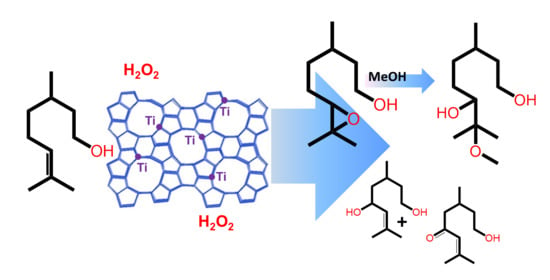
Selective Oxidation of Citronellol over Titanosilicate Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
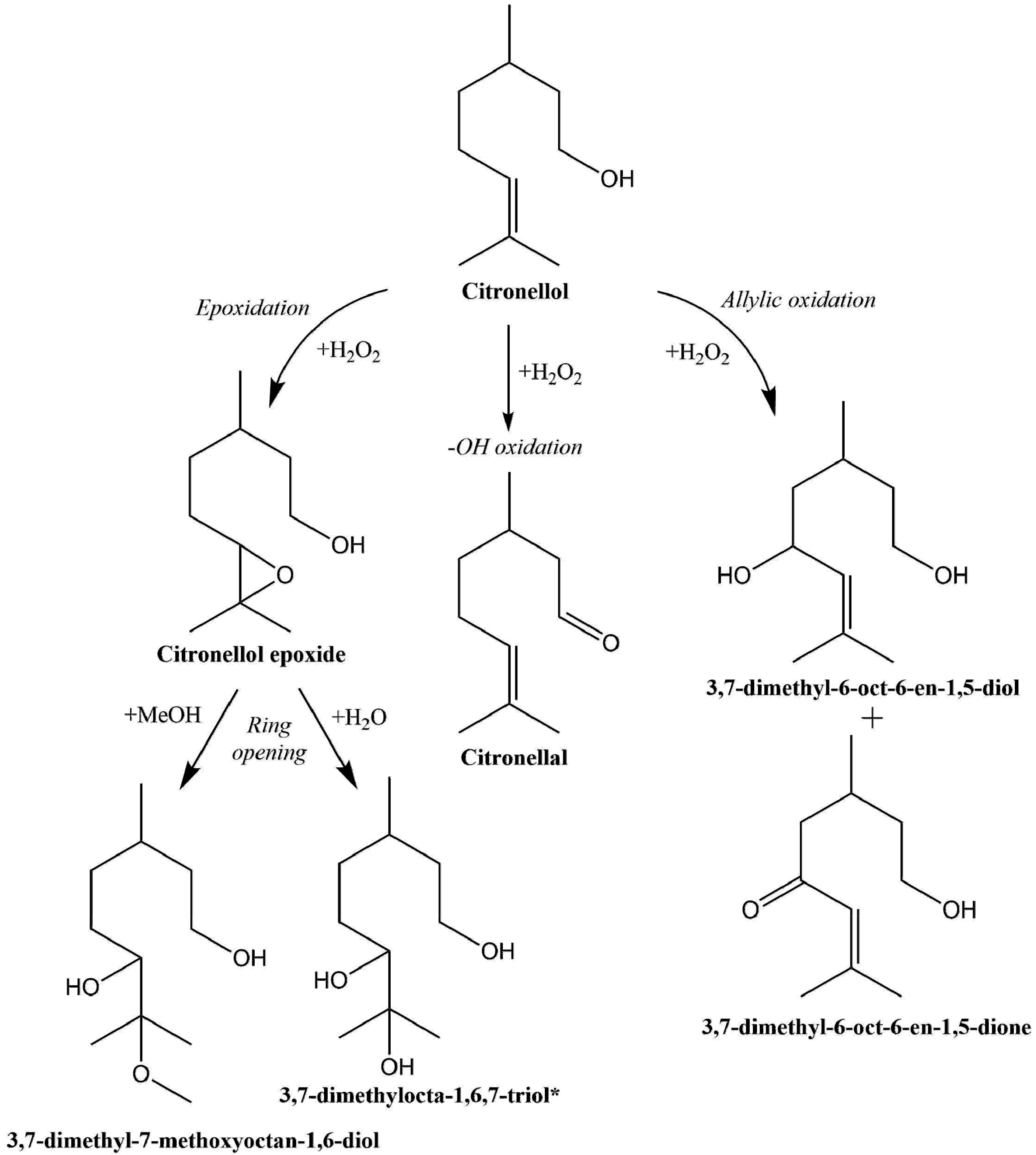
2.2. Selective Catalytic Oxidation of Citronellol
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.2.1. Mesoporous TS-1
3.2.2. Layered TS-1
3.2.3. Silica-Titania Pillared TS-1
3.3. Instrumentation
3.4. Catalytic Experiments
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sell, C.S. Fundamentals of Fragrance Chemistry; Wiley-VCH: Weinheim, Germany, 2019. [Google Scholar]
- Davis, S.E.; Ide, M.S.; Davis, R.J. Selective oxidation of alcohols and aldehydes over supported metal nanoparticles. Green Chem. 2013, 15, 17–45. [Google Scholar] [CrossRef]
- Mallat, T.; Baiker, A. Oxidation of alcohols with molecular oxygen on platinum metal catalysts in aqueous solutions. Catal. Today 1994, 19, 247–283. [Google Scholar] [CrossRef]
- Murahashi, S.; Naota, T.; Hirai, N. Aerobic oxidation of alcohols with ruthenium-cobalt bimetallic catalyst in the presence of aldehydes. J. Org. Chem. 1993, 58, 7318–7319. [Google Scholar] [CrossRef]
- Higashimoto, S.; Kitao, N.; Yoshida, N.; Sakura, T.; Azuma, M.; Ohue, H.; Sakata, Y. Selective photocatalytic oxidation of benzyl alcohol and its derivatives into corresponding aldehydes by molecular oxygen on titanium dioxide under visible light irradiation. J. Catal. 2009, 266, 279–285. [Google Scholar] [CrossRef]
- Přech, J. Catalytic performance of advanced titanosilicate selective oxidation catalysts—a review. Catal. Rev. 2018, 60, 71–131. [Google Scholar] [CrossRef]
- Velusamy, S.; Ahamed, M.; Punniyamurthy, T. Novel polyaniline-supported molybdenum-catalyzed aerobic oxidation of alcohols to aldehydes and ketones. Org. Lett. 2004, 6, 4821–4824. [Google Scholar] [CrossRef] [PubMed]
- Choudary, B.M.; Kantam, M.L.; Rahman, A.; Reddy, C.V.; Rao, K.K. The First Example of Activation of Molecular Oxygen by Nickel in Ni-Al Hydrotalcite: A Novel Protocol for the Selective Oxidation of Alcohols. Angew. Chem. Int. Ed. Engl. 2001, 40, 763–766. [Google Scholar] [CrossRef]
- Torbina, V.V.; Vodyankin, A.A.; Ten, S.; Mamontov, G.V.; Salaev, M.A.; Sobolev, V.I.; Vodyankina, O.V. Ag-based catalysts in heterogeneous selective oxidation of alcohols: A review. Catalysts 2018, 8, 447. [Google Scholar] [CrossRef] [Green Version]
- Suib, S.L.; Přech, J.; Čejka, J.; Kuwahara, Y.; Mori, K.; Yamashita, H. Some novel porous materials for selective catalytic oxidations. Mater. Today 2020, 32, 244–259. [Google Scholar] [CrossRef]
- Perego, C.; Carati, A.; Ingallina, P.; Mantegazza, M.A.; Bellussi, G. Production of titanium containing molecular sieves and their application in catalysis. Appl. Catal. A Gen. 2001, 221, 63–72. [Google Scholar] [CrossRef]
- Mintova, S.; Barrier, N. Verified Synthesis of Zeolitic Materials, 3rd ed.; Elsevier (On behalf of Synthesis Commission of the International Zeolite Association): Amsterdam, The Netherlands, 2016. [Google Scholar]
- Prech, J.; Pizarro, P.; Serrano, D.P.; Cejka, J. From 3D to 2D zeolite catalytic materials. Chemical Society Reviews. Chem. Soc. Rev. 2018, 47, 8263–8306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taramasso, M.; Perego, G.; Notari, B. Preparation of Porous Crystalline Synthetic Material Comprised of Silicon and Titanium Oxides. U.S. Patent 4,410,501, 18 October 1983. [Google Scholar]
- Perez-Ramirez, J.; Christensen, C.H.; Egeblad, K.; Christensen, C.H.; Groen, J.C. Hierarchical zeolites: Enhanced utilisation of microporous crystals in catalysis by advances in materials design. Chem. Soc. Rev. 2008, 37, 2530–2542. [Google Scholar] [CrossRef] [PubMed]
- Na, K.; Jo, C.; Kun, J.; Ahn, W.S.; Ryoo, R. MFI Titanosilicate Nanosheets with Single-Unit-Cell Thickness as an Oxidation Catalyst Using Peroxides. ACS Catal. 2011, 1, 901–907. [Google Scholar] [CrossRef]
- Surburg, H.; Panten, J. Common Fragrance and Flavor Materials: Preparation, Properties and Uses; Wiley-VCH: Weinheim, Germany, 2016. [Google Scholar]
- Schofield, L.J.; Kerton, O.J.; McMorn, P.; Bethell, D.; Ellwood, S.; Hutchings, G.J. Oxidation of α-hydroxy containing monoterpenes using titanium silicate catalysts: Comments on regioselectivity and the role of acidity. J. Chem. Soc. Perkin Trans. 2 2002, 8, 1475–1481. [Google Scholar] [CrossRef]
- Přech, J.; Eliášová, P.; Aldhayan, D.; Kubů, M. Epoxidation of bulky organic molecules over pillared titanosilicates. Catal. Today 2015, 243, 134–140. [Google Scholar] [CrossRef]
- Choi, M.; Na, K.; Kim, J.; Sakamoto, Y.; Terasaki, O.; Ryoo, R. Stable single-unit-cell nanosheets of zeolite MFI as active and long-lived catalysts. Nature 2009, 461, 828. [Google Scholar] [CrossRef]
- Ratnasamy, P.; Srinivas, D.; Knözinger, H. Active Sites and Reactive Intermediates in Titanium Silicate Molecular Sieves. Adv. Catal. 2004, 48, 1–169. [Google Scholar]
- van der Waal, J.C.; Lin, P.; Rigutto, M.S.; van Bekkum, H. Synthesis of Aluminium Free Titanium Silicate with the BEA Structure Using a New and Selective Template and Its Use as a Catalyst in Epoxidations; Chon, S.-K.I.H., Sun, U.Y., Eds.; Elsevier: Amsterdam, The Netherlands, 1997; Volume 105, pp. 1093–1100. [Google Scholar]
- van der Waal, J.C.; Rigutto, M.S.; van Bekkum, H. Zeolite titanium beta as a selective catalyst in the epoxidation of bulky alkenes. Appl. Catal. A Gen. 1998, 167, 331–342. [Google Scholar] [CrossRef]
- Přech, J.; Kim, J.; Mazur, M.; Ryoo, R.; Čejka, J. Nanosponge TS-1: A Fully Crystalline Hierarchical Epoxidation Catalyst. Adv. Mater. Interfaces 2020, in press. [Google Scholar]
- Tatsumi, T.; Yako, M.; Nakamura, M.; Yuhara, Y.; Tominaga, H. Effect of alkene structure on selectivity in the oxidation of unsaturated alcohols with titanium silicalite-1 catalyst. J. Mol. Catal. A 1993, 78, L41–L45. [Google Scholar] [CrossRef]
- Kubota, Y.; Inagaki, S. High-Performance Catalysts with MSE-Type Zeolite Framework. Top. Catal. 2015, 58, 480–493. [Google Scholar] [CrossRef]
- Wilde, N.; Přech, J.; Pelz, M.; Kubů, M.; Čejka, J.; Gläser, R. Accessibility enhancement of TS-1-based catalysts for improving the epoxidation of plant oil-derived substrates. Catal. Sci. Technol. 2016, 6, 7280–7288. [Google Scholar] [CrossRef] [Green Version]
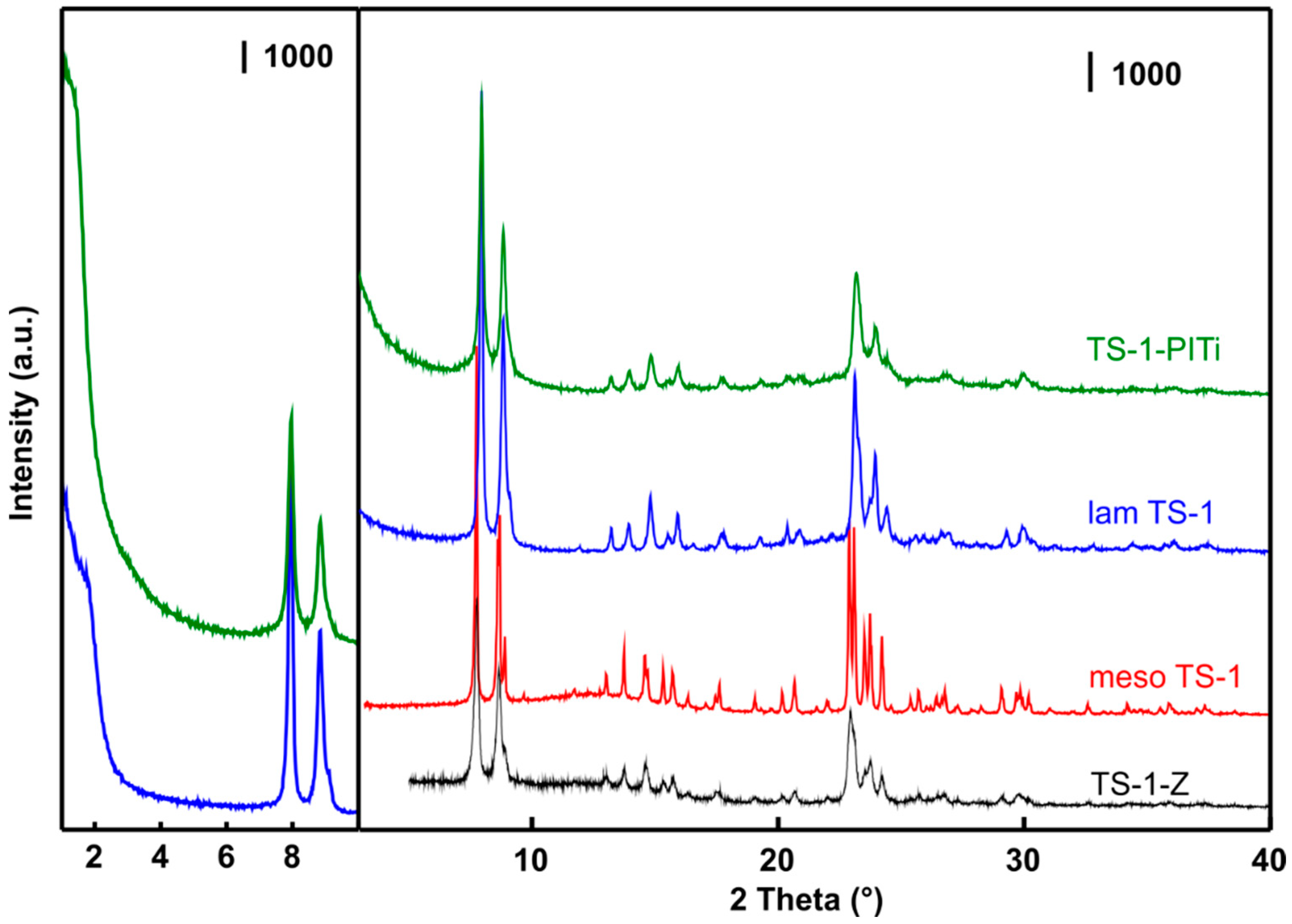
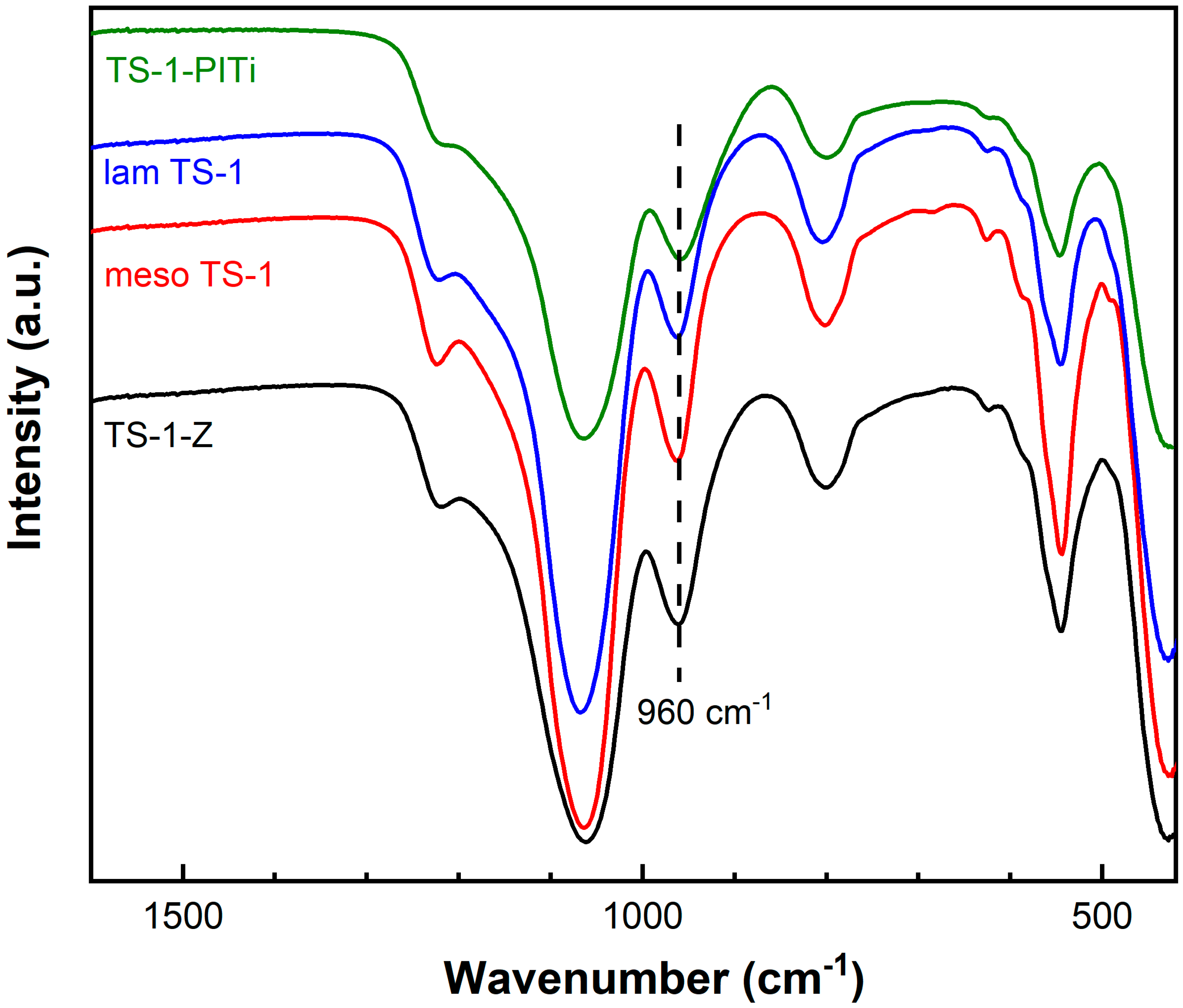
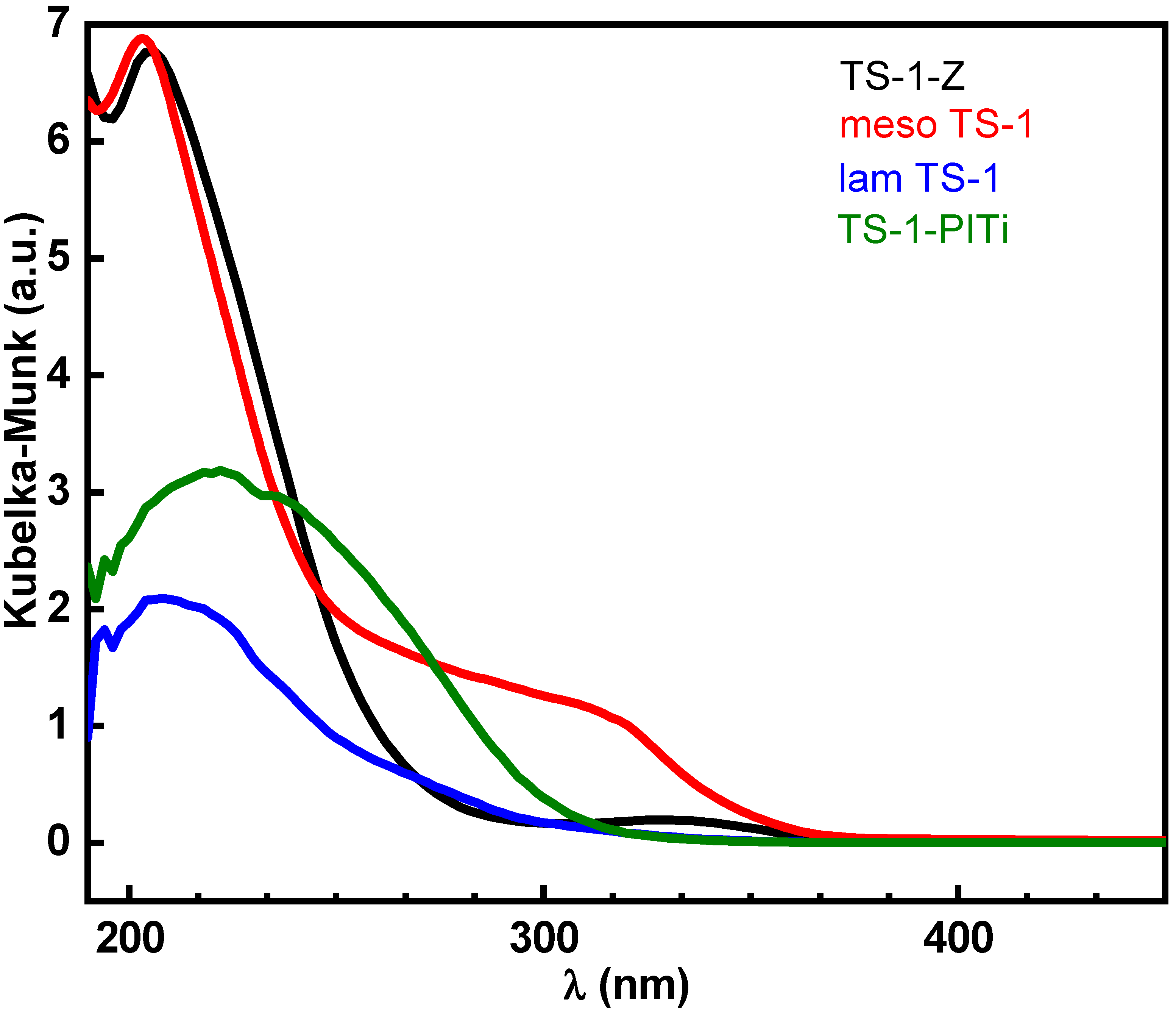


| Catalyst | Morphology | Si/Ti (mol/mol) | SBET (m2/g) | Vmic (cm3/g) | Vtot (cm3/g) | Sext (m2/g) |
|---|---|---|---|---|---|---|
| TS-1-Z | Conventional | 28 | 510 | 0.10 | 0.28 | 31 |
| Meso-TS-1 | Hierarchical | 43 | 432 | 0.09 | 0.31 | 29 |
| Lam-TS-1 | Layered | 49 | 440 | 0.09 | 0.34 | 238 |
| TS-1-PITi | Layered-pillared | 22 | 580 | 0.09 | 0.35 | 369 |
| Solvent | Conversion (%) | Epox. Yield (%) | Selectivity (%) |
|---|---|---|---|
| Methanol | 15.1 | 7.2 | 47 |
| 2-propanol | 11.4 | 4.0 | 36 |
| 1,4-dioxane | 9.9 | 2.1 | 31 |
| Acetonitrile | 16.6 | 9.1 | 55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldhayan, D.; Kalíková, K.; Shaik, M.R.; Siddiqui, M.R.H.; Přech, J. Selective Oxidation of Citronellol over Titanosilicate Catalysts. Catalysts 2020, 10, 1284. https://doi.org/10.3390/catal10111284
Aldhayan D, Kalíková K, Shaik MR, Siddiqui MRH, Přech J. Selective Oxidation of Citronellol over Titanosilicate Catalysts. Catalysts. 2020; 10(11):1284. https://doi.org/10.3390/catal10111284
Chicago/Turabian StyleAldhayan, Daifallah, Květa Kalíková, Mohammed Rafi Shaik, Mohammed Rafiq H. Siddiqui, and Jan Přech. 2020. "Selective Oxidation of Citronellol over Titanosilicate Catalysts" Catalysts 10, no. 11: 1284. https://doi.org/10.3390/catal10111284