Sulfonic Acids Supported on UiO-66 as Heterogeneous Catalysts for the Esterification of Fatty Acids for Biodiesel Production
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of UiO-66 and UiO-66-Supported Acids
2.2. Effect of Immersion Time on the Content of Organo-Sulfonic Acids in Catalysts
2.3. The Catalytic Activity of Acids Supported UiO-66 towards the Esterification of Palmitic Acid with Methanol
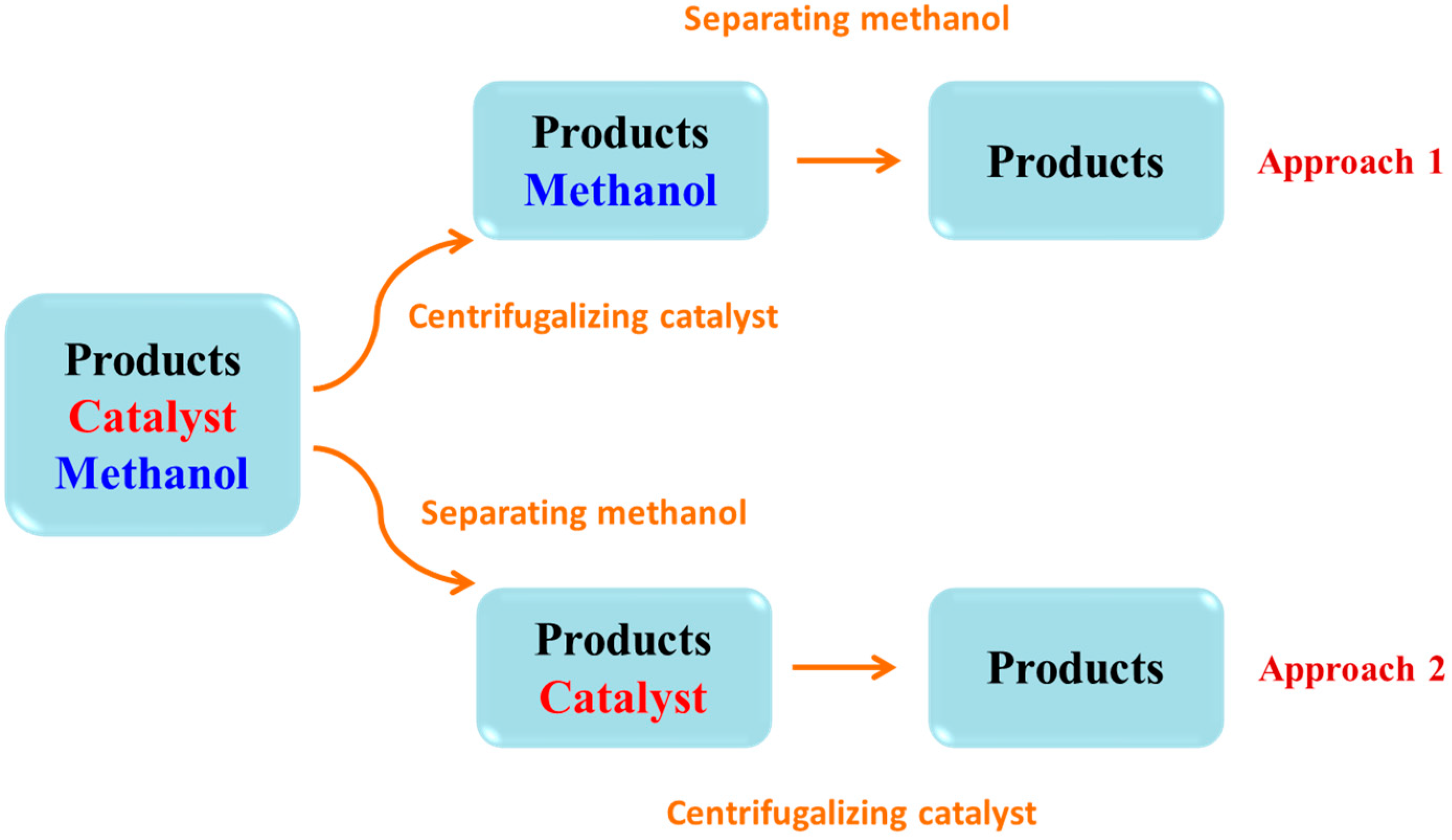
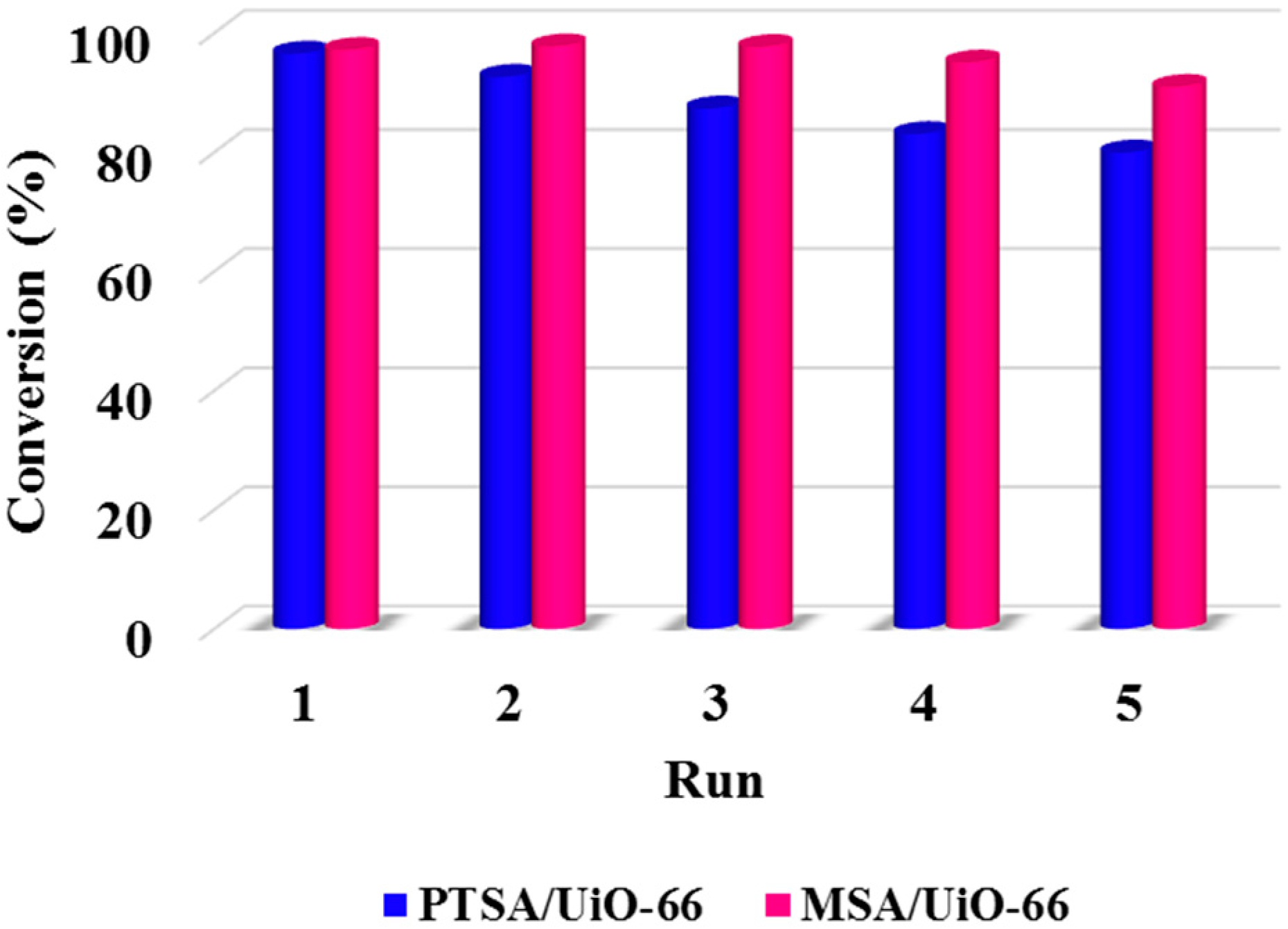
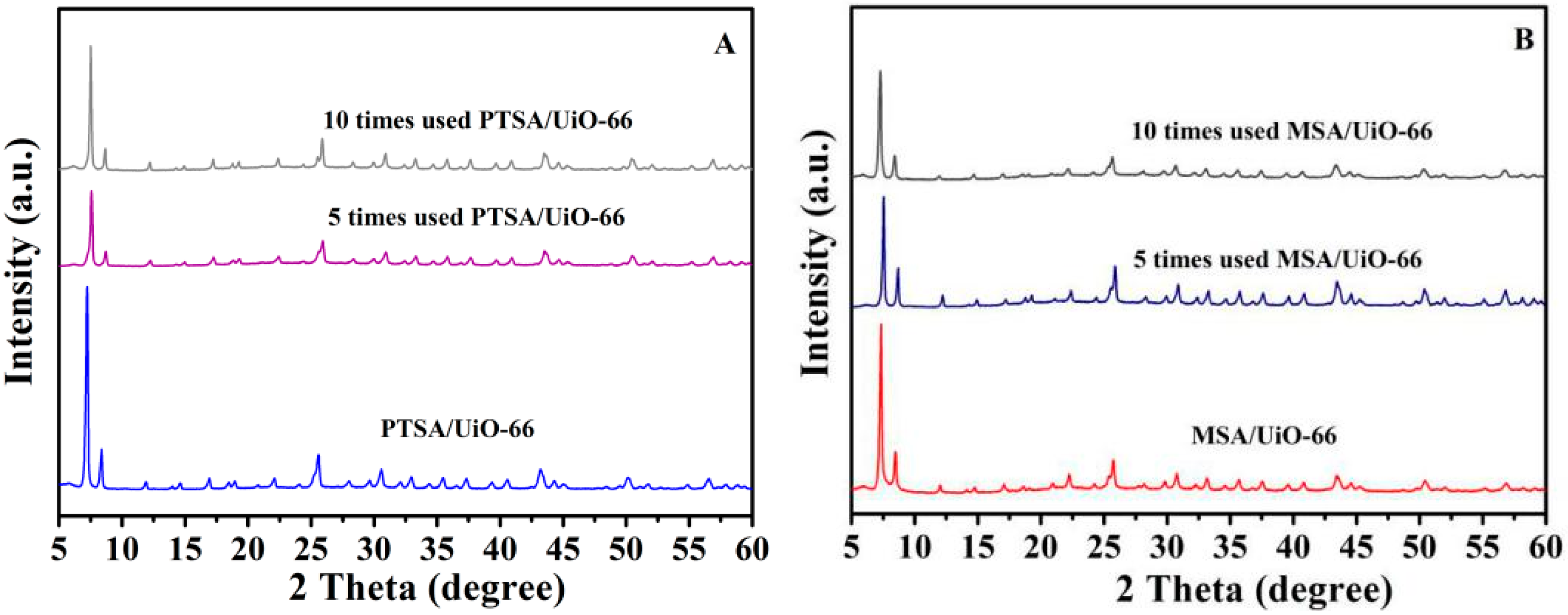
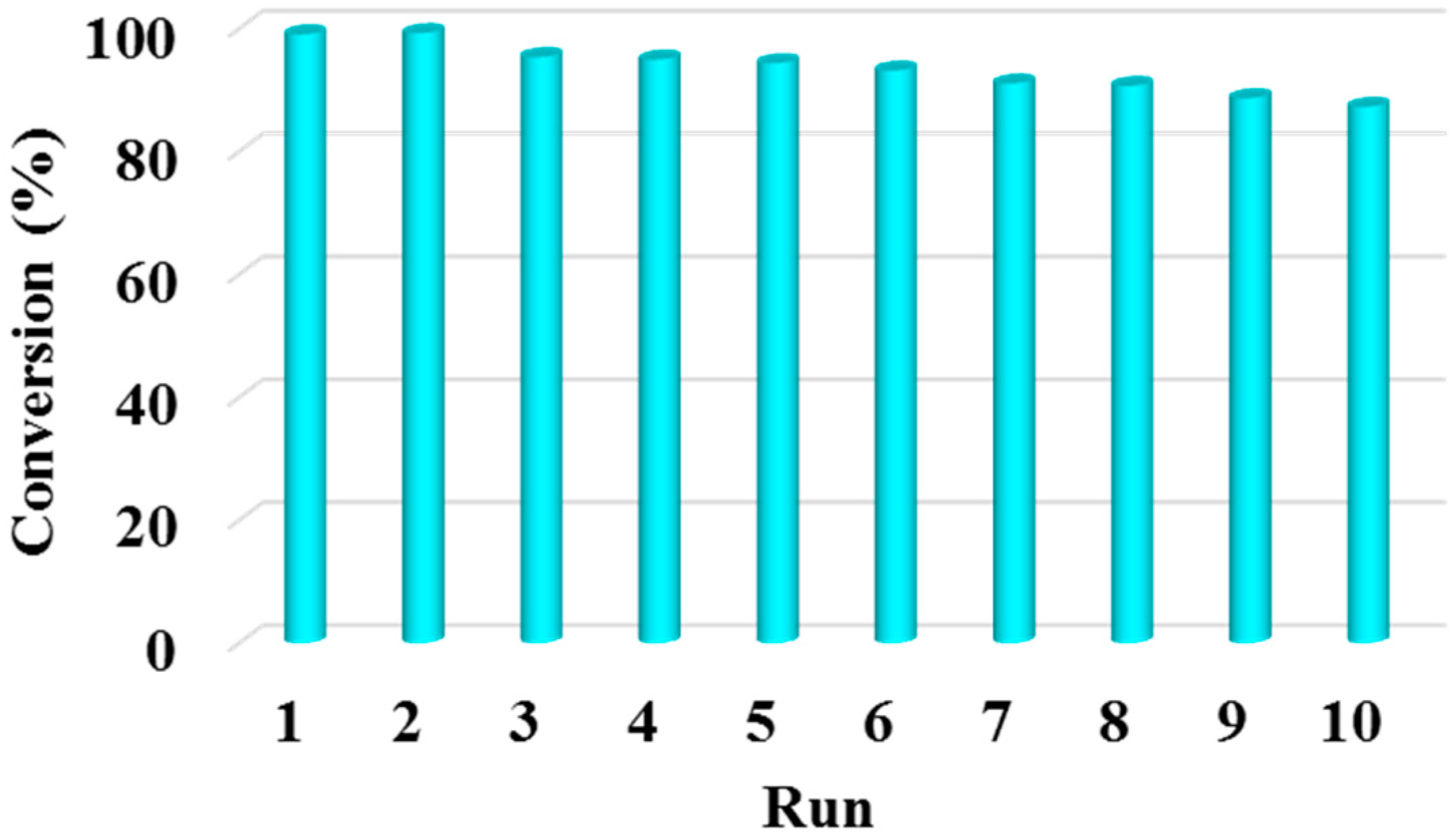
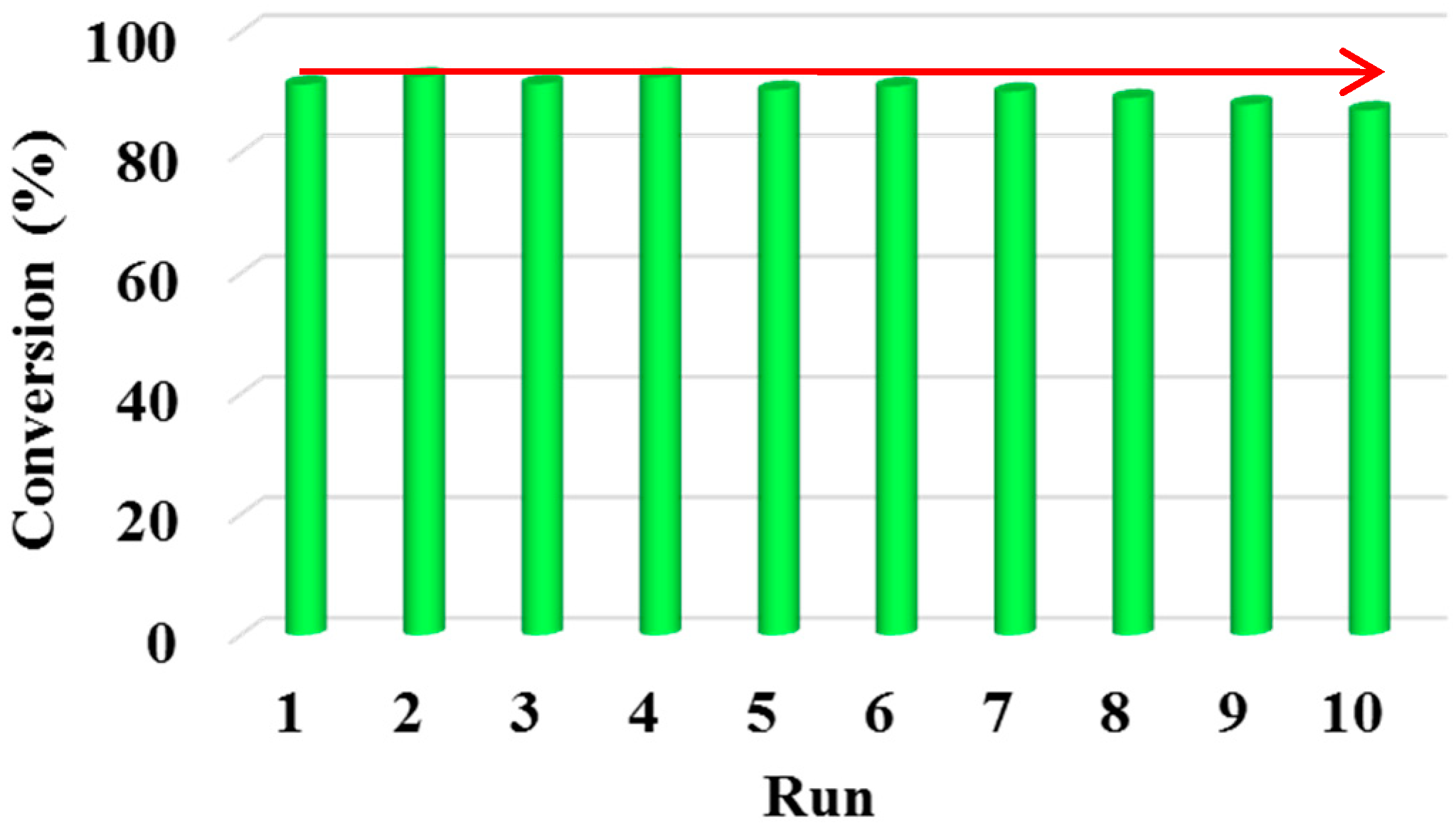
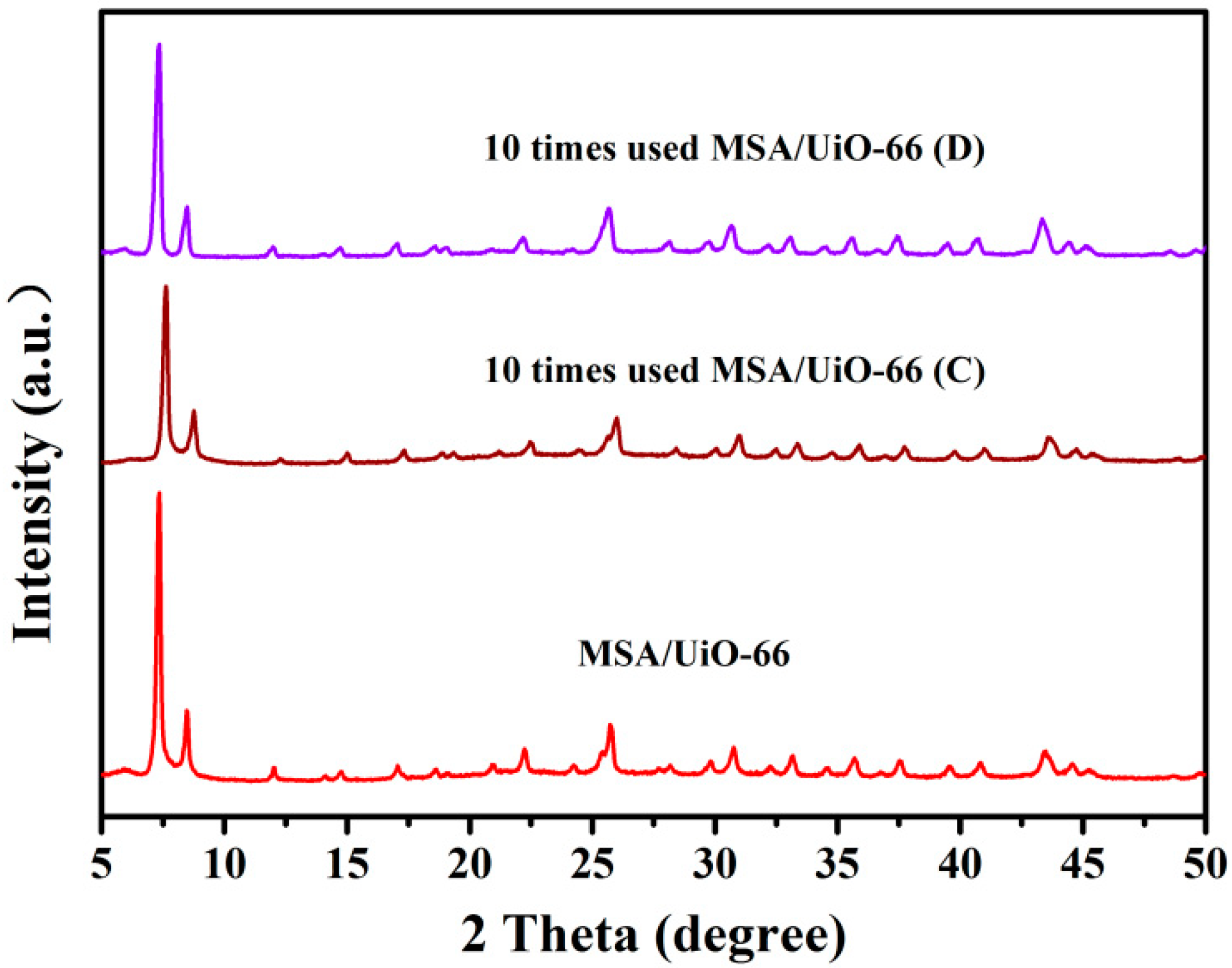
2.4. The Reusability of Acids Supported UiO-66 towards the Esterification of Palmitic Acid with Methanol
2.5. The Catalytic Activity of Acids Supported UiO-66 towards the Esterification of Palmitic Acid with n-Butanol
2.6. The Catalytic Activity of Acids Supported UiO-66 towards the Synthesis of n-Decyl Palmitate
2.7. Reaction Mechanism for Acids Supported UiO-66 towards the Esterification
3. Experimental
3.1. Materials
3.2. Preparation of Supported Catalyst
3.3. Characterization of Sulfonic Acid Supported UiO-66
3.4. Esterification Reactions of Fatty Acid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Maag, H. Fatty acid derivatives: Important surfactants for household, cosmetic and industrial purposes. J. Am. Oil Chem. Soc. 1984, 61, 259–267. [Google Scholar] [CrossRef]
- Xu, H.; Li, P.; Ma, K.; Welbourn, R.J.L.; Doutch, J.; Penfold, J.; Thomas, R.K.; Roberts, D.W.; Petkov, J.T.; Choo, K.L.; et al. Adsorption and self-assembly in methyl ester sulfonate surfactants, their eutectic mixtures and the role of electrolyte. J. Colloid Interface Sci. 2018, 516, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Tian, S.; Guo, J.; Ren, X.; Li, X.; Gao, S. Synthesis, Characterization and Exploratory Application of Anionic Surfactant Fatty Acid Methyl Ester Sulfonate from Waste Cooking Oil. J. Surfactants Deterg. 2016, 19, 467–475. [Google Scholar] [CrossRef]
- Wildes, S. Methyl soyate: A new green alternative solvent. Chem. Health Saf. 2002, 9, 24–26. [Google Scholar] [CrossRef]
- Drown, D.C.; Harper, K.; Frame, E. Screening vegetable oil alcohol esters as fuel lubricity enhancers. J. Am. Oil Chem. Soc. 2001, 78, 579–584. [Google Scholar] [CrossRef]
- Lee, D.-W.; Lee, K.-Y. Heterogeneous Solid Acid Catalysts for Esterification of Free Fatty Acids. Catal. Surv. Asia 2014, 18, 55–74. [Google Scholar] [CrossRef]
- Meher, L.; Sagar, D.V.; Naik, S. Technical aspects of biodiesel production by transesterification—A review. Renew. Sustain. Energy Rev. 2006, 10, 248–268. [Google Scholar] [CrossRef]
- Ma, F.; Hanna, M.A. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Veillette, M.; Giroir-Fendler, A.; Faucheux, N.; Heitz, M. Esterification of free fatty acids with methanol to biodiesel using heterogeneous catalysts: From model acid oil to microalgae lipids. Chem. Eng. J. 2016, 308, 101–109. [Google Scholar] [CrossRef]
- Hommes, A.; De Wit, T.; Euverink, G.J.W.; Yue, J. Enzymatic Biodiesel Synthesis by the Biphasic Esterification of Oleic Acid and 1-Butanol in Microreactors. Ind. Eng. Chem. Res. 2019, 58, 15432–15444. [Google Scholar] [CrossRef] [Green Version]
- Xie, W.; Wan, F. Immobilization of polyoxometalate-based sulfonated ionic liquids on UiO-66-2COOH metal-organic frameworks for biodiesel production via one-pot transesterification-esterification of acidic vegetable oils. Chem. Eng. J. 2019, 365, 40–50. [Google Scholar] [CrossRef]
- Jacobson, K.; Gopinath, R.; Meher, L.C.; Dalai, A.K. Solid acid catalyzed biodiesel production from waste cooking oil. Appl. Catal. B 2008, 85, 86–91. [Google Scholar] [CrossRef]
- Mansir, N.; Taufiq-Yap, Y.H.; Rashid, U.; Lokman, I.M. Investigation of heterogeneous solid acid catalyst performance on low grade feedstocks for biodiesel production: A review. Energy Convers. Manag. 2017, 141, 171–182. [Google Scholar] [CrossRef]
- Di Serio, M.; Tesser, R.; Pengmei, L.; Santacesaria, E. Heterogeneous Catalysts for Biodiesel Production. Energy Fuels 2008, 22, 207–217. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Singh, B.; Korstad, J. Advancements in solid acid catalysts for ecofriendly and economically viable synthesis of biodiesel. Biofuels Bioprod. Biorefining 2011, 5, 69–92. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Kango, H.; Yamashita, H. Catalytic Transfer Hydrogenation of Biomass-Derived Levulinic Acid and Its Esters to γ-Valerolactone over Sulfonic Acid-Functionalized UiO-66. ACS Sustain. Chem. Eng. 2017, 5, 1141–1152. [Google Scholar] [CrossRef]
- Du, M.; Agrawal, A.M.; Chakraborty, S.; Garibay, S.J.; Limvorapitux, R.; Choi, B.; Madrahimov, S.; Nguyen, S.T. Matching the Activity of Homogeneous Sulfonic Acids: The Fructose-to-HMF Conversion Catalyzed by Hierarchically Porous Sulfonic-Acid-Functionalized Porous Organic Polymer (POP) Catalysts. ACS Sustain. Chem. Eng. 2019, 7, 8126–8135. [Google Scholar] [CrossRef]
- Ravi, S.; Roshan, R.; Tharun, J.; Kathalikkattil, A.C.; Park, D.-W. Sulfonic acid functionalized mesoporous SBA-15 as catalyst for styrene carbonate synthesis from CO2 and styrene oxide at moderate reaction conditions. J. CO2 Util. 2015, 10, 88–94. [Google Scholar] [CrossRef]
- Jin, H.; Ansari, M.B.; Park, S.-E. Sulfonic acid functionalized mesoporous ZSM-5: Synthesis, characterization and catalytic activity in acidic catalysis. Catal. Today 2015, 245, 116–121. [Google Scholar] [CrossRef]
- Davis, M.E. Ordered porous materials for emerging applications. Nature 2002, 417, 813–821. [Google Scholar] [CrossRef]
- Rác, B.; Mulas, G.; Csongrádi, A.; Lóki, K.; Árpád, M. SiO2-supported dodecatungstophosphoric acid and Nafion-H prepared by ball-milling for catalytic application. Appl. Catal. A 2005, 282, 255–265. [Google Scholar] [CrossRef]
- Schwegler, M.A.; Vinke, P.; van der Eijk, M.; van Bekkum, H. Activated carbon as asupport for heteropolyanion catalysts. Appl. Catal. A 1992, 80, 41–57. [Google Scholar] [CrossRef]
- Chapman, D.M.; Roe, A. Synthesis, characterization and crystal chemistry of microporous titanium-silicate materials. Zeolites 1990, 10, 730–737. [Google Scholar] [CrossRef]
- Liang, J.; Liang, Z.; Zou, R.; Zhao, Y. Heterogeneous Catalysis in Zeolites, Mesoporous Silica, and Metal-Organic Frameworks. Adv. Mater. 2017, 29, 1701139. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Moler, D.B.; Li, H.; Chen, B.; Reineke, T.M.; O’Keeffe, M.; Yaghi, O.M. Modular Chemistry: Secondary Building Units as a Basis for the Design of Highly Porous and Robust Metal−Organic Carboxylate Frameworks. Acc. Chem. Res. 2001, 34, 319–330. [Google Scholar] [CrossRef]
- Farha, O.K.; Eryazici, I.; Jeong, N.C.; Hauser, B.G.; Wilmer, C.E.; Sarjeant, A.A.; Snurr, R.Q.; Nguyen, S.T.; Yazaydın, A.Ö.; Hupp, J.T. Metal–Organic Framework Materials with Ultrahigh Surface Areas: Is the Sky the Limit? J. Am. Chem. Soc. 2012, 134, 15016–15021. [Google Scholar] [CrossRef] [Green Version]
- Deng, H.; Grunder, S.; Cordova, K.E.; Valente, C.; Furukawa, H.; Hmadeh, M.; Gándara, F.; Whalley, A.C.; Liu, Z.; Asahina, S.; et al. Large-Pore Apertures in a Series of Metal-Organic Frameworks. Science 2012, 336, 1018–1023. [Google Scholar] [CrossRef] [Green Version]
- Cirujano, F.; Corma, A.; I Xamena, F.X.X.L. Zirconium-containing metal organic frameworks as solid acid catalysts for the esterification of free fatty acids: Synthesis of biodiesel and other compounds of interest. Catal. Today 2015, 257, 213–220. [Google Scholar] [CrossRef]
- Ramos-Fernandez, E.V.; Pieters, C.; van der Linden, B.; Juan-Alcañiz, J.; Serra-Crespo, P.; Verhoeven, M.W.G.M.; Niemantsverdriet, H.; Gascon, J.; Kapteijn, F. Highly dispersed platinum in metal organic framework NH2-MIL-10l(Al)containing phosphotungstic acid-characterization and catalytic performance. J. Catal. 2012, 289, 42–52. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, X.; Gao, Z.; Liu, D. Effective Removal of Naphthalenesulfonic Acid from Water Using Functionalized Metal–Organic Frameworks. J. Chem. Eng. Data 2018, 63, 3061–3067. [Google Scholar] [CrossRef]
- Abbasi, A.R.; Rizvandi, M. Influence of the ultrasound-assisted synthesis of Cu–BTC metal–organic frameworks nanoparticles on uptake and release properties of rifampicin. Ultrason. Sonochem. 2018, 40, 465–471. [Google Scholar] [CrossRef]
- Horcajada, P.; Serre, C.; Maurin, G.; Ramsahye, N.A.; Balas, F.; Vallet-Regí, M.; Sebban, M.; Taulelle, F.; Férey, G. Flexible porous metal-organic frameworks for a controlled drug delivery. J. Am. Chem. Soc. 2008, 130, 6774–6780. [Google Scholar] [CrossRef]
- Cychosz, K.A.; Wong-Foy, A.G.; Matzger, A.J. Liquid Phase Adsorption by Microporous Coordination Polymers: Removal of Organosulfur Compounds. J. Am. Chem. Soc. 2008, 130, 6938–6939. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- Piscopo, C.G.; Polyzoidis, A.; Schwarzer, M.; Loebbecke, S. Stability of UiO-66 under acidic treatment: Opportunities and limitations for post-synthetic modifications. Microporous Mesoporous Mater. 2015, 208, 30–35. [Google Scholar] [CrossRef]
- Han, Y.; Liu, M.; Zuo, Y.; Zhang, G.; Guo, X. Preparation and application of high stability metal-organic framework UiO-66. Chin. J. Appl. Chem. 2016, 33, 367–378. [Google Scholar]
- Katz, M.J.; Brown, Z.J.; Colón, Y.J.; Siu, P.W.; Scheidt, K.A.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K. A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem. Commun. 2013, 49, 9449–9451. [Google Scholar] [CrossRef]
- Bavykina, A.; Kolobov, N.; Khan, I.S.; Bau, J.A.; Ramirez, A.; Gascon, J. Metal-Organic Frameworks in Heterogeneous Catalysis: Recent Progress, New Trends, and Future Perspectives. Chem. Rev. 2020, 120, 8468–8535. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Astruc, D. State of the Art and Prospects in Metal–Organic Framework (MOF)-Based and MOF-Derived Nanocatalysis. Chem. Rev. 2020, 120, 1438–1511. [Google Scholar] [CrossRef] [PubMed]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On A Theory of The Vander Waals Adsorption of Gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Howarth, A.J.; Peters, A.W.; Vermeulen, N.A.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Best Practices for the Synthesis, Activation, and Characterization of Metal–Organic Frameworks. Chem. Mater. 2016, 29, 26–39. [Google Scholar] [CrossRef]
- Mondloch, J.E.; Karagiaridi, O.; Farha, O.K.; Hupp, J.T. Activation of metal–organic framework materials. CrystEngComm 2013, 15, 9258–9264. [Google Scholar] [CrossRef]
- Khan, N.R.; Gawas, S.D.; Rathod, V.K. Enzyme-catalysed production of n-butyl palmitate using ultrasound-assisted esterification of palmitic acid in a solvent-free system. Bioprocess Biosyst. Eng. 2018, 41, 1621–1634. [Google Scholar] [CrossRef]
- Bouaid, A.; El Boulifi, N.; Hahati, K.; Martínez, M.; Aracil, J. Biodiesel production from biobutanol. Improvement of cold flow properties. Chem. Eng. J. 2014, 238, 234–241. [Google Scholar] [CrossRef]
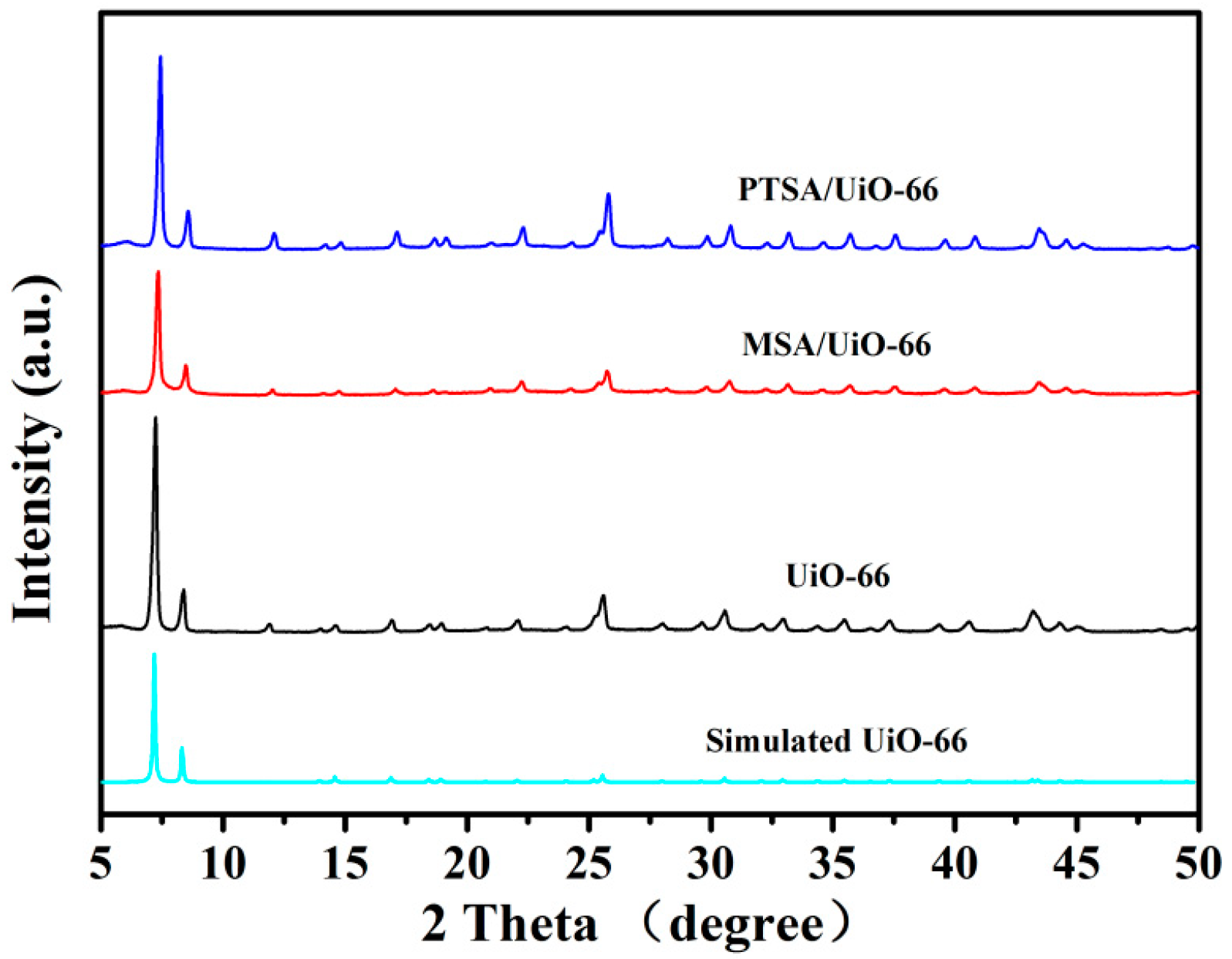
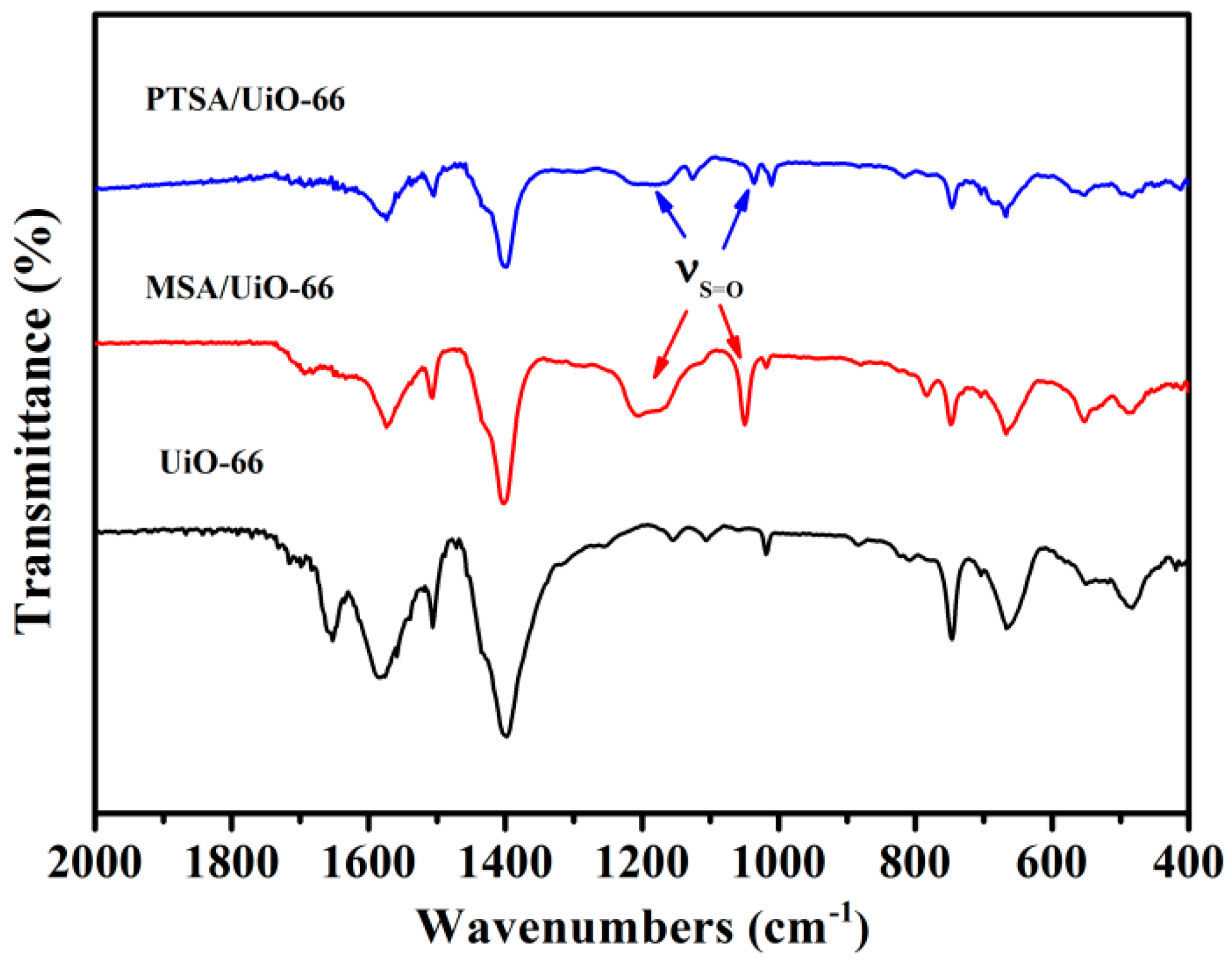
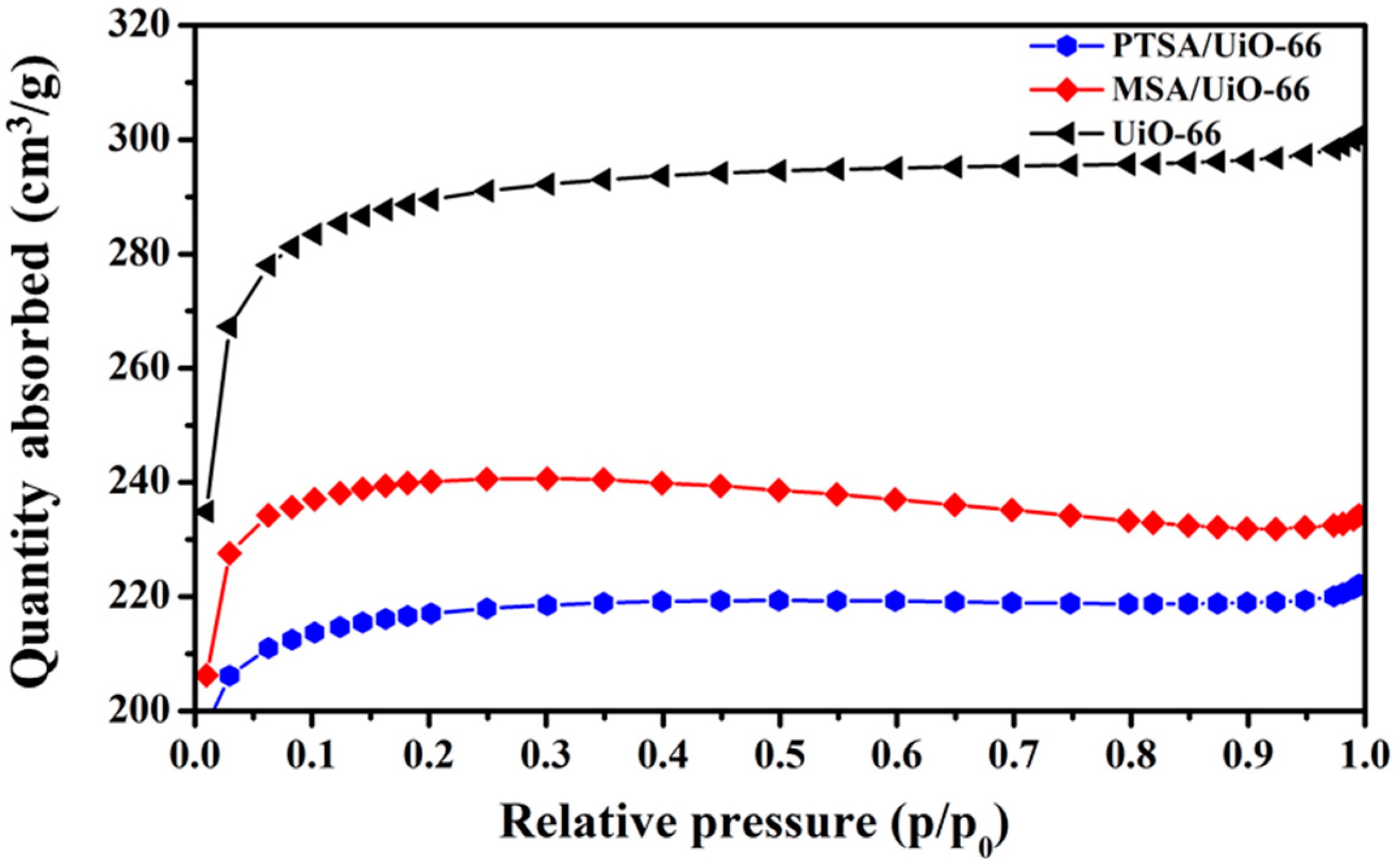



| Catalyst | SLa/(m2/g) | Vtotalb/(m3/g) | Waveragec/nm |
|---|---|---|---|
| UiO-66 | 1284.8 | 0.46 | 1.90 |
| PTSA/UiO-66 | 958.5 | 0.34 | 1.87 |
| MSA/UiO-66 | 1058.9 | 0.36 | 1.79 |
| Entry | Catalyst | MeOH/PA (mol/mol) | Time (h) | Temp. (°C) | Conv. (%) b |
|---|---|---|---|---|---|
| 1 | PTSA/UiO-66 | 3 | 4 | 100 | 88.2 |
| 2 | PTSA/UiO-66 | 5 | 4 | 100 | 94.6 |
| 3 | PTSA/UiO-66 | 8 | 4 | 100 | 97.1 |
| 4 | PTSA/UiO-66 | 12 | 4 | 100 | 97.1 |
| 5 | PTSA/UiO-66 | 8 | 2 | 100 | 92.8 |
| 6 | PTSA/UiO-66 | 8 | 4 | 80 | 93.5 |
| 7 | PTSA/UiO-66-Br | 8 | 4 | 100 | 95.5 |
| 8 | PTSA/UiO-66-NO2 | 8 | 4 | 100 | 97.0 |
| 9 | PTSA/UiO-67 | 8 | 4 | 100 | 97.7 |
| 10 | MSA/UiO-66 | 8 | 4 | 100 | 97.0 |
| 11 | PTSA/C | 8 | 4 | 100 | 44.0 |
| 12 | MSA/C | 8 | 4 | 100 | 37.5 |
| 13 | UiO-66 | 8 | 4 | 100 | 25.2 |
| Catalyst | SLa/(m2/g) | Vtotalb/(m3/g) | Waveragec/nm |
|---|---|---|---|
| UiO-66 | 1284.8 | 0.46 | 1.9 |
| PTSA/UiO-66 (used 10 times) | 1032.9 | 0.38 | 1.94 |
| MSA/UiO-66 (used 10 times) | 1056.3 | 0.43 | 2.16 |
| Entry | Content of Catalyst (g/mol) | n-butanol/PA (mol/mol) | Time (h) | Temp. (°C) | Conv. (%) |
|---|---|---|---|---|---|
| 1 | 20 | 3 | 2 | 80 | 64.1 |
| 2 | 20 | 3 | 1 | 100 | 91.3 |
| 3 | 20 | 3 | 2 | 100 | 98.7 |
| 4 | 20 | 3 | 3 | 100 | 99.0 |
| 5 | 20 | 3 | 3 | 100 | 99.0 |
| 6 | 25 | 3 | 2 | 100 | 99.2 |
| 7 | 25 | 1.5 | 2 | 100 | 93.3 |
| 8 | 20 | 3 | 2 | 110 | 98.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Wang, F.; Meng, P.; Zang, S.-Q. Sulfonic Acids Supported on UiO-66 as Heterogeneous Catalysts for the Esterification of Fatty Acids for Biodiesel Production. Catalysts 2020, 10, 1271. https://doi.org/10.3390/catal10111271
Liu W, Wang F, Meng P, Zang S-Q. Sulfonic Acids Supported on UiO-66 as Heterogeneous Catalysts for the Esterification of Fatty Acids for Biodiesel Production. Catalysts. 2020; 10(11):1271. https://doi.org/10.3390/catal10111271
Chicago/Turabian StyleLiu, Wei, Fang Wang, Pengcheng Meng, and Shuang-Quan Zang. 2020. "Sulfonic Acids Supported on UiO-66 as Heterogeneous Catalysts for the Esterification of Fatty Acids for Biodiesel Production" Catalysts 10, no. 11: 1271. https://doi.org/10.3390/catal10111271
APA StyleLiu, W., Wang, F., Meng, P., & Zang, S.-Q. (2020). Sulfonic Acids Supported on UiO-66 as Heterogeneous Catalysts for the Esterification of Fatty Acids for Biodiesel Production. Catalysts, 10(11), 1271. https://doi.org/10.3390/catal10111271






