Pt-Co3O4 Superstructures by One-Pot Reduction/Precipitation in Bicontinuous Microemulsion for Electrocatalytic Oxygen Evolution Reaction
Abstract
:1. Introduction
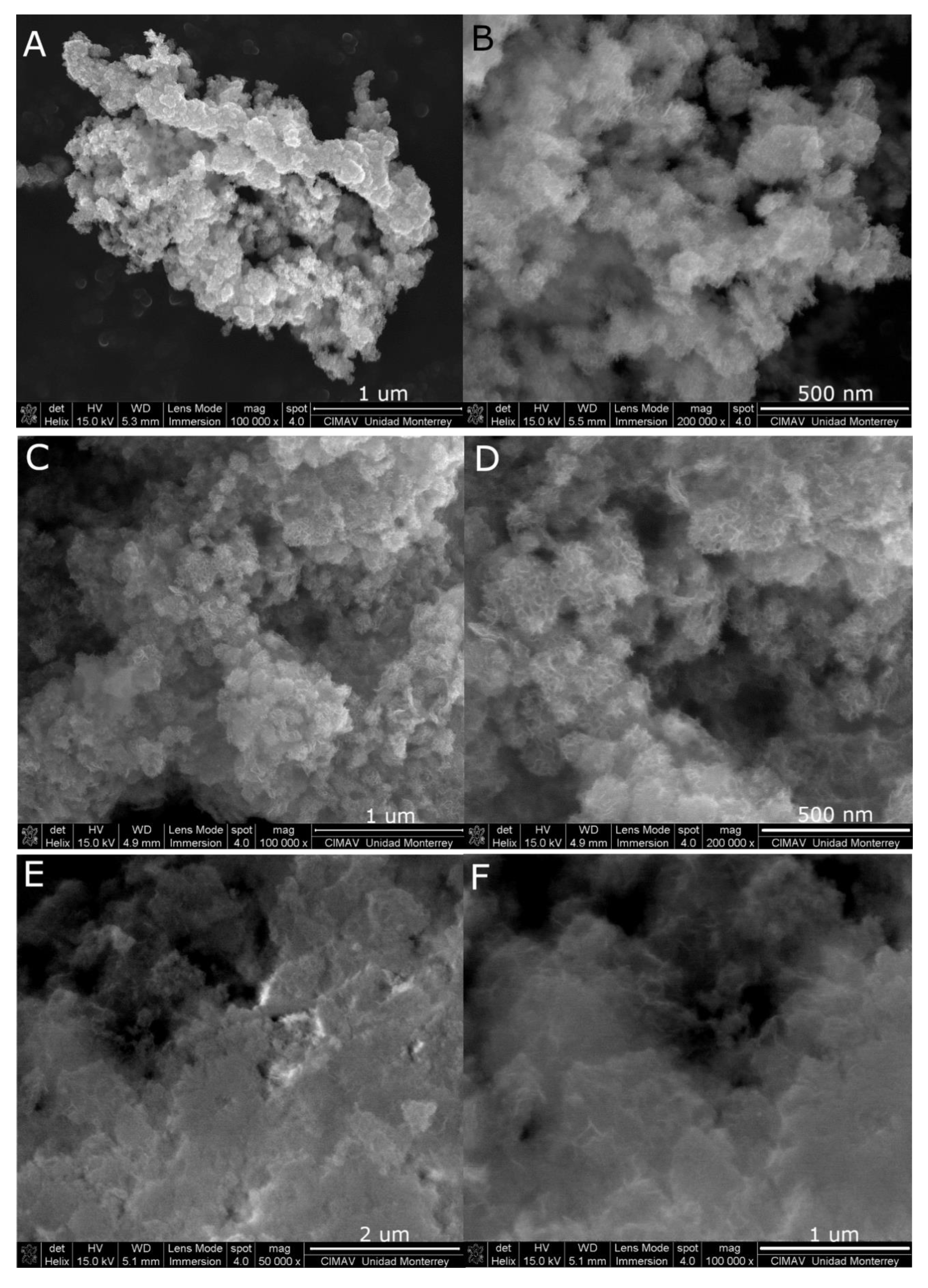
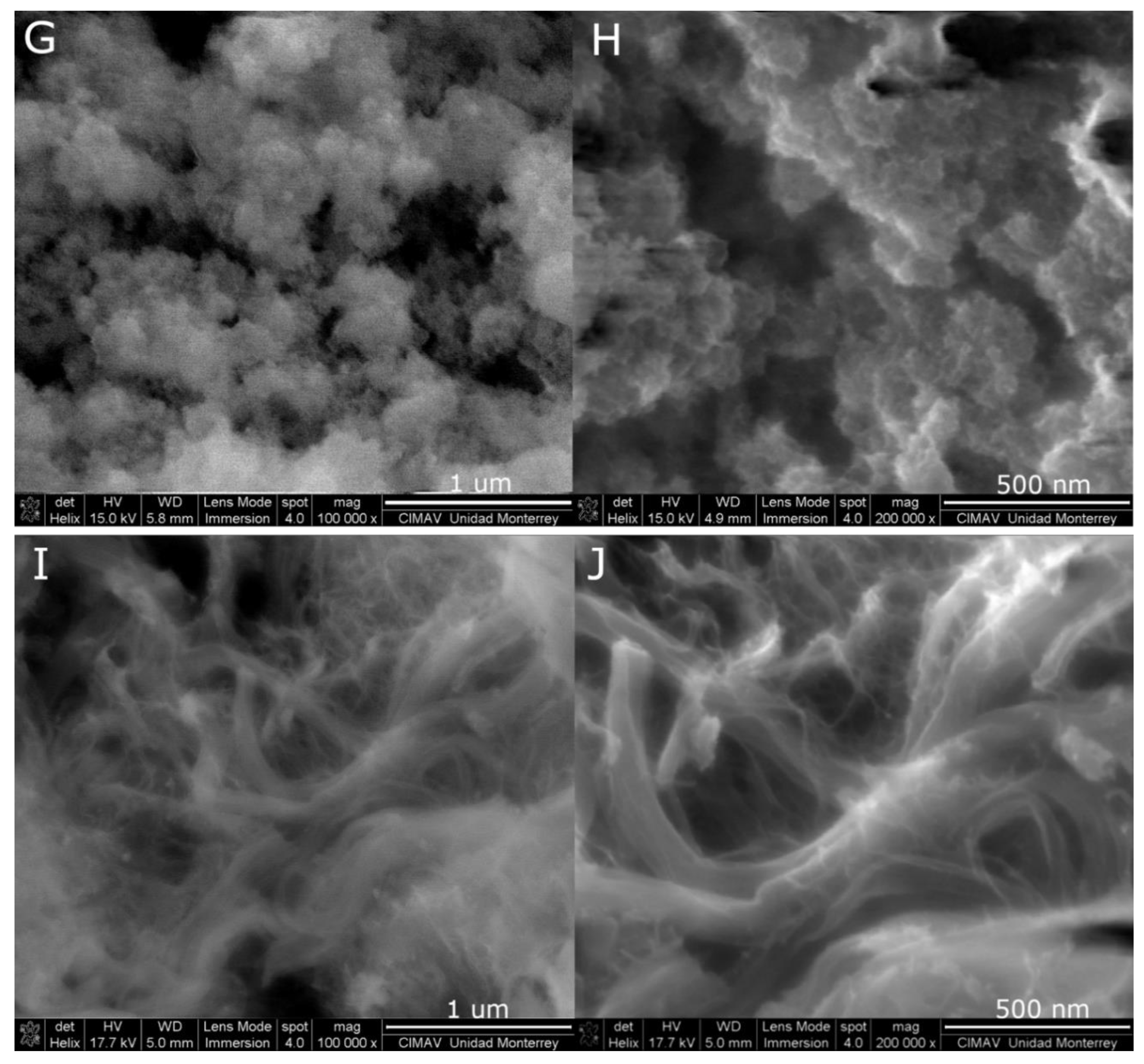
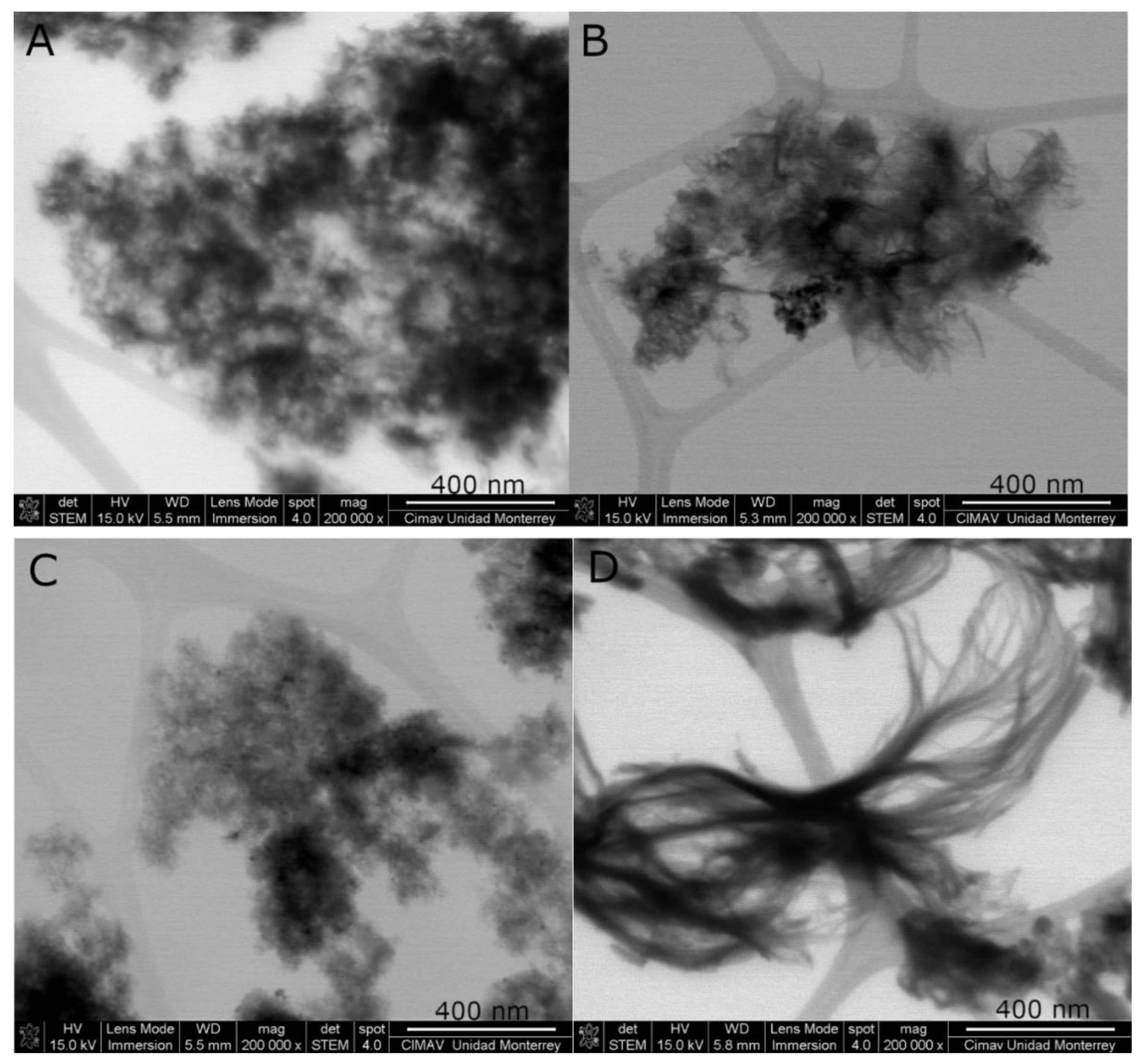
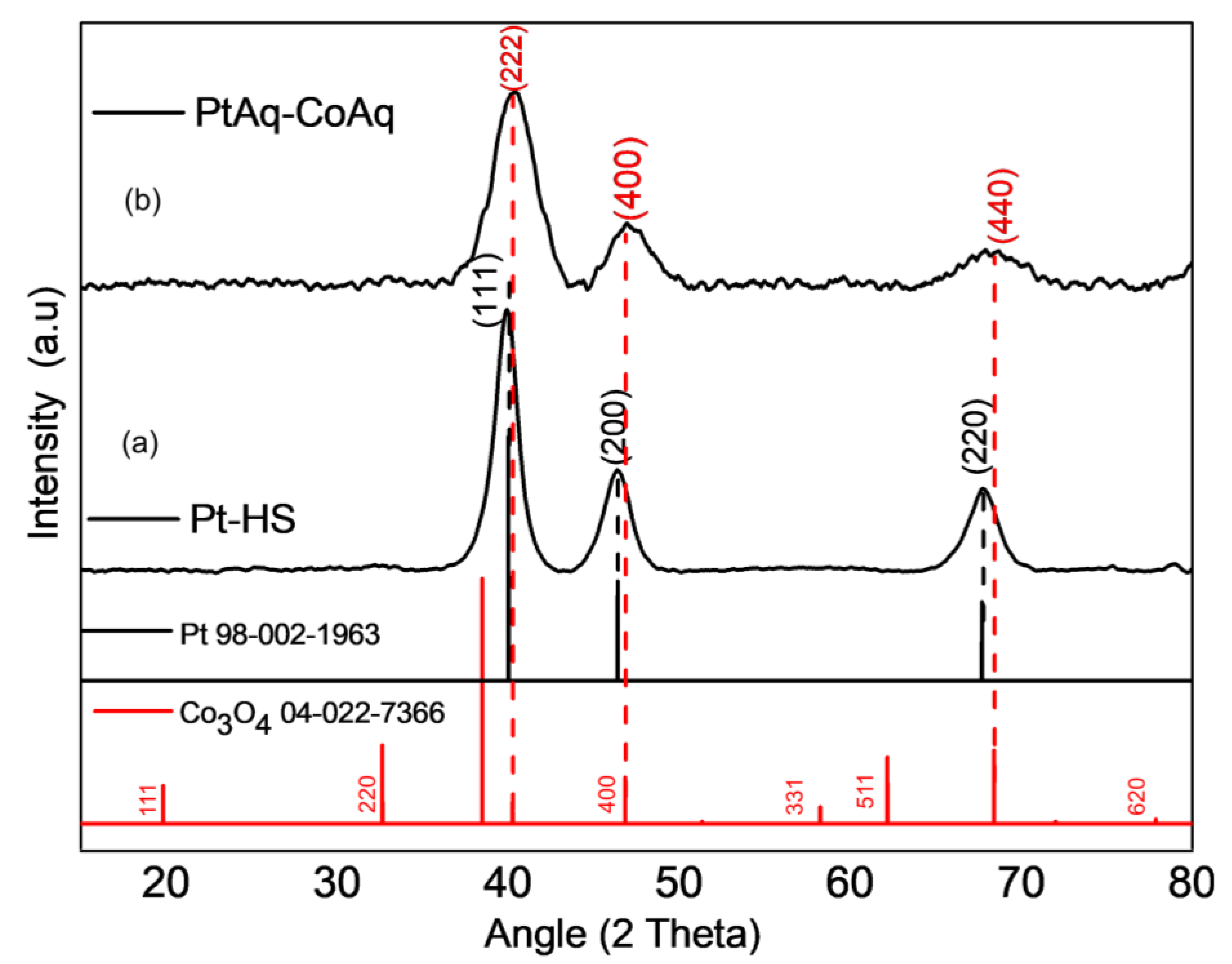
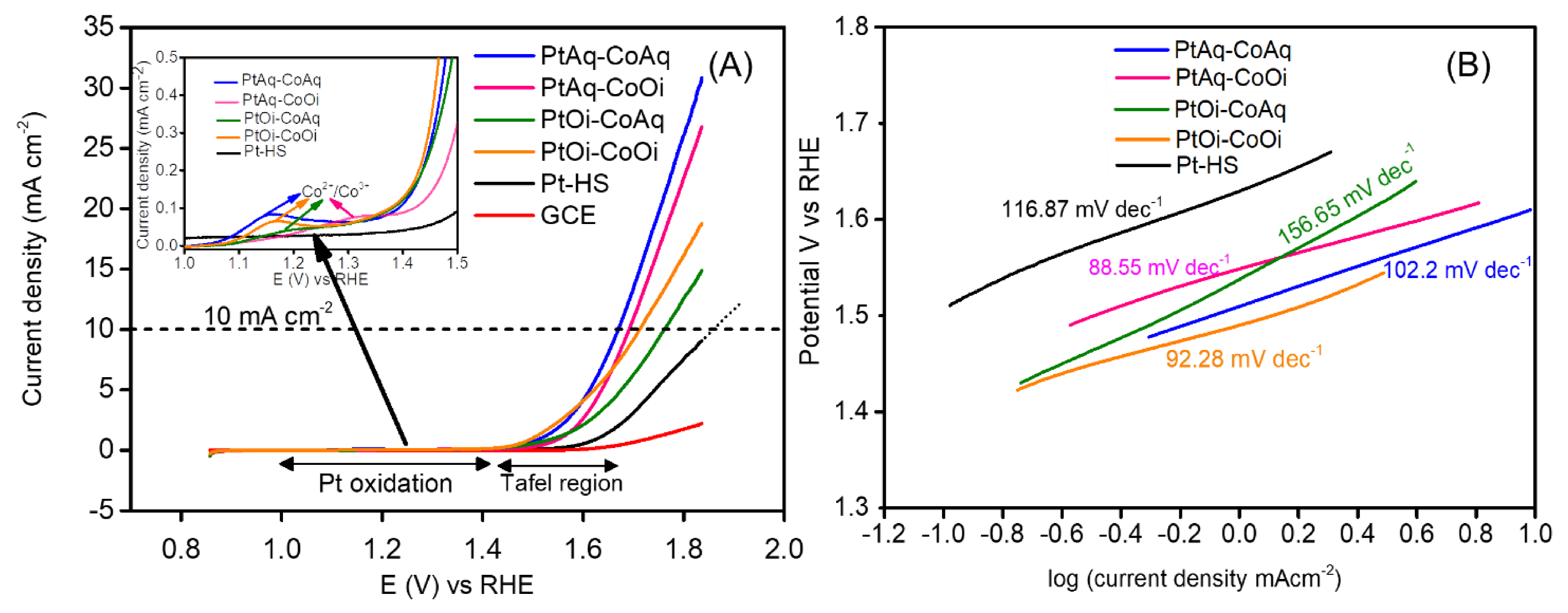
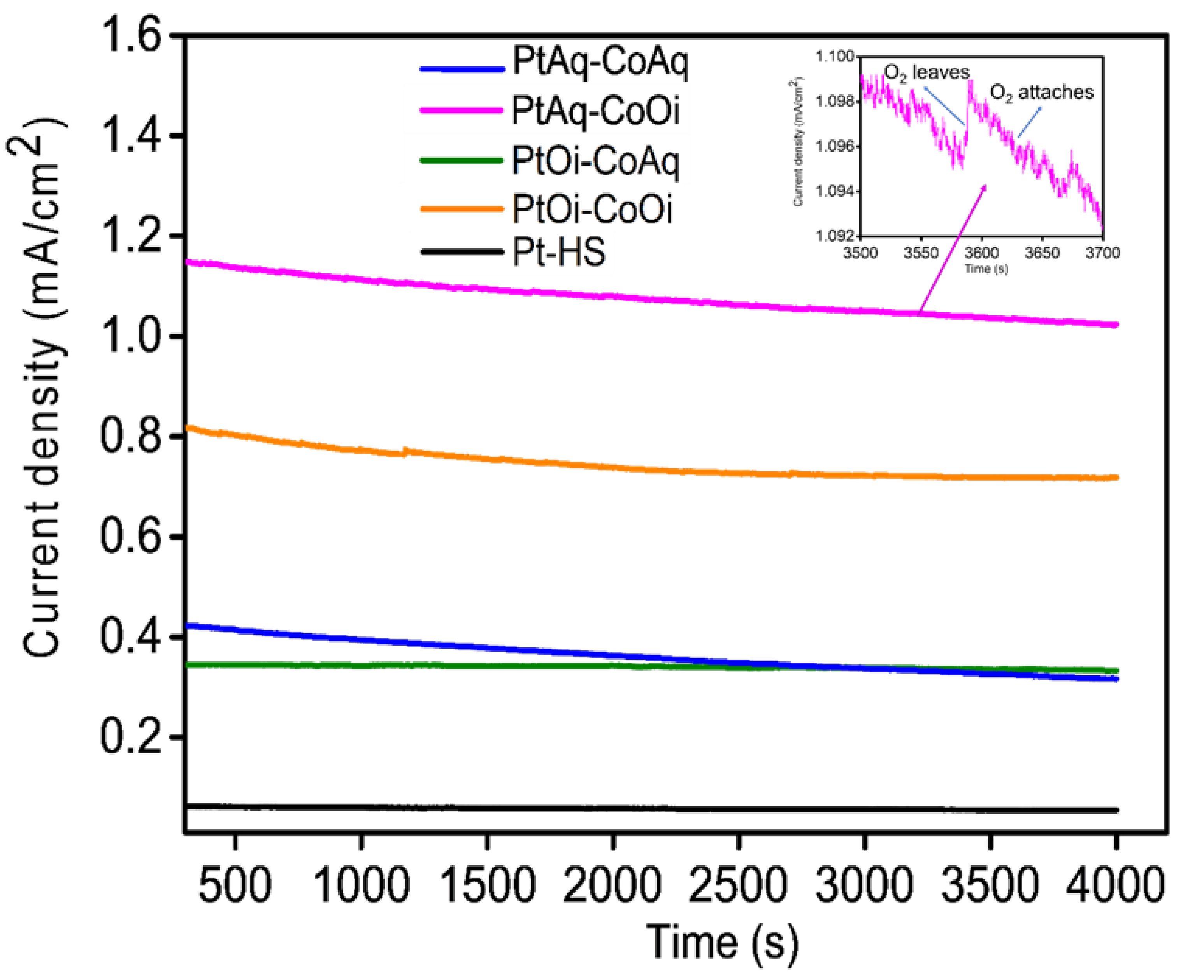
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of Pt-Co3O4-HSs and Pt-HS
- I.
- Pt and Co aqueous precursors both present in the aqueous phase is referred as PtAq-CoAq
- II.
- Pt and Co organic precursors both present in the organic phase is referred to as PtOi-CoOi
- III.
- Pt aqueous precursor and Co organic precursor is referred to as PtAq-CoOi
- IV.
- Pt organic precursor and Co aqueous precursor is referred to as PtOi-CoAq
3.3. Recovery and Isolation of Pt-Co3O4-HSs and Pt-HS
3.4. Characterization of Pt-Co3O4-HSs and Pt-HS
3.5. Electrocatalytic Characteristics of Pt-Co3O4-HSs and Pt-HS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ozoemena, K.I. Nanostructured platinum-free electrocatalysts in alkaline direct alcohol fuel cells: Catalyst design, principles and applications. RSC Adv. 2016, 6, 89523–89550. [Google Scholar] [CrossRef] [Green Version]
- Lim, T.; Sung, M.; Kim, J. Oxygen evolution reaction at microporous pt layers: Differentiated electrochemical activity between acidic and basic media. Sci. Rep. 2017, 7, 15382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.U.; Kim, B.J.; Chen, Z. One-pot synthesis of a mesoporous nico2o4 nanoplatelet and graphene hybrid and its oxygen reduction and evolution activities as an efficient bi-functional electrocatalyst. J. Mater. Chem. A 2013, 1, 4754–4762. [Google Scholar] [CrossRef]
- Qu, Q.; Zhang, J.H.; Wang, J.; Li, Q.Y.; Xu, C.W.; Lu, X. Three-dimensional ordered mesoporous co3o4 enhanced by pd for oxygen evolution reaction. Sci. Rep. 2017, 7, 41542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, M.; Sheng, W.; Zhuang, Z.; Fang, Q.; Gu, S.; Jiang, J.; Yan, Y. Efficient water oxidation using nanostructured alpha-nickel-hydroxide as an electrocatalyst. J. Am. Chem. Soc. 2014, 136, 7077–7084. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Ge, L.; Yang, Y.; Li, M.; Jia, Y.; Yao, X.; Zhu, Z. Ultrathin iron-cobalt oxide nanosheets with abundant oxygen vacancies for the oxygen evolution reaction. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, C.W.; Zhang, L.; Lee, K.; Liu, H.; Marques, A.L.; Marques, E.P.; Wang, H.; Zhang, J. A review of fe–n/c and co–n/c catalysts for the oxygen reduction reaction. Electrochim. Acta 2008, 53, 4937–4951. [Google Scholar] [CrossRef]
- Wu, L.; Liu, Z.; Xu, M.; Zhang, J.; Yang, X.; Huang, Y.; Lin, J.; Sun, D.; Xu, L.; Tang, Y. Facile synthesis of ultrathin pd–pt alloy nanowires as highly active and durable catalysts for oxygen reduction reaction. Int. J. Hydrogen Energy 2016, 41, 6805–6813. [Google Scholar] [CrossRef]
- Yuan, N.; Jiang, Q.; Li, J.; Tang, J. A review on non-noble metal based electrocatalysis for the oxygen evolution reaction. Arab. J. Chem. 2020, 13, 4294–4309. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Ye, K.-H.; Zhong, Q.-S.; Zhang, C.-J.; Shi, S.-T.; Xu, C.-W. Au-co3o4/c as an efficient electrocatalyst for the oxygen evolution reaction. ChemPlusChem 2014, 79, 1569–1572. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, Q.; Xiao, Z.; Li, X.; Huo, J.; Wang, S.; Dai, L. Plasma-engraved co3 o4 nanosheets with oxygen vacancies and high surface area for the oxygen evolution reaction. Angew. Chem. Int. Ed. Engl. 2016, 55, 5277–5281. [Google Scholar] [CrossRef]
- Ceyssens, F.; Sree, S.P.; Martens, J.; Puers, R. Fabrication of nanostructured platinum with multilevel porosity for low impedance biomedical recording and stimulation electrodes. Procedia Eng. 2015, 120, 355–359. [Google Scholar] [CrossRef] [Green Version]
- McAlpin, J.G.; Surendranath, Y.; Dinca, M.; Stich, T.A.; Stoian, S.A.; Casey, W.H.; Nocera, D.G.; Britt, R.D. Epr evidence for co (iv) species produced during water oxidation at neutral ph. J. Am. Chem. Soc. 2010, 132, 6882–6883. [Google Scholar] [CrossRef]
- Yeo, B.S.; Bell, A.T. Enhanced activity of gold-supported cobalt oxide for the electrochemical evolution of oxygen. J. Am. Chem. Soc. 2011, 133, 5587–5593. [Google Scholar] [CrossRef]
- Hao, Y.; Xu, Y.; Liu, J.; Sun, X. Nickel–cobalt oxides supported on co/n decorated graphene as an excellent bifunctional oxygen catalyst. J. Mater. Chem. A 2017, 5, 5594–5600. [Google Scholar] [CrossRef]
- Qu, Q.; Pan, G.-L.; Lin, Y.-T.; Xu, C.-W. Boosting the electrocatalytic performance of pt, pd and au embedded within mesoporous cobalt oxide for oxygen evolution reaction. Int. J. Hydrogen Energy 2018, 43, 14252–14264. [Google Scholar] [CrossRef]
- Zhuang, Z.; Sheng, W.; Yan, Y. Synthesis of monodispere au@co3o4 core-shell nanocrystals and their enhanced catalytic activity for oxygen evolution reaction. Adv. Mater. 2014, 26, 3950–3955. [Google Scholar] [CrossRef]
- Xiao, Z.; Huang, Y.-C.; Dong, C.-L.; Xie, C.; Liu, Z.; Du, S.; Chen, W.; Yan, D.; Tao, L.; Shu, Z.; et al. Operando identification of the dynamic behavior of oxygen vacancy-rich co3o4 for oxygen evolution reaction. J. Am. Chem. Soc. 2020, 142, 12087–12095. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, L.; Min, Y.; Zhang, Y. Facile synthesis of noble metal (au,pt)/co3o4 nanocomposite microspheres and their catalytic activity. Mater. Chem. Phys. 2010, 124, 1166–1171. [Google Scholar] [CrossRef]
- Grewe, T.; Deng, X.; Tüysüz, H. Influence of fe doping on structure and water oxidation activity of nanocast co3o4. Chem. Mater. 2014, 26, 3162–3168. [Google Scholar] [CrossRef]
- Lu, X.; Ng, Y.H.; Zhao, C. Gold nanoparticles embedded within mesoporous cobalt oxide enhance electrochemical oxygen evolution. ChemSusChem 2014, 7, 82–86. [Google Scholar] [CrossRef]
- Rosen, J.; Hutchings, G.S.; Jiao, F. Ordered mesoporous cobalt oxide as highly efficient oxygen evolution catalyst. J. Am. Chem. Soc. 2013, 135, 4516–4521. [Google Scholar] [CrossRef]
- Deng, X.; Ozturk, S.; Weidenthaler, C.; Tuysuz, H. Iron-induced activation of ordered mesoporous nickel cobalt oxide electrocatalyst for the oxygen evolution reaction. ACS Appl. Mater. Interfaces 2017, 9, 21225–21233. [Google Scholar] [CrossRef]
- Zhan, T.; Lu, S.; Liu, X.; Teng, H.; Hou, W. Alginate derived co3o4/co nanoparticles decorated in n-doped porous carbon as an efficient bifunctional catalyst for oxygen evolution and reduction reactions. Electrochim. Acta 2018, 265, 681–689. [Google Scholar] [CrossRef]
- Carlo, G.D.; Lualdi, M.; Venezia, A.M.; Boutonnet, M.; Sanchez-Dominguez, M. Design of cobalt nanoparticles with tailored structural and morphological properties via o/w and w/o microemulsions and their deposition onto silica. Catalysts 2015, 5, 442–459. [Google Scholar] [CrossRef]
- König, R.Y.; Schwarze, M.; Schomäcker, R.; Stubenrauch, C. Catalytic activity of mono-and bi-metallic nanoparticles synthesized via microemulsions. Catalysts 2014, 4, 256–275. [Google Scholar] [CrossRef] [Green Version]
- Serrà, A.; Vallés, E. Microemulsion-based one-step electrochemical fabrication of mesoporous catalysts. Catalysts 2018, 8, 395. [Google Scholar] [CrossRef] [Green Version]
- Adesuji, E.T.; Khalil-Cruz, L.E.; Videa, M.; Sánchez-Domínguez, M. From nano to macro: Hierarchical platinum superstructures synthesized using bicontinuous microemulsion for hydrogen evolution reaction. Electrochim. Acta 2020, 354, 136608. [Google Scholar] [CrossRef]
- Schlesinger, H.I.; Brown, H.C.; Finholt, A.E.; Gilbreath, J.R.; Hoekstra, H.R.; Hyde, E.K. Sodium borohydride, its hydrolysis and its use as a reducing agent and in the generation of hydrogen1. J. Am. Chem. Soc. 1953, 75, 215–219. [Google Scholar] [CrossRef]
- Liu, B.H.; Li, Z.P. A review: Hydrogen generation from borohydride hydrolysis reaction. J. Power Sources 2009, 187, 527–534. [Google Scholar] [CrossRef]
- Brack, P.; Dann, S.E.; Wijayantha, K.G.U. Heterogeneous and homogenous catalysts for hydrogen generation by hydrolysis of aqueous sodium borohydride (nabh4) solutions. Energy Sci. Eng. 2015, 3, 174–188. [Google Scholar] [CrossRef] [Green Version]
- Demirci, U.B.; Miele, P. Reaction mechanisms of the hydrolysis of sodium borohydride: A discussion focusing on cobalt-based catalysts. Comptes Rendus Chim. 2014, 17, 707–716. [Google Scholar] [CrossRef]
- Kaufman, C.M.; Sen, B. Hydrogen generation by hydrolysis of sodium tetrahydroborate: Effects of acids and transition metals and their salts. J. Chem. Soc. Dalton Trans. 1985, 307–313. [Google Scholar] [CrossRef]
- Folgueiras-Amador, A.A.; Jolley, K.E.; Birkin, P.R.; Brown, R.C.D.; Pletcher, D.; Pickering, S.; Sharabi, M.; de Frutos, O.; Mateos, C.; Rincón, J.A. The influence of non-ionic surfactants on electrosynthesis in extended channel, narrow gap electrolysis cells. Electrochem. Commun. 2019, 100, 6–10. [Google Scholar] [CrossRef]
- Ohki, T.; Harada, M.; Okada, T. Solvation of ions in hydrophilic layer of polyoxyethylated nonionic micelle. Cooperative approach by electrophoresis and ion-transfer voltammetry. J. Phys. Chem. B 2006, 110, 15486–15492. [Google Scholar] [CrossRef]
- Chang, L.; Cao, Y.; Fan, G.; Li, C.; Peng, W. A review of the applications of ion floatation: Wastewater treatment, mineral beneficiation and hydrometallurgy. RSC Adv. 2019, 9, 20226–20239. [Google Scholar] [CrossRef]
- Boutonnet, M.; Kizling, J.; Stenius, P.; Maire, G. The preparation of monodisperse colloidal metal particles from microemulsions. Colloids Surf. 1982, 5, 209–225. [Google Scholar] [CrossRef]
- Henglein, A.; Giersig, M. Reduction of pt(ii) by h2: Effects of citrate and naoh and reaction mechanism. J. Phys. Chem. B 2000, 104, 6767–6772. [Google Scholar] [CrossRef]
- Zeng, J. A simple eco-friendly solution phase reduction method for the synthesis of polyhedra platinum nanoparticles with high catalytic activity for methanol electrooxidation. J. Mater. Chem. 2012, 22, 3170–3176. [Google Scholar] [CrossRef]
- Sanchez-Dominguez, M.; Pemartin, K.; Boutonnet, M. Preparation of inorganic nanoparticles in oil-in-water microemulsions: A soft and versatile approach. Curr. Opin. Colloid Interface Sci. 2012, 17, 297–305. [Google Scholar] [CrossRef]
- Huang, H.; Hu, X.; Zhang, J.; Su, N.; Cheng, J. Facile fabrication of platinum-cobalt alloy nanoparticles with enhanced electrocatalytic activity for a methanol oxidation reaction. Sci. Rep. 2017, 7, 45555. [Google Scholar] [CrossRef]
- Chattot, R.; Asset, T.; Bordet, P.; Drnec, J.; Dubau, L.; Maillard, F. Beyond strain and ligand effects: Microstrain-induced enhancement of the oxygen reduction reaction kinetics on various ptni/c nanostructures. ACS Catal. 2017, 7, 398–408. [Google Scholar] [CrossRef]
- Maniammal, K.; Madhu, G.; Biju, V. X-ray diffraction line profile analysis of nanostructured nickel oxide: Shape factor and convolution of crystallite size and microstrain contributions. Phys. E Low Dimens. Syst. Nanostruct. 2017, 85, 214–222. [Google Scholar] [CrossRef] [Green Version]
- Clementi, E.; Raimondi, D.L.; Reinhardt, W.P. Atomic screening constants from scf functions. Ii. Atoms with 37 to 86 electrons. J. Chem. Phys. 1967, 47, 1300–1307. [Google Scholar] [CrossRef]
- Wang, J.; Gao, R.; Zhou, D.; Chen, Z.; Wu, Z.; Schumacher, G.; Hu, Z.; Liu, X. Boosting the electrocatalytic activity of co3o4 nanosheets for a li-o2 battery through modulating inner oxygen vacancy and exterior CO3+/CO2+ ratio. ACS Catal. 2017, 7, 6533–6541. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, X.; Mu, J.; Fan, S.; Chen, X.; Wang, L.; Yin, Z.; Tadé, M.; Liu, S. Oxygen vacancy-rich porous co3o4 nanosheets toward boosted no reduction by co and co oxidation: Insights into the structure–activity relationship and performance enhancement mechanism. ACS Appl. Mater. Interfaces 2019, 11, 41988–41999. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Fu, L.; Jiang, D.; Ouyang, J.; Hu, Y.; Yang, H.; Xi, Y. Nanoclay-modulated oxygen vacancies of metal oxide. Commun. Chem. 2019, 2, 11. [Google Scholar] [CrossRef]
- Khajehbashi, S.M.B.; Li, J.; Wang, M.; Xu, L.; Zhao, K.; Wei, Q.; Shi, C.; Tang, C.; Huang, L.; Wang, Z.; et al. A crystalline/amorphous cobalt(ii,iii) oxide hybrid electrocatalyst for lithium-air batteries. Energy Technol. 2017, 5, 568–579. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Shi, S.-T.; Zhong, Q.-S.; Zhang, C.-J.; Xu, C.-W. Pt-mn 3 o 4/c as efficient electrocatalyst for oxygen evolution reaction in water electrolysis. Electrochim. Acta 2014, 146, 119–124. [Google Scholar] [CrossRef]
- Peuckert, M.; Bonzel, H.P. Characterization of oxidized platinum surfaces by X-ray photoelectron spectroscopy. Surf. Sci. 1984, 145, 239–259. [Google Scholar] [CrossRef]
- Eberhardt, W.; Fayet, P.; Cox, D.M.; Fu, Z.; Kaldor, A.; Sherwood, R.; Sondericker, D. Photoemission from mass-selected monodispersed pt clusters. Phys. Rev. Lett. 1990, 64, 780–783. [Google Scholar] [CrossRef]
- Alexander, V.N.; Anna, K.; Stephen, W.G.; Cedric, J.P. Nist X-ray photoelectron spectroscopy database. In NIST Standard Reference Database 20, Version 4.1; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Li, Z.-Y.; Liu, Z.-L.; Liang, J.-C.; Xu, C.-W.; Lu, X. Facile synthesis of pd–mn3o4/c as high-efficient electrocatalyst for oxygen evolution reaction. J. Mater. Chem. A 2014, 2, 18236–18240. [Google Scholar] [CrossRef]
- Payne, B.P.; Biesinger, M.C.; McIntyre, N.S. X-ray photoelectron spectroscopy studies of reactions on chromium metal and chromium oxide surfaces. J. Electron. Spectrosc. Relat. Phenom. 2011, 184, 29–37. [Google Scholar] [CrossRef]
- Aricò, A.S.; Shukla, A.K.; Kim, H.; Park, S.; Min, M.; Antonucci, V. An xps study on oxidation states of pt and its alloys with co and cr and its relevance to electroreduction of oxygen. Appl. Surf. Sci. 2001, 172, 33–40. [Google Scholar] [CrossRef]
- Xin, W.-L.; Lu, K.-K.; Zhu, D.-R.; Zeng, H.-B.; Zhang, X.-J.; Marks, R.-S.; Shan, D. Highly reactive n,n’-carbonyldiimidazole-tailored bifunctional electrocatalyst for oxygen reduction and oxygen evolution. Electrochim. Acta 2019, 307, 375–384. [Google Scholar] [CrossRef]
- Kuznetsov, D.A.; Naeem, M.A.; Kumar, P.V.; Abdala, P.M.; Fedorov, A.; Müller, C.R. Tailoring lattice oxygen binding in ruthenium pyrochlores to enhance oxygen evolution activity. J. Am. Chem. Soc. 2020, 142, 7883–7888. [Google Scholar] [CrossRef]
- Ramos-Moore, E.; Ferrari, P.; Diaz-Droguett, D.E.; Lederman, D.; Evans, J.T. Raman and X-ray photoelectron spectroscopy study of ferroelectric switching in pb(nb,zr,ti)o3 thin films. J. Appl. Phys. 2012, 111, 014108. [Google Scholar] [CrossRef] [Green Version]
- Bajdich, M.; Garcia-Mota, M.; Vojvodic, A.; Norskov, J.K.; Bell, A.T. Theoretical investigation of the activity of cobalt oxides for the electrochemical oxidation of water. J. Am. Chem. Soc. 2013, 135, 13521–13530. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhou, T.; Jiang, K.; Da, P.; Peng, Z.; Tang, J.; Kong, B.; Cai, W.-B.; Yang, Z.; Zheng, G. Reduced mesoporous co3o4nanowires as efficient water oxidation electrocatalysts and supercapacitor electrodes. Adv. Energy Mater. 2014, 4. [Google Scholar] [CrossRef]
- Kunhiraman, A.K. Hydrogen evolution reaction catalyzed by platinum nanoislands decorated on three-dimensional nanocarbon hybrid. Ionics 2019, 25, 3787–3797. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Ferrer, I.M.; Chatman, S.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices. J. Am. Chem. Soc. 2015, 137, 4347–4357. [Google Scholar] [CrossRef] [Green Version]
- Gong, H.; Zhang, W.; Li, F.; Yang, R. Enhanced electrocatalytic performance of self-supported aucuco for oxygen reduction and evolution reactions. Electrochim. Acta 2017, 252, 261–267. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wang, W.; Zhang, L.; Jiang, D. Surface oxygen vacancies on co3o4 mediated catalytic formaldehyde oxidation at room temperature. Catal. Sci. Technol. 2016, 6, 3845–3853. [Google Scholar] [CrossRef]
- Han, X.; Cheng, F.; Zhang, T.; Yang, J.; Hu, Y.; Chen, J. Hydrogenated uniform pt clusters supported on porous camno(3) as a bifunctional electrocatalyst for enhanced oxygen reduction and evolution. Adv. Mater. 2014, 26, 2047–2051. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Ji, Y.; Xie, M.; You, C.; Yang, L.; Liu, Z.; Asiri, A.M.; Sun, X. Mno2-cop3 nanowires array: An efficient electrocatalyst for alkaline oxygen evolution reaction with enhanced activity. Electrochem. Commun. 2018, 86, 161–165. [Google Scholar] [CrossRef]
- Zhong, L.; Bao, Y.; Feng, L. Fe-doping effect on cote catalyst with greatly boosted intrinsic activity for electrochemical oxygen evolution reaction. Electrochim. Acta 2019, 321. [Google Scholar] [CrossRef]
- Su, Y.; Zhu, Y.; Jiang, H.; Shen, J.; Yang, X.; Zou, W.; Chen, J.; Li, C. Cobalt nanoparticles embedded in n-doped carbon as an efficient bifunctional electrocatalyst for oxygen reduction and evolution reactions. Nanoscale 2014, 6, 15080–15089. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Jiang, R.; Chen, X.; Chen, Y.; Wang, L. 3d nickel-cobalt diselenide nanonetwork for highly efficient oxygen evolution. Sci. Bull. 2017, 62, 1373–1379. [Google Scholar] [CrossRef]


| Sample | Pt/Co Mole Ratio | pH BCME | ||
|---|---|---|---|---|
| Nominal | Experimental (ICP-AES) | Before NaBH4 Addition | After NaBH4 Addition | |
| PtAq-CoAq | 0.30 | 1.55 | 1.57 | 6.36 |
| PtAq-CoOi | 0.12 | 0.43 | 6.60 | 8.66 |
| PtOi-CoAq | 0.76 | 0.17 | 4.10 | 10.17 |
| PtOi-CoOi | 0.30 | 0.12 | 7.34 | 10.30 |
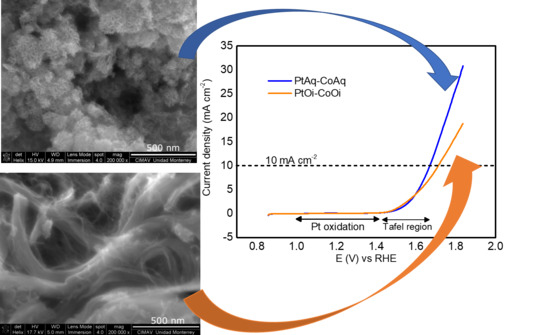
| Electrocatalyst | Overpotential at 10 mA cm−2 (V) η10 | Onset (V) vs. RHE | Tafel Slope mV dec−1 |
|---|---|---|---|
| PtAq-CoAq | 0.381 | 1.49 | 102 |
| PtAq-CoOi | 0.406 | 1.50 | 89 |
| PtOi-CoAq | 0.513 | 1.48 | 157 |
| PtOi-CoOi | 0.411 | 1.46 | 92 |
| Pt-HS | 0.627 | 1.56 | 117 |
| Electrocatalysts | Electrolyte KOH | Onset Potential (V vs. RHE) | Overpotential (V) at 10 mA cm−2 | Tafel Slope (mV dec−1) | Reference |
|---|---|---|---|---|---|
| PtAq-CoAq | 0.1 M | 1.49 | 0.381 | 102 | This work |
| PtAq-CoOi | 1.50 | 0.406 | 89 | This work | |
| PtOi-CoAq | 1.48 | 0.513 | 157 | This work | |
| PtOi-CoOi | 1.46 | 0.411 | 92 | This work | |
| Pt-HS | 1.56 | 0.627 | 117 | This work | |
| CoOx/Co-N-C | 0.1 M | 1.52 | 0.421 | 124 | [56] |
| Co-N-C | 1.60 | 0.561 | 143 | ||
| AuCuCo | 0.1 M | 1.57 | 0.596 | 160 | [63] |
| AuCu | 1.63 | 0.724 | 238 | ||
| Pt/C | 1.74 | - | 233 | ||
| Co3O4/Co-NPC | 0.1 M | 1.45 | 0.277 | 105 | [24] |
| IrO2 | 1.52 | 0.394 | 169 | ||
| Pristine-Co3O4 | 0.1 M | 1.50 | 0.541 | 234 | [11] |
| Plasma-Co3O4 | 1.45 | 0.301 | 68 | ||
| NixCoyO4/Co-NG | 0.1 M | 1.52 | 0.4 | 77 | [15] |
| NixCoyO4/Co-NG | 1.59 | 0.448 | 100 | ||
| Pt | 1.70 | 0.497 | 151 | ||
| Pt-Co3O4 | 0.1 M | 1.383 | 0.254 | 58 | [16] |
| Pd-Co3O4 | 1.388 | - | 61 | ||
| Au-Co3O4 | 1.395 | - | 68 | ||
| Co3O4 | 1.481 | - | 72 | ||
| NiCo2O4 | 0.1 M | 1.593 | 0.424 | 205 | [3] |
| NiCo2O4-G | 1.523 | 0.564 | 161 | ||
| CaMnO3 | 0.1 M | 1.66 | - | [66] | |
| H-Pt/CaMnO3 | 1.58 | 0.581 | - | ||
| Pt/CaMnO3 | 1.58 | 0.631 | - | ||
| MnO2/Ti | 1.0 M | 1.6 | 0.408 | 241 | [67] |
| CoP3/Ti | 1.54 | 0.322 | 276 | ||
| RuO2/Ti | 1.45 | 0.24 | - | ||
| MnO2-CoP3/Ti | 1.545 | 0.288 | 65 | ||
| CoTe | 1.0 M | 1.6 | 0.445 | 93 | [68] |
| Fe-CoTe | 1.5 | 0.3 | 45 | ||
| Au/C | 0.1 M | 1.568 | - | - | [10] |
| Co3O4/C | 1.518 | - | - | ||
| Au-Co3O4/C | 1.507 | - | - | ||
| Fe1Co1-ONS | 0.1 M | 1.46 | 0.308 | 37 | [6] |
| Fe1Co1-ONP | 1.56 | 0.4 | 72 | ||
| Co3O4 | 0.1 M | 1.481 | - | 72 | [4] |
| Pd-Co3O4 | 1.388 | - | 61 | ||
| Co/N-C-800 | 0.1 M | 1.53 | 0.371 | 61 | [69] |
| Co/N-C-800-250 | 1.53 | 0.424 | 116 | ||
| Co/N-C-800-450 | 1.52 | 0.398 | 74 | ||
| Pt/C | 1.65 | 0.629 | 170 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adesuji, E.T.; Guardado-Villegas, E.; Fuentes, K.M.; Sánchez-Domínguez, M.; Videa, M. Pt-Co3O4 Superstructures by One-Pot Reduction/Precipitation in Bicontinuous Microemulsion for Electrocatalytic Oxygen Evolution Reaction. Catalysts 2020, 10, 1311. https://doi.org/10.3390/catal10111311
Adesuji ET, Guardado-Villegas E, Fuentes KM, Sánchez-Domínguez M, Videa M. Pt-Co3O4 Superstructures by One-Pot Reduction/Precipitation in Bicontinuous Microemulsion for Electrocatalytic Oxygen Evolution Reaction. Catalysts. 2020; 10(11):1311. https://doi.org/10.3390/catal10111311
Chicago/Turabian StyleAdesuji, Elijah T., Esther Guardado-Villegas, Keyla M. Fuentes, Margarita Sánchez-Domínguez, and Marcelo Videa. 2020. "Pt-Co3O4 Superstructures by One-Pot Reduction/Precipitation in Bicontinuous Microemulsion for Electrocatalytic Oxygen Evolution Reaction" Catalysts 10, no. 11: 1311. https://doi.org/10.3390/catal10111311