Asymmetric Synthesis of Optically Pure Aliphatic Amines with an Engineered Robust ω-Transaminase
Abstract
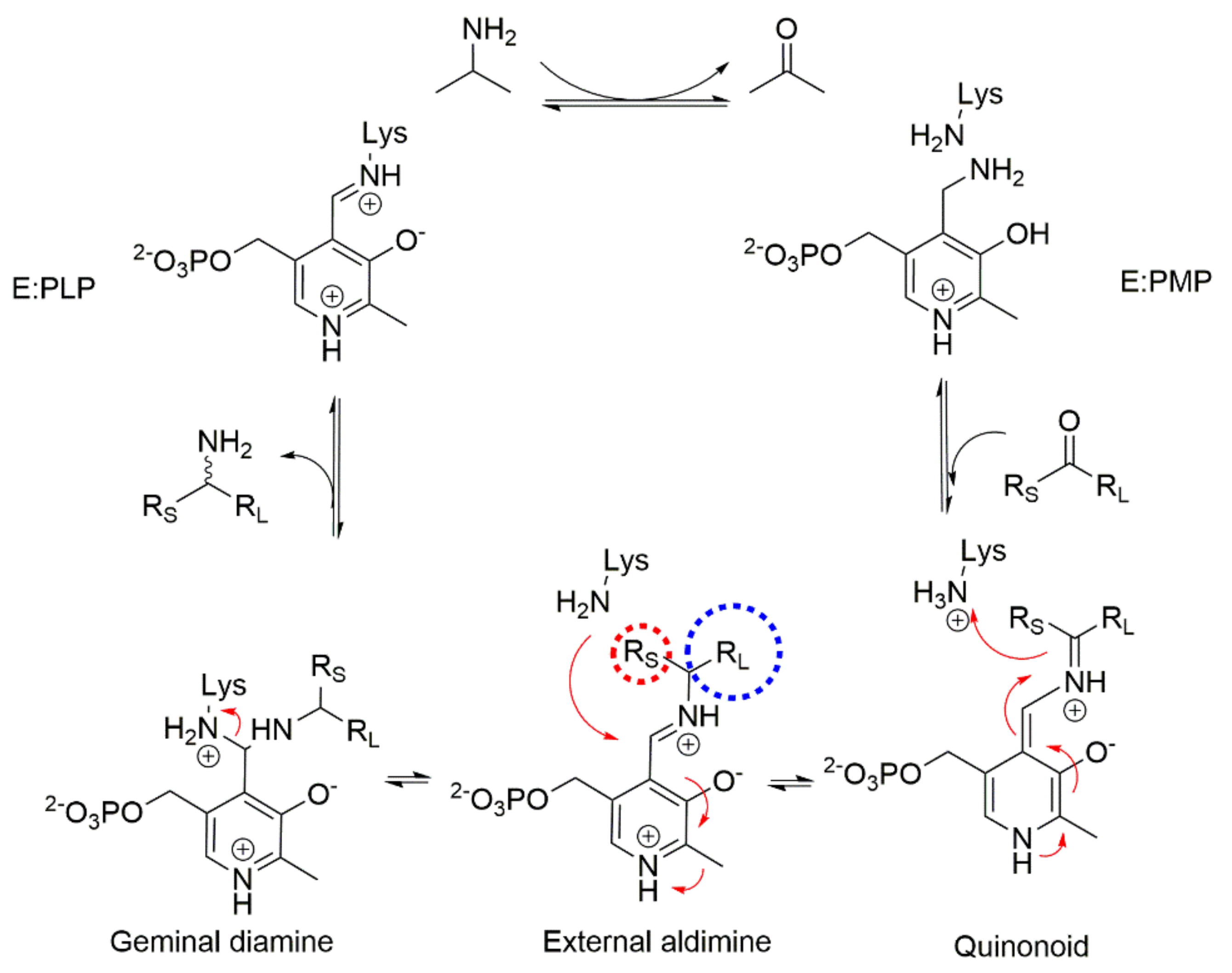
1. Introduction
2. Results and Discussion
2.1. Asymmetric Synthesis of Aliphatic Amines
2.2. Role of Switching Arginine
2.3. Structure–Activity Relationship Obtained by Docking
3. Materials and Methods
3.1. Materials
3.2. Enzyme Expression and Purification
3.3. Thermal Shift Assay
3.4. Amination Reactions and Product Derivatization
3.5. Quantification and Optical Purity
3.6. Computational Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koszelewski, D.; Lavandera, I.; Clay, D.; Rozzell, D.; Kroutil, W. Asymmetric Synthesis of Optically Pure Pharmacologically Relevant Amines Employing ω-Transaminases. Adv. Synth. Catal. 2008, 350, 2761–2766. [Google Scholar] [CrossRef]
- Ghislieri, D.; Turner, N.J. Biocatalytic Approaches to the Synthesis of Enantiomerically Pure Chiral Amines. Top. Catal. 2014, 57, 284–300. [Google Scholar] [CrossRef]
- Patil, M.D.; Grogan, G.; Bommarius, A.; Yun, H. Recent Advances in ω-Transaminase-Mediated Biocatalysis for the Enantioselective Synthesis of Chiral Amines. Catalysts 2018, 8, 254. [Google Scholar] [CrossRef]
- Rösler, M.; Anand, R.; Cicin-Sain, A.; Gauthier, S.; Agid, Y.; Dal-Bianco, P.; Stähelin, H.B.; Hartman, R. Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: International randomised controlled trial. BMJ 1999, 318, 8. [Google Scholar] [CrossRef] [PubMed]
- Hoye, T.R.; Chen, M. Total synthesis of (ent)-korupensamine D. Tetrahedron Lett. 1996, 37, 3099–3100. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; De Paolis, M.; Zhu, J. Synthetic Studies toward Ecteinascidin 743. J. Org. Chem. 2005, 70, 4397–4408. [Google Scholar] [CrossRef]
- Kelly, S.A.; Pohle, S.; Wharry, S.; Mix, S.; Allen, C.C.R.; Moody, T.S.; Gilmore, B.F. Application of ω-Transaminases in the Pharmaceutical Industry. Chem. Rev. 2018, 118, 349–367. [Google Scholar] [CrossRef]
- Simon, R.C.; Richter, N.; Busto, E.; Kroutil, W. Recent Developments of Cascade Reactions Involving ω-Transaminases. ACS Catal. 2014, 4, 129–143. [Google Scholar] [CrossRef]
- Eliot, A.C.; Kirsch, J.F. Pyridoxal Phosphate Enzymes: Mechanistic, Structural, and Evolutionary Considerations. Annu. Rev. Biochem. 2004, 73, 383–415. [Google Scholar] [CrossRef]
- Slabu, I.; Galman, J.L.; Lloyd, R.C.; Turner, N.J. Discovery, Engineering, and Synthetic Application of Transaminase Biocatalysts. ACS Catal. 2017, 7, 8263–8284. [Google Scholar] [CrossRef]
- Voss, M.; Das, D.; Genz, M.; Kumar, A.; Kulkarni, N.; Kustosz, J.; Kumar, P.; Bornscheuer, U.T.; Höhne, M. In Silico Based Engineering Approach to Improve Transaminases for the Conversion of Bulky Substrates. ACS Catal. 2018, 8, 11524–11533. [Google Scholar] [CrossRef]
- Han, S.-W.; Kim, J.; Cho, H.-S.; Shin, J.-S. Active Site Engineering of ω-Transaminase Guided by Docking Orientation Analysis and Virtual Activity Screening. ACS Catal. 2017, 7, 3752–3762. [Google Scholar] [CrossRef]
- Sirin, S.; Kumar, R.; Martinez, C.; Karmilowicz, M.J.; Ghosh, P.; Abramov, Y.A.; Martin, V.; Sherman, W. A Computational Approach to Enzyme Design: Predicting ω-Aminotransferase Catalytic Activity Using Docking and MM-GBSA Scoring. J. Chem. Inf. Model. 2014, 54, 2334–2346. [Google Scholar] [CrossRef] [PubMed]
- Dourado, D.F.A.R.; Pohle, S.; Carvalho, A.T.P.; Dheeman, D.S.; Caswell, J.M.; Skvortsov, T.; Miskelly, I.; Brown, R.T.; Quinn, D.J.; Allen, C.C.R.; et al. Rational Design of a (S)-Selective-Transaminase for Asymmetric Synthesis of (1S)-1-(1,1′-biphenyl-2-yl)ethanamine. ACS Catal. 2016, 6, 7749–7759. [Google Scholar] [CrossRef]
- Cassimjee, K.E.; Manta, B.; Himo, F. A quantum chemical study of the ω-transaminase reaction mechanism. Org. Biomol. Chem. 2015, 13, 8453–8464. [Google Scholar] [CrossRef]
- Koszelewski, D.; Tauber, K.; Faber, K.; Kroutil, W. ω-Transaminases for the synthesis of non-racemic α-chiral primary amines. Trends Biotechnol. 2010, 28, 324–332. [Google Scholar] [CrossRef]
- Park, E.-S.; Malik, M.S.; Dong, J.-Y.; Shin, J.-S. One-Pot Production of Enantiopure Alkylamines and Arylalkylamines of Opposite Chirality Catalyzed by ω-Transaminase. ChemCatChem 2013, 5, 1734–1738. [Google Scholar] [CrossRef]
- Liu, T.-L.; Wang, C.-J.; Zhang, X. Synthesis of Chiral Aliphatic Amines through Asymmetric Hydrogenation. Angew. Chem. Int. Ed. 2013, 52, 8416–8419. [Google Scholar] [CrossRef]
- Shin, J.-S.; Kim, B.-G. Asymmetric Synthesis of Chiral Amines with ω-Transaminase. Biotechnol. Bioeng. 1999, 65, 206–211. [Google Scholar] [CrossRef]
- Fuchs, M.; Farnberger, J.E.; Kroutil, W. The Industrial Age of Biocatalytic Transamination: The Industrial Age of Biocatalytic Transamination. Eur. J. Org. Chem. 2015, 2015, 6965–6982. [Google Scholar] [CrossRef]
- Tufvesson, P.; Lima-Ramos, J.; Jensen, J.S.; Al-Haque, N.; Neto, W.; Woodley, J.M. Process considerations for the asymmetric synthesis of chiral amines using transaminases. Biotechnol. Bioeng. 2011, 108, 1479–1493. [Google Scholar] [CrossRef]
- Otzen, M.; Palacio, C.; Janssen, D.B. Characterization of the caprolactam degradation pathway in Pseudomonas jessenii using mass spectrometry-based proteomics. Appl. Microbiol. Biotechnol. 2018, 102, 6699–6711. [Google Scholar] [CrossRef] [PubMed]
- Palacio, C.M.; Rozeboom, H.J.; Lanfranchi, E.; Meng, Q.; Otzen, M.; Janssen, D.B. Biochemical properties of a Pseudomonas aminotransferase involved in caprolactam metabolism. FEBS J. 2019, 286, 4086–4102. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Capra, N.; Palacio, C.M.; Lanfranchi, E.; Otzen, M.; van Schie, L.Z.; Rozeboom, H.J.; Thunnissen, A.-M.W.H.; Wijma, H.J.; Janssen, D.B. Robust ω-Transaminases by Computational Stabilization of the Subunit Interface. ACS Catal. 2020, 10, 2915–2928. [Google Scholar] [CrossRef]
- Calvelage, S.; Dörr, M.; Höhne, M.; Bornscheuer, U.T. A Systematic Analysis of the Substrate Scope of (S)- and (R)-Selective Amine Transaminases. Adv. Synth. Catal. 2017, 359, 4235–4243. [Google Scholar] [CrossRef]
- Huisman, G.W.; Collier, S.J. On the development of new biocatalytic processes for practical pharmaceutical synthesis. Curr. Opin. Chem. Biol. 2013, 17, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.-K.; Park, H.-Y.; Seo, J.-H.; Kim, J.; Kang, T.-J.; Lee, B.-S.; Kim, B.-G. Redesigning the substrate specificity of ω-aminotransferase for the kinetic resolution of aliphatic chiral amines. Biotechnol. Bioeng. 2008, 99, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Nobili, A.; Steffen-Munsberg, F.; Kohls, H.; Trentin, I.; Schulzke, C.; Höhne, M.; Bornscheuer, U.T. Engineering the Active Site of the Amine Transaminase from Vibrio fluvialis for the Asymmetric Synthesis of Aryl-Alkyl Amines and Amino Alcohols. ChemCatChem 2015, 7, 757–760. [Google Scholar] [CrossRef]
- Genz, M.; Melse, O.; Schmidt, S.; Vickers, C.; Dörr, M.; van den Bergh, T.; Joosten, H.-J.; Bornscheuer, U.T. Engineering the Amine Transaminase from Vibrio fluvialis towards Branched-Chain Substrates. ChemCatChem 2016, 8, 3199–3202. [Google Scholar] [CrossRef]
- Midelfort, K.S.; Kumar, R.; Han, S.; Karmilowicz, M.J.; McConnell, K.; Gehlhaar, D.K.; Mistry, A.; Chang, J.S.; Anderson, M.; Villalobos, A.; et al. Redesigning and characterizing the substrate specificity and activity of Vibrio fluvialis aminotransferase for the synthesis of imagabalin. Protein Eng. Des. Sel. 2013, 26, 25–33. [Google Scholar] [CrossRef]
- Ericsson, U.B.; Hallberg, B.M.; DeTitta, G.T.; Dekker, N.; Nordlund, P. Thermofluor-based high-throughput stability optimization of proteins for structural studies. Anal. Biochem. 2006, 357, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Boivin, S.; Kozak, S.; Meijers, R. Optimization of protein purification and characterization using Thermofluor screens. Protein Expr. Purif. 2013, 91, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Krieger, E.; Vriend, G. YASARA View—Molecular Graphics for 1139 all Devices—from Smartphones to Workstations. Bioinformatics 2014, 30, 2981–2982. [Google Scholar] [CrossRef] [PubMed]
- Conway, P.; Tyka, M.D.; DiMaio, F.; Konerding, D.E.; Baker, D. Relaxation of backbone bond geometry improves protein energy landscape modeling. Protein Sci. 2014, 23, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Richter, F.; Leaver-Fay, A.; Khare, S.D.; Bjelic, S.; Baker, D. De Novo Enzyme Design using Rosetta3. PLoS ONE 2011, 6, e19230. [Google Scholar] [CrossRef]




| Amine Target Code | Amine Structure | PjTA-R6 | CvTA | VfTA | |||
|---|---|---|---|---|---|---|---|
| Yield 1 (%) | e.e. 2 (%) | Yield (%) | e.e. (%) | Yield (%) | e.e. (%) | ||
| A1 |  | 70 | 94 | 40 | 86 | 5 | n.d. 3 |
| A2 |  | 80 | 98 | 34 | 91 | 12 | 41 |
| A3 |  | 51 | >99 | 23 | 91 | 14 | 88 |
| A4 |  | 51 | >99 | 44 | 90 | 41 | 92 |
| A5 |  | 42 | >99 | 46 | 88 | 35 | 94 |
| B1 |  | 95 | >99 | 3 | n.d. | 1 | n.d. |
| B2 |  | 81 | >99 | 3 | n.d. | 2 | n.d. |
| B3 |  | 48 | >99 | <1 | n.d. | 2 | n.d. |
| C1 |  | 42 | >99 | 1 | n.d. | <1 | n.d. |
| C2 |  | 63 | >99 | <1 | n.d. | 1 | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, L.; Meng, Q.; Ramírez-Palacios, C.; Wijma, H.J.; Marrink, S.J.; Janssen, D.B. Asymmetric Synthesis of Optically Pure Aliphatic Amines with an Engineered Robust ω-Transaminase. Catalysts 2020, 10, 1310. https://doi.org/10.3390/catal10111310
Dong L, Meng Q, Ramírez-Palacios C, Wijma HJ, Marrink SJ, Janssen DB. Asymmetric Synthesis of Optically Pure Aliphatic Amines with an Engineered Robust ω-Transaminase. Catalysts. 2020; 10(11):1310. https://doi.org/10.3390/catal10111310
Chicago/Turabian StyleDong, Linhan, Qinglong Meng, Carlos Ramírez-Palacios, Hein J. Wijma, Siewert J. Marrink, and Dick B. Janssen. 2020. "Asymmetric Synthesis of Optically Pure Aliphatic Amines with an Engineered Robust ω-Transaminase" Catalysts 10, no. 11: 1310. https://doi.org/10.3390/catal10111310
APA StyleDong, L., Meng, Q., Ramírez-Palacios, C., Wijma, H. J., Marrink, S. J., & Janssen, D. B. (2020). Asymmetric Synthesis of Optically Pure Aliphatic Amines with an Engineered Robust ω-Transaminase. Catalysts, 10(11), 1310. https://doi.org/10.3390/catal10111310






