Preparation of Immobilized Lipase on Silica Clay as a Potential Biocatalyst on Synthesis of Biodiesel
Abstract
:1. Introduction
2. Results and Discussion
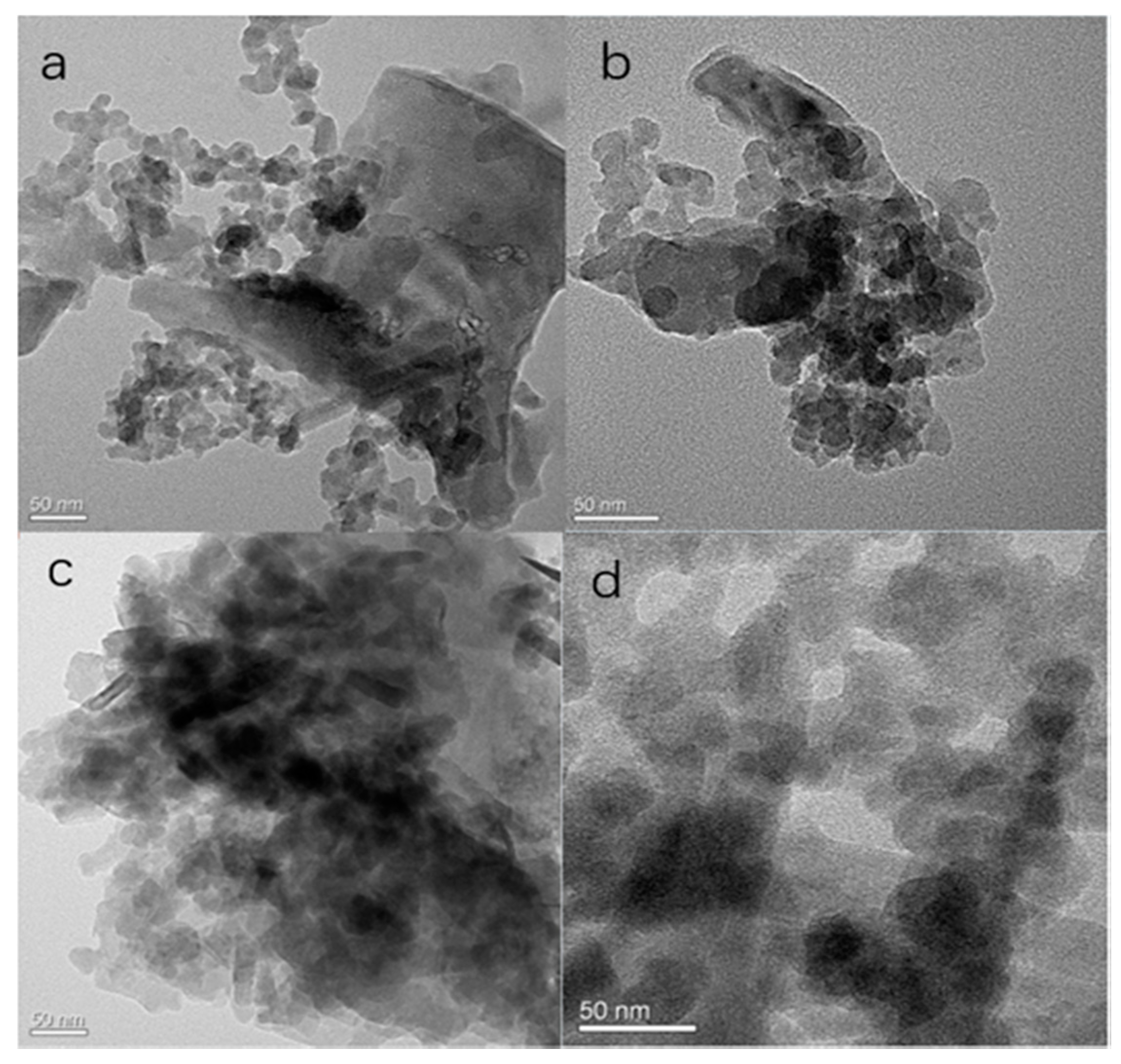
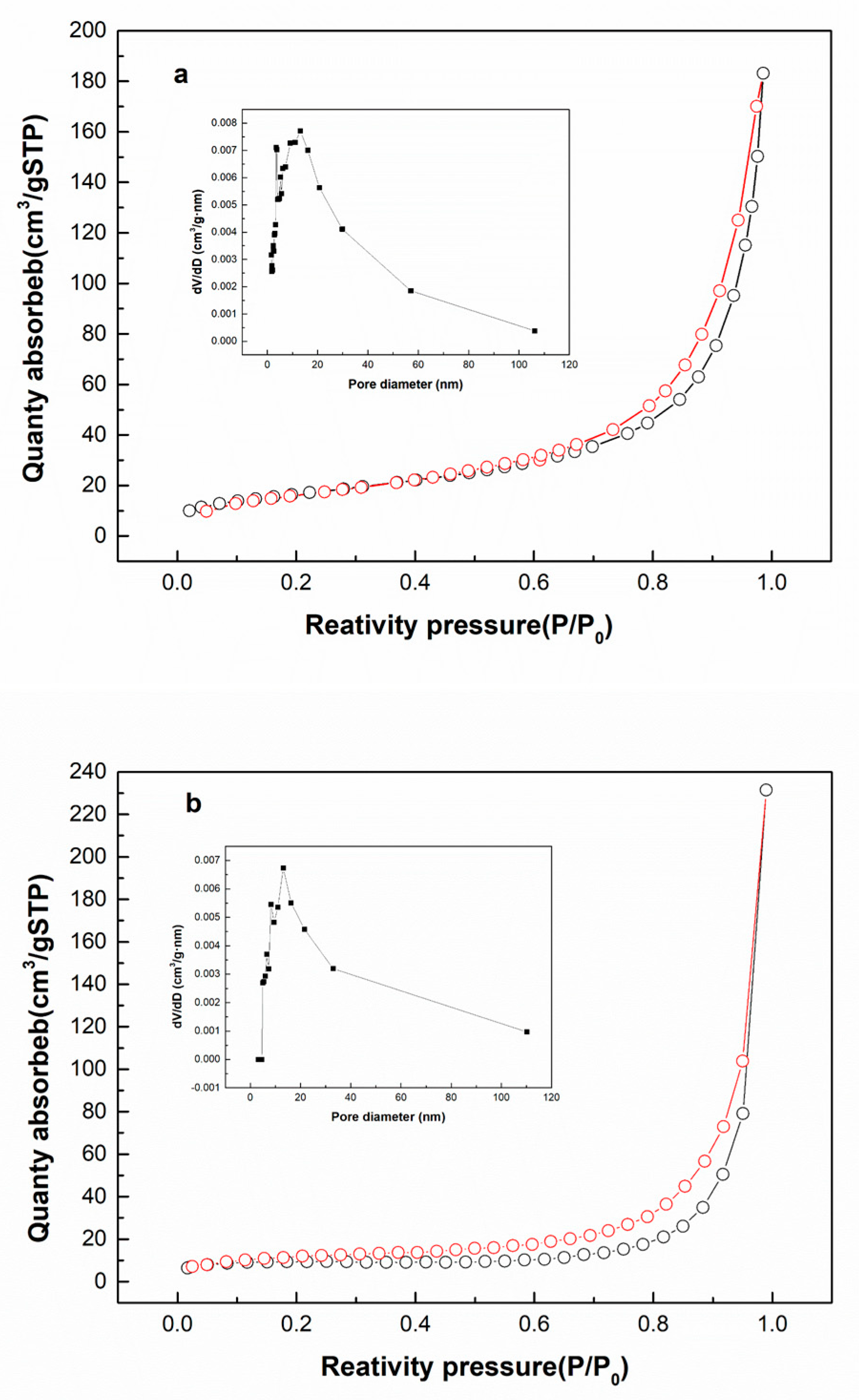
2.1. Characterization of the Modification and Immobilization
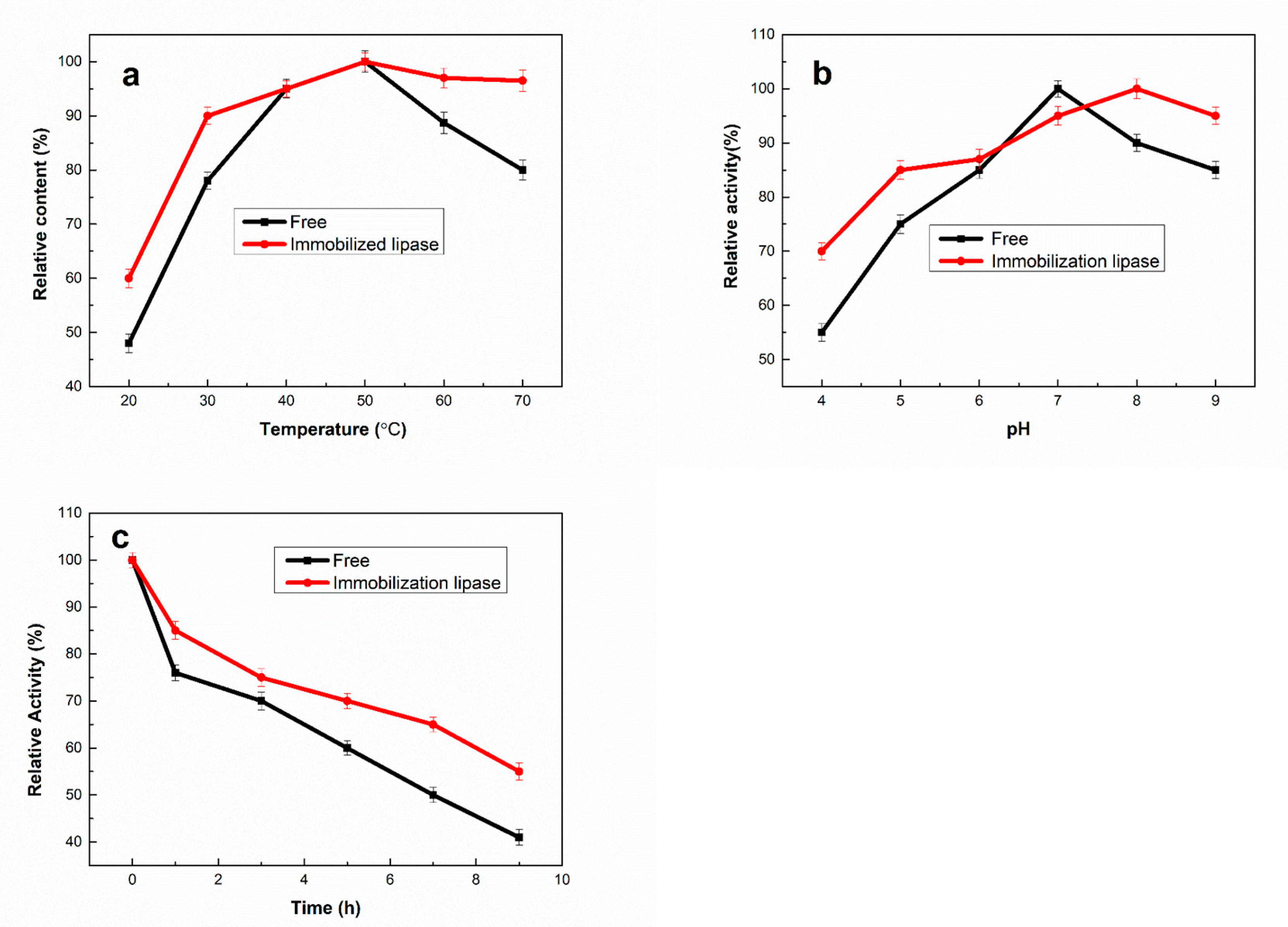
2.2. Optimization of the Immobilization Process
2.3. Immolization of Lipase on Modified Silica Clay
2.4. Application of Lip–Glu–A-SC for Synthesis of Biodiesel
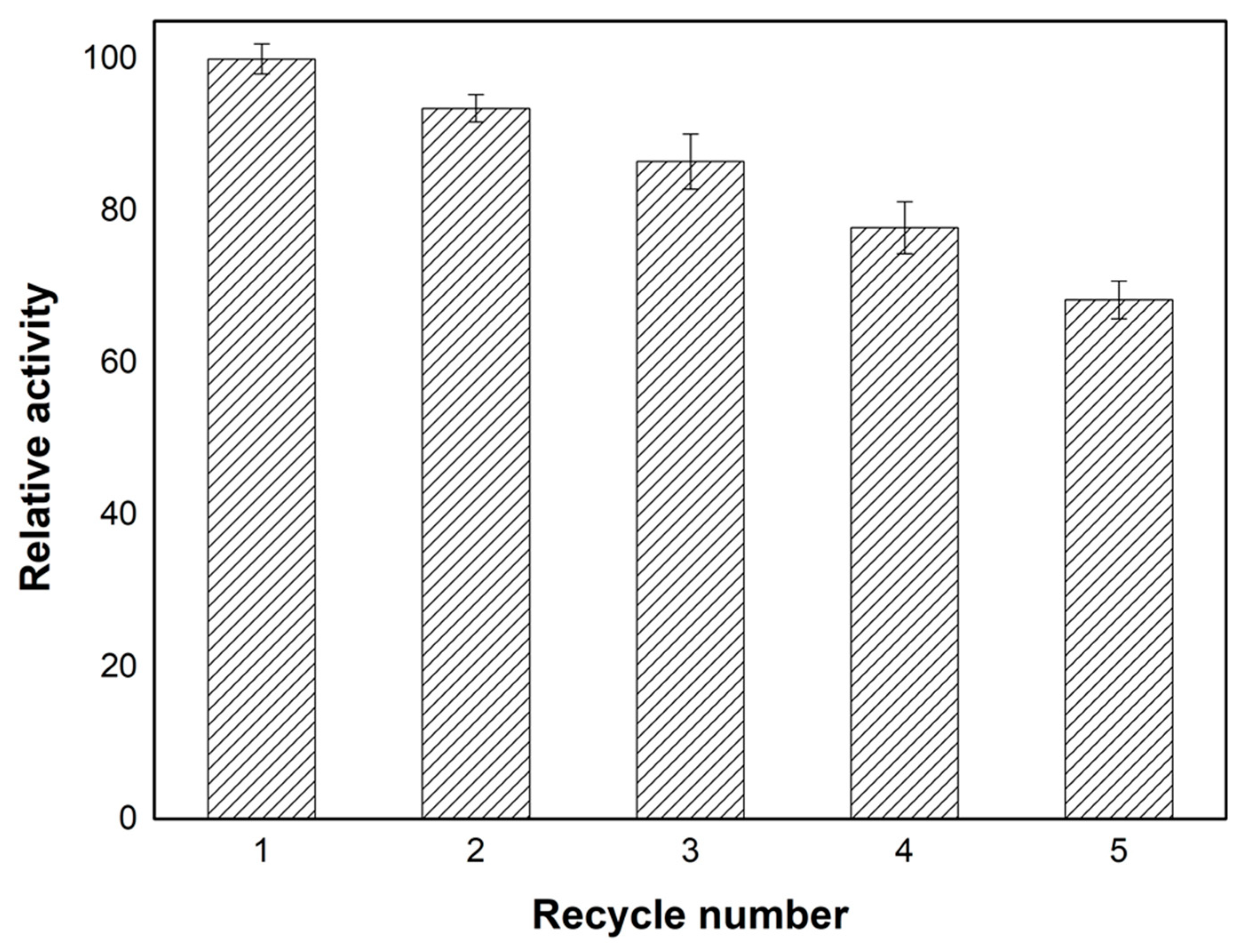
2.5. Reusability of Lip–Glu–A-SC
3. Materials and Methods
3.1. Materials and Reagents
3.2. Modification of Silica Clay and Immobilization of Lipase
3.2.1. Modification of Silica Clay
3.2.2. Immobilization Process
3.3. Application of Lip–Glu–A-SC for Biodiesel Preparation
3.4. Hydrolytic Activity Assay
3.5. Determination of Immobilization Efficiency
3.6. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Dubé, M.; McLean, D.; Kates, M. Biodiesel production from waste cooking oil: 1. Process design and technological assessment. Bioresour. Technol. 2003, 89, 1–16. [Google Scholar] [CrossRef]
- Xie, W.; Huang, M. Immobilization of Candida rugosa lipase onto graphene oxide Fe 3 O 4 nanocomposite: Characterization and application for biodiesel production. Energy Convers. Manag. 2018, 159, 42–53. [Google Scholar] [CrossRef]
- Nayak, M.G.; Vyas, A.P. Optimization of microwave-assisted biodiesel production from Papaya oil using response surface methodology. Renew. Energy 2019, 138, 18–28. [Google Scholar] [CrossRef]
- Halek, F.S.; Delavari, A.; Kavousi-Rahim, A. Production of biodiesel as a renewable energy source from castor oil. Clean Technol. Environ. Policy 2012, 15, 1063–1068. [Google Scholar] [CrossRef]
- Miladinović, M.R.; Stojković, I.J.; Veličković, A.V.; Stamenković, O.S.; Banković-Ilić, I.B.; Veljković, V.B. Optimization and kinetic modeling of waste lard methanolysis in a continuous reciprocating plate reactor. Chin. J. Chem. Eng. 2019, 27, 2481–2490. [Google Scholar] [CrossRef]
- Chakraborty, R.; Gupta, A.; Chowdhury, R. Conversion of slaughterhouse and poultry farm animal fats and wastes to biodiesel: Parametric sensitivity and fuel quality assessment. Renew. Sustain. Energy Rev. 2014, 29, 120–134. [Google Scholar] [CrossRef]
- Soufi, M.D.; Ghobadian, B.; Mousavi, S.M.; Najafi, G.; Aubin, J. Valorization of waste cooking oil based biodiesel for biolubricant production in a vertical pulsed column: Energy efficient process approach. Energy 2019, 189, 116266. [Google Scholar] [CrossRef]
- Dhawane, S.H.; Kumar, T.; Halder, G. Process optimisation and parametric effects on synthesis of lipase immobilised carbonaceous catalyst for conversion of rubber seed oil to biodiesel. Energy Convers. Manag. 2018, 176, 55–68. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Fang, Z.; Zhang, F. Esterification of oleic acid to biodiesel catalyzed by a highly acidic carbonaceous catalyst. Catal. Today 2019, 319, 172–181. [Google Scholar] [CrossRef]
- Kumar, D.; Das, T.; Giri, B.S.; Verma, B. Preparation and characterization of novel hybrid bio-support material immobilized from Pseudomonas cepacia lipase and its application to enhance biodiesel production. Renew. Energy 2020, 147, 11–24. [Google Scholar] [CrossRef]
- Nicoletti, G.; Cipolatti, E.P.; Valério, A.; Carbonera, N.G.; Soares, N.S.; Theilacker, E.; Ninow, J.L.; Oliveira, D. Evaluation of different methods for immobilization of Candida antarctica lipase B (CalB lipase) in polyurethane foam and its application in the production of geranyl propionate. Bioprocess Biosyst. Eng. 2015, 38, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Sun, B.; Lin, T.; Feng, Y.; Jia, S. Enzyme shielding by mesoporous organosilica shell on Fe3O4@silica yolk-shell nanospheres. Int. J. Biol. Macromol. 2018, 117, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, M.; Ali, A. Immobilization of Candida rugosa lipase on MCM-41 for the transesterification of cotton seed oil. J. Oleo Sci. 2012, 61, 469–475. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Li, K. Biodiesel production from phoenix tree seed oil catalyzed by liquid lipozyme TL100L. Renew. Energy 2020, 151, 152–160. [Google Scholar] [CrossRef]
- Norjannah, B.; Ong, H.C.; Masjuki, H.H.; Juan, J.C.; Chong, W.T. Enzymatic transesterification for biodiesel production: A comprehensive review. RSC Adv. 2016, 6, 60034–60055. [Google Scholar] [CrossRef]
- Datta, S.; Christena, L.R.; Rajaram, Y.R.S. Enzyme immobilization: An overview on techniques and support materials. 3 Biotech 2013, 3, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noureddini, H.; Gao, X.; Philkana, R. Immobilized Pseudomonas cepacia lipase for biodiesel fuel production from soybean oil. Bioresour. Technol. 2005, 96, 769–777. [Google Scholar] [CrossRef]
- Çakmak, R.; Topal, G.; Çınar, E. Covalent Immobilization of Candida rugosa Lipase on Epichlorohydrin-Coated Magnetite Nanoparticles: Enantioselective Hydrolysis Studies of Some Racemic Esters and HPLC Analysis. Appl. Biochem. Biotechnol. 2020, 191, 1411–1431. [Google Scholar] [CrossRef]
- Bonet-Ragel, K.; Canet, A.; Benaiges, M.D.; Valero, F. Synthesis of biodiesel from high FFA alperujo oil catalysed by immobilized lipase. Fuel 2015, 161, 12–17. [Google Scholar] [CrossRef]
- Song, D.W.; Chen, M.; Cheng, H.M. Collagen-immobilized lipases show good activity and reusability for butyl butyrate synthesis. Appl. Biochem. Biotechnol. 2016, 15, 1–15. [Google Scholar]
- Xie, W.; Ma, N. Immobilized Lipase on Fe3O4Nanoparticles as Biocatalyst for Biodiesel Production. Energy Fuels 2009, 23, 1347–1353. [Google Scholar] [CrossRef]
- Machado, N.B.; Miguez, J.P.; Bolina, I.C.; Salviano, A.B.; Gomes, R.A.; Tavano, O.L.; Luiz, J.H.; Tardioli, P.W.; Érika, C.C.; Mendes, A.A. Preparation, functionalization and characterization of rice husk silica for lipase immobilization via adsorption. Enzym. Microb. Technol. 2019, 128, 9–21. [Google Scholar] [CrossRef]
- Mendes, A.A.; Giordano, R.C.; Giordano, R.D.L.C.; De Castro, H.F. Immobilization and stabilization of microbial lipases by multipoint covalent attachment on aldehyde-resin affinity: Application of the biocatalysts in biodiesel synthesis. J. Mol. Catal. B Enzym. 2011, 68, 109–115. [Google Scholar] [CrossRef]
- Liu, C.-H.; Huang, C.-C.; Wang, Y.-W.; Lee, D.S.; Chang, J.-S. Biodiesel production by enzymatic transesterification catalyzed by Burkholderia lipase immobilized on hydrophobic magnetic particles. Appl. Energy 2012, 100, 41–46. [Google Scholar] [CrossRef]
- Yagiz, F.; Kazan, D.; Akin, A.N. Biodiesel production from waste oils by using lipase immobilized on hydrotalcite and zeolites. Chem. Eng. J. 2007, 134, 262–267. [Google Scholar] [CrossRef]
- You, Q.; Yin, X.; Zhao, Y.; Zhang, Y. Biodiesel production from jatropha oil catalyzed by immobilized Burkholderia cepacia lipase on modified attapulgite. Bioresour. Technol. 2013, 148, 202–207. [Google Scholar] [CrossRef]
- Ulker, C.; Gokalp, N.; Guvenilir, Y. Immobilization of Candida antarctica lipase B (CALB) on surface-modified rice husk ashes (RHA) via physical adsorption and cross-linking methods. Biocatal. Biotransformation 2016, 34, 172–180. [Google Scholar] [CrossRef]
- Bandikari, R.; Qian, J.; Baskaran, R.; Liu, Z.; Wu, G. Bio-affinity mediated immobilization of lipase onto magnetic cellulose nanospheres for high yield biodiesel in one time addition of methanol. Bioresour. Technol. 2018, 249, 354–360. [Google Scholar] [CrossRef]
- Zheng, R.; Ren, Z.; Gao, H.; Zhang, A.; Bian, Z. Effects of calcination on silica phase transition in diatomite. J. Alloy. Compd. 2018, 757, 364–371. [Google Scholar] [CrossRef]
- Liu, X. Preparation of porous hollow Fe3O4/P (GMA-DVB-St) microspheres and application for lipase immobilization. Bioprocess. Biosyst. Eng. 2018, 41, 771–779. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Song, D.; Chen, M.; Cheng, H. Immobilization of Lipases on Magnetic Collagen Fibers and Its Applications for Short-Chain Ester Synthesis. Catalysts 2017, 7, 178. [Google Scholar] [CrossRef] [Green Version]
- Mu, Y.; Cui, M.; Zhang, S.; Zhao, J.; Meng, C.; Sun, Q. Comparison study between a series of new type functional diatomite on methane adsorption performance. Microporous Mesoporous Mater. 2018, 267, 203–211. [Google Scholar] [CrossRef]
- Li, Q.; Zhai, G.; Xu, Y.; Odoom-Wubah, T.; Jia, L.; Huang, J.; Sun, D.; Li, Q. Diatomite Supported Pt Nanoparticles as Efficient Catalyst for Benzene Removal. Ind. Eng. Chem. Res. 2019, 58, 14008–14015. [Google Scholar] [CrossRef]
- Arica, M.Y.; Soydogan, H.; Bayramoglu, G. Reversible immobilization of Candida rugosa lipase on fibrous polymer grafted and sulfonated p(HEMA/EDGMA) beads. Bioprocess. Biosyst. Eng. 2010, 33, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Yang, L.; Wang, Z.; Luo, W.; Li, H.; Lv, P.; Yuan, Z. Lipase immobilization on amino-silane modified superparamagnetic Fe3O4 nanoparticles as biocatalyst for biodiesel production. Fuel 2018, 224, 774–782. [Google Scholar] [CrossRef]
- Kleiner, B.; Fleischer, P.; Schörken, U. Biocatalytic synthesis of biodiesel utilizing deep eutectic solvents: A two-step-one-pot approach with free lipases suitable for acidic and used oil processing. Process. Biochem. 2016, 51, 1808–1816. [Google Scholar] [CrossRef]
- Yasin, Y. Optimization of biocatalytic biodiesel production from pomace oil using response surface methodology. Fuel Process. Technol. 2012, 99, 97–102. [Google Scholar]





| Samples | N (%) | C (%) | H (%) | S (%) |
|---|---|---|---|---|
| SC | 0.88 | 0.27 | 0.46 | 0 |
| A-SC | 1.85 | 3.78 | 1.08 | 0 |
| Glu–A-SC | 1.23 | 4.68 | 1.36 | 0 |
| Lip–Glu–A-SC | 2.06 | 5.21 | 1.73 | 0.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, T.; Duan, Y.-d.; Wang, Q.-e.; Cheng, H.-m. Preparation of Immobilized Lipase on Silica Clay as a Potential Biocatalyst on Synthesis of Biodiesel. Catalysts 2020, 10, 1266. https://doi.org/10.3390/catal10111266
Zou T, Duan Y-d, Wang Q-e, Cheng H-m. Preparation of Immobilized Lipase on Silica Clay as a Potential Biocatalyst on Synthesis of Biodiesel. Catalysts. 2020; 10(11):1266. https://doi.org/10.3390/catal10111266
Chicago/Turabian StyleZou, Ting, You-dan Duan, Qiao-e Wang, and Hai-ming Cheng. 2020. "Preparation of Immobilized Lipase on Silica Clay as a Potential Biocatalyst on Synthesis of Biodiesel" Catalysts 10, no. 11: 1266. https://doi.org/10.3390/catal10111266
APA StyleZou, T., Duan, Y.-d., Wang, Q.-e., & Cheng, H.-m. (2020). Preparation of Immobilized Lipase on Silica Clay as a Potential Biocatalyst on Synthesis of Biodiesel. Catalysts, 10(11), 1266. https://doi.org/10.3390/catal10111266




