In Situ Thermal-Stage Fitted-STEM Characterization of Spherical-Shaped Co/MoS2 Nanoparticles for Conversion of Heavy Crude Oils
Abstract
:1. Introduction
2. Results and Discussion
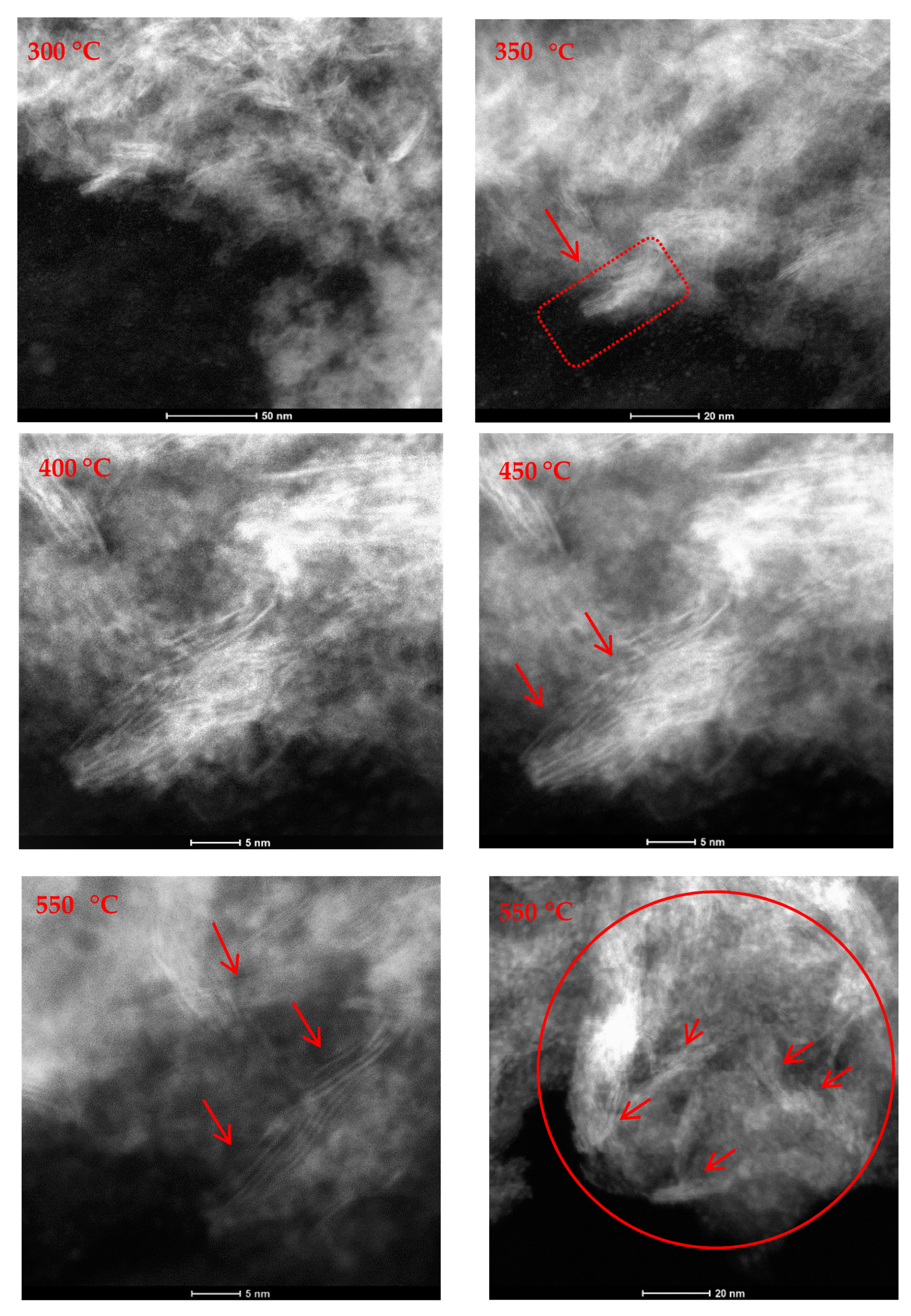
2.1. In-Situ Scanning Transmission Electron Microscopy (STEM)
2.2. In-Situ Hydrodesulfurization Activity
Saturates, Asphaltenes, Resins and Aromatics (SARA) Analysis
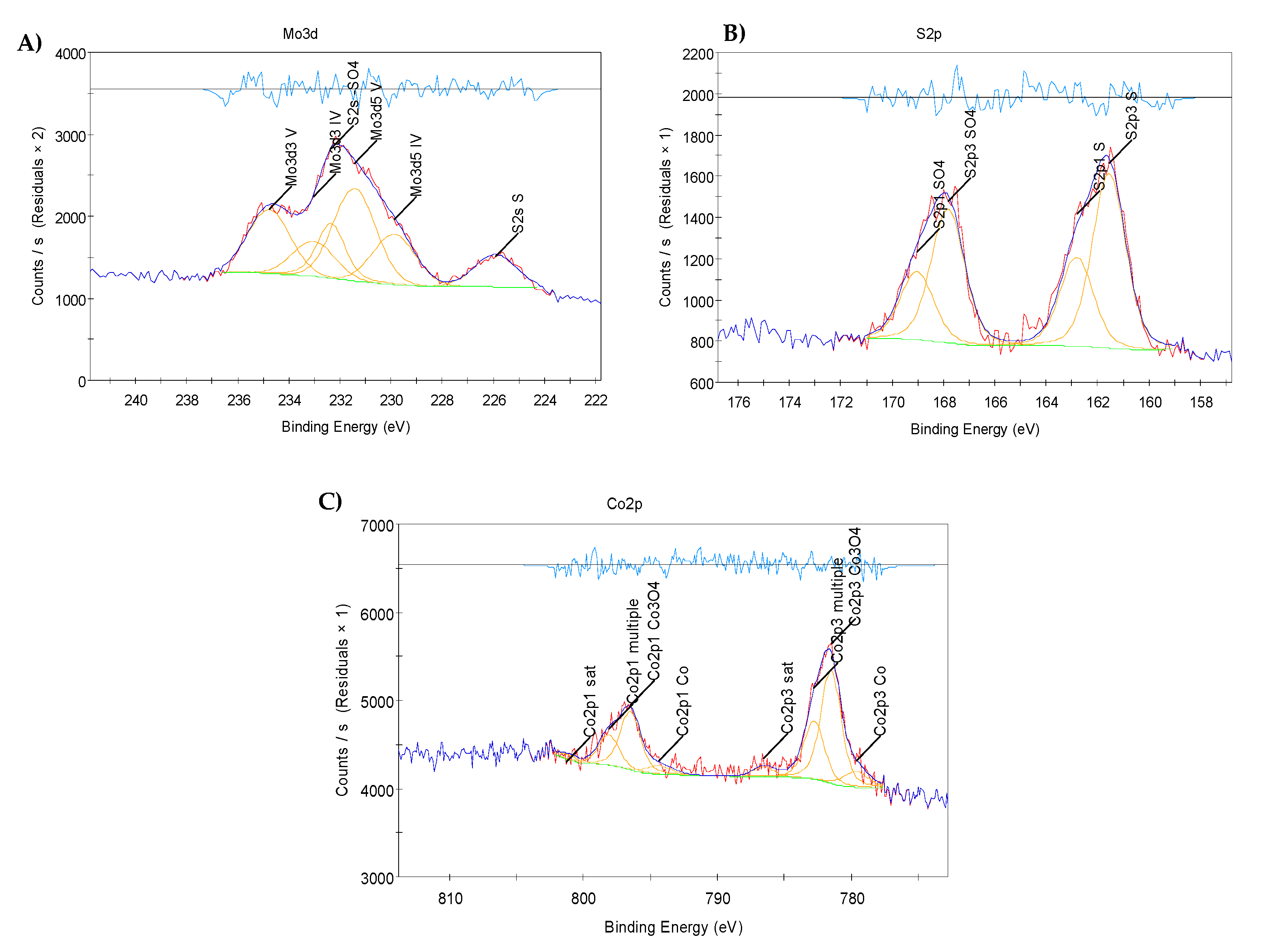
2.3. X-Ray Photoelectron Spectroscopy (XPS) Results
3. Materials and Methods
3.1. Co/MoS2 Catalyst Preparation
3.2. Catalytic Activity in the Conversion of Golden Lane Crude Oil
3.3. SARA Analysis
3.4. In-Situ STEM
3.5. X-Ray Photoelectron Spectroscopy (XPS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chianelli, R.R.; Berhault, G.; Torres, B. Unsupported transition metal sulfide catalysts: 100 years of science and application. Catal. Today 2009, 147, 275–286. [Google Scholar] [CrossRef]
- Chianelli, R.R.; Berhault, G.; Raybaud, P.; Kasztelan, S.; Hafner, J.; Toulhoat, H. Periodic trends in hydrodesulfurization: In support of the Sabatier principle. Appl. Catal. A Gen. 2002, 227, 83–96. [Google Scholar] [CrossRef]
- Topsøe, H.; Candia, R.; Topsøe, N.Y.; Clausen, B.S. On the state of the Co–Mo–S Model. Bull. Sociétés Chim. Belg. 1984, 93, 783–806. [Google Scholar] [CrossRef]
- MCharoo, S.; Wani, M.F.; Hanief, M.; Rather, M.A. Tribological Properties of MoS2 Particles as Lubricant Additive on EN31 Alloy Steel and AISI 52100 Steel Ball. Mater. Today Proc. 2017, 4, 9967–9971. [Google Scholar] [CrossRef]
- Ramos, M.; Nogan, J.; Ortiz-Díaz, M.; Enríquez-Carrejo, J.L.; Rodríguez-González, C.A.; Mireles-Jr-García, J.; Ornelas, C.; Hurtado-Macias, A. Mechanical properties of RF-sputtering MoS2 thin films. IOP Surf. Topogr. Metrol. Prop. 2017, 5, 025003. [Google Scholar] [CrossRef]
- Daage, M.; Chianelli, R.R. Structure-Function Relations in Molybdenum Sulfide Catalysts: The Rim-Edge Model. J. Catal. 1994, 149, 414–427. [Google Scholar] [CrossRef]
- Zhu, Y.; Ramasse, Q.M.; Brorson, M.; Moses, P.G.; Hansen, L.P.; Topsøe, H.; Kisielowski, C.F.; Helveg, S. Location of Co and Ni promoter atoms in multi-layer MoS2 nanocrystals for hydrotreating catalysis. Catal. Today 2016, 261, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Lin, H.; Zheng, Y. Application of Uniform Design Method in the Optimization of Hydrothermal Synthesis for Nano MoS2 Catalyst with High HDS Activity. Catalysts 2018, 8, 654. [Google Scholar] [CrossRef] [Green Version]
- Lauritsen, J.V.; Nyberg, M.; Vang, R.T.; Bollinger, M.V.; Clausen, B.S.; Topsøe, H.; Jacobsen, K.W.; Lægsgaard, E.; Nørskov, J.K.; Besenbacher, F. Chemistry of one-dimensional metallic edge states in MoS2 nanoclusters. IOP Nanotechnol. 2003, 14, 385–389. [Google Scholar] [CrossRef]
- José-Yacamán, M.; Díaz, G.; Gómez, A. Electron microscopy of catalysts; the present, the future and the hopes. Catal. Today 1995, 23, 161–199. [Google Scholar] [CrossRef]
- Deepak, F.L.; Esparza, R.; Borges, B.; Lopez-Lozano, X.; Jose-Yacaman, M. Direct Imaging and Identification of Individual Dopant Atoms in MoS2 and WS2 Catalysts by Aberration Corrected Scanning Transmission Electron Microscopy. ACS Catal. 2011, 1, 537–543. [Google Scholar] [CrossRef]
- Hansen, L.P.; Johnson, E.; Brorson, M.; Helveg, S. Growth Mechanism for Single- and Multi-Layer MoS2 Nanocrystals. J. Phys. Chem. C 2014, 118, 22768–22773. [Google Scholar] [CrossRef]
- Ramos, M.; Ferrer, D.; Martinez-Soto, E.; Lopez-Lippmann, H.; Torres, B.; Berhault, G.; Chianelli, R.R. In-situ HRTEM study of the reactive carbide phase of Co/MoS2 catalyst. Ultramicroscopy 2013, 127, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Berhault, G.; Mehta, A.; Pavel, A.C.; Yang, J.; Rendon, L.; Yácaman, M.J.; Araiza, L.C.; Moller, A.D.; Chianelli, R.R. The Role of Structural Carbon in Transition Metal Sulfides Hydrotreating Catalysts. J. Catal. 2001, 198, 9–19. [Google Scholar] [CrossRef]
- Albiter, M.A.; Huirache-Acuña, R.; Paraguay-Delgado, F.; Rico, J.L.; Alonso-Nuñez, G. Synthesis of MoS2 nanorods and their catalytic test in the HDS of dibenzothiophene. Nanotechnology 2006, 17, 3473–3481. [Google Scholar] [CrossRef]
- Ramos, M.; Galindo-Hernández, F.; Arslan, I.; Sanders, T.; Domínguez, J.M. Electron tomography and fractal aspects of MoS2 and MoS2/Co spheres. Sci. Rep. 2017, 7, 12322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Y.; Han, J.; Zhang, Y.; Zhang, X.; Xu, P.; Yuan, Q.; Samad, L.; Wang, X.; Wang, Y.; Zhang, Z.; et al. Contributions of Phase, Sulfur Vacancies, and Edges to the Hydrogen Evolution Reaction Catalytic Activity of Porous Molybdenum Disulfide Nanosheets. J. Am. Chem. Soc. 2015, 138, 7965–7972. [Google Scholar] [CrossRef]
- Ruiz-Morales, Y.; Miranda-Olvera, A.D.; Portales-Martínez, B.; Domínguez, J.M. Experimental and Theoretical Approach To Determine the Average Asphaltene Structure of a Crude Oil from the Golden Lane (Faja de Oro) of Mexico. Energy Fuels 2020, 34, 7985–8006. [Google Scholar] [CrossRef]
- Elahi, S.M.; Scott, C.E.; Chen, Z.; Pereira-Almao, P. In-situ upgrading and enhanced recovery of heavy oil from carbonate reservoirs using nano-catalysts: Upgrading reactions analysis. Fuel 2019, 252, 262–271. [Google Scholar] [CrossRef]
- Pereira-Almao, P. In situ upgrading of bitumen and heavy oils via nanocatalysis. Can. J. Chem. Eng. 2012, 90, 320–329. [Google Scholar] [CrossRef]
- Chianelli, R.R.; Ruppert, A.F.; José-Yacamán, M.; Váquez-Zavala, A. HREM studies of layered transition metal sulfide catalytic materials. Catal. Today 1995, 23, 269–2581. [Google Scholar] [CrossRef]
- Joe, J.; Bae, C.; Kim, E.; Ho, T.A.; Yang, H.; Park, J.H.; Shin, H. Mixed-Phase (2H and 1T) MoS2 Catalyst for a Highly Efficient and Stable Si Photocathode. Catalysts 2018, 8, 580. [Google Scholar] [CrossRef] [Green Version]
- Scott, C.E.; Carbognani-Ortega, L.; Pereira-Almao, P. Situ Upgrading via Hot Fluid and Nanocatalyst Injection. In Advanced Catalytic Materials: Current Status and Future Progress; Domínguez-Esquivel, J., Ramos, M., Eds.; Springer: Cham, Switzerland, 2019; pp. 129–149. [Google Scholar] [CrossRef]
- Afanasiev, P. The influence of reducing and sulfiding conditions on the properties of unsupported MoS2-based catalysts. J. Catal. 2010, 269, 269–280. [Google Scholar] [CrossRef]
- Fan, T.; Wang, J.; Buckley, J.S. SPE 75228. In SPE/DOE Improved Oil Recovery Symposium; Society of Petroleum Engineers: Tulsa, OK, USA, 2002. [Google Scholar]
- Andersen, S.I.; Speight, J.G. Petroleum resins: Separation, character, and role in petroleum. Pet. Sci. Technol. 2001, 19, 1–34. [Google Scholar] [CrossRef]
- Yen, A.; Yin, Y.R.; Asomaning, S. Evaluating asphaltene inhibitors: Laboratory tests and field studies. In SPE International Symposium on Oilfield Chemistry; SPE 65376; Society of Petroleum Engineers: Houston, TX, USA, 2001; pp. 613–619. [Google Scholar]
- Stauffer, E.; Dolan, J.A.; Newman, R. Chapter 7—Flammable and Combustible Liquids. In Fire Debris Anaysis; Elsevier: Amsterdam, The Netherlands, 2008; pp. 199–233. [Google Scholar]

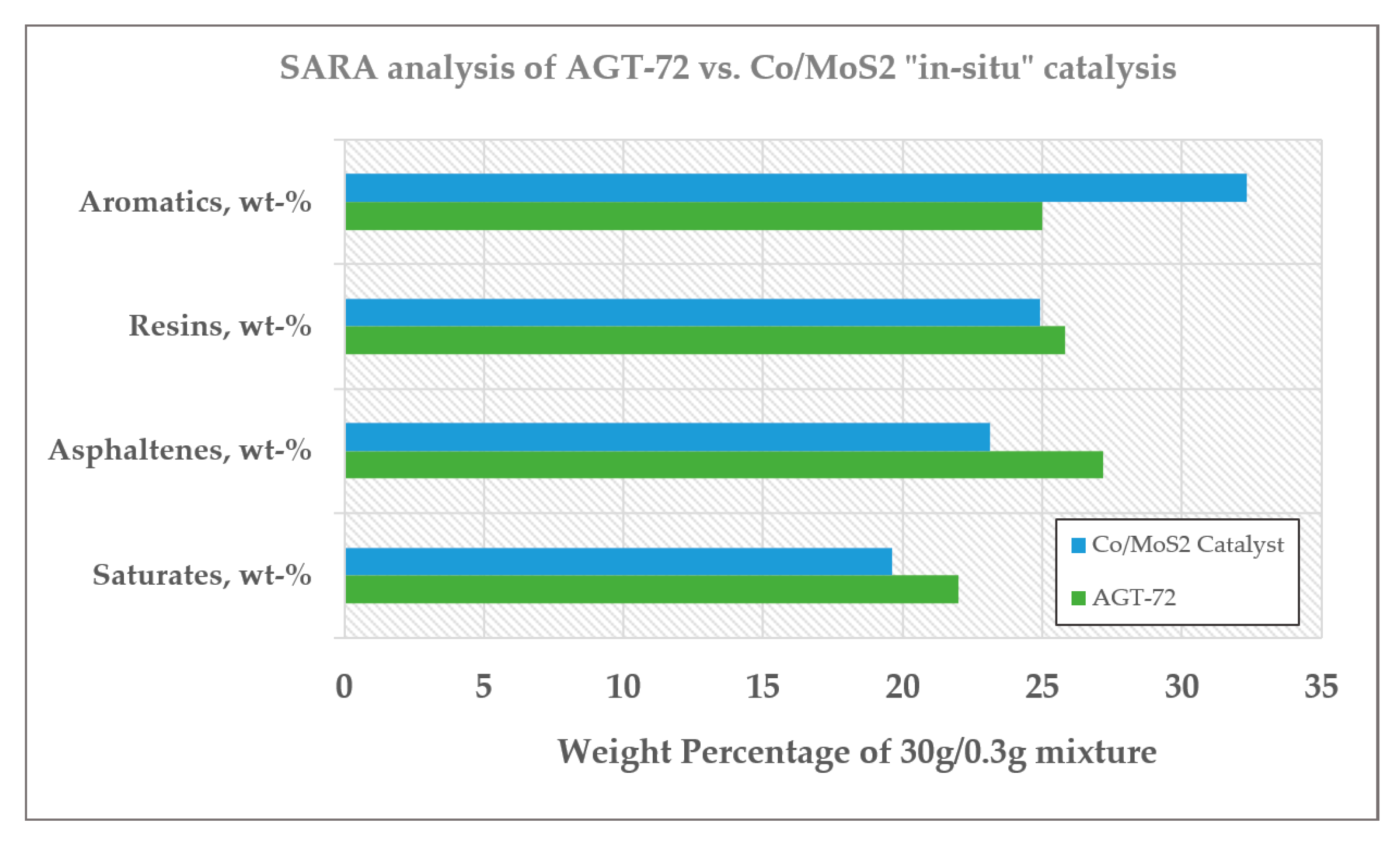
| Properties. | AGT-72 | “in-situ” Catalysis with Co/MoS2 | % Difference |
|---|---|---|---|
| Sulfur content, wt-% | 5.199 | 4.813 | −7.43 |
| Saturates, wt-% | 22.000 | 19.63 | −10.88 |
| Asphaltenes, wt-% | 27.180 | 23.14 | −15.87 |
| Resins, wt-% | 25.810 | 24.90 | −3.53 |
| Aromatics, wt-% | 25.010 | 32.33 | +22.65 |
| CII Index | 0.967729 | 0.747335314 | −0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, M.; Galindo-Hernández, F.; Torres, B.; Domínguez-Esquivel, J.M.; Heilmaier, M. In Situ Thermal-Stage Fitted-STEM Characterization of Spherical-Shaped Co/MoS2 Nanoparticles for Conversion of Heavy Crude Oils. Catalysts 2020, 10, 1239. https://doi.org/10.3390/catal10111239
Ramos M, Galindo-Hernández F, Torres B, Domínguez-Esquivel JM, Heilmaier M. In Situ Thermal-Stage Fitted-STEM Characterization of Spherical-Shaped Co/MoS2 Nanoparticles for Conversion of Heavy Crude Oils. Catalysts. 2020; 10(11):1239. https://doi.org/10.3390/catal10111239
Chicago/Turabian StyleRamos, Manuel, Félix Galindo-Hernández, Brenda Torres, José Manuel Domínguez-Esquivel, and Martin Heilmaier. 2020. "In Situ Thermal-Stage Fitted-STEM Characterization of Spherical-Shaped Co/MoS2 Nanoparticles for Conversion of Heavy Crude Oils" Catalysts 10, no. 11: 1239. https://doi.org/10.3390/catal10111239







