Slope (mV dec−1) | Reference |
|---|---|---|---|
| WS2 (cysteine 1:2) | 130 @ 10 mA cm−2 | 45 | This work |
| N-WS2-H2 | 197 @ 100 mA cm−2 | 69 | 15 |
| N-doped MoS2 | 164 @ 10 mA cm−2 | 71 | 19 |
| Pd-WS2 NF | 175 @ 10 mA cm−2 | 54 | 20 |
| V0.0065-WS2/CC | 148 @ 10 mA cm−2 | 72 | 21 |
| Te-doped WS2 | 213 @ 10 mA cm−2 | 94 | 22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nayak, A.K.; Enhtuwshin, E.; Kim, S.J.; Han, H. Facile Synthesis of N-Doped WS2 Nanosheets as an Efficient and Stable Electrocatalyst for Hydrogen Evolution Reaction in Acidic Media. Catalysts 2020, 10, 1238. https://doi.org/10.3390/catal10111238
Nayak AK, Enhtuwshin E, Kim SJ, Han H. Facile Synthesis of N-Doped WS2 Nanosheets as an Efficient and Stable Electrocatalyst for Hydrogen Evolution Reaction in Acidic Media. Catalysts. 2020; 10(11):1238. https://doi.org/10.3390/catal10111238
Chicago/Turabian StyleNayak, Arpan Kumar, Enhbayar Enhtuwshin, So Jung Kim, and HyukSu Han. 2020. "Facile Synthesis of N-Doped WS2 Nanosheets as an Efficient and Stable Electrocatalyst for Hydrogen Evolution Reaction in Acidic Media" Catalysts 10, no. 11: 1238. https://doi.org/10.3390/catal10111238






