Recent Advances in Continuous-Flow Reactions Using Metal-Free Homogeneous Catalysts
Abstract
:1. Introduction
2. Continuous-Flow Reactions Using Metal-Free Homogeneous Catalysts
2.1. Acidic Catalysts
2.1.1. Tropylium-Catalyzed Acetalization Reactions
2.1.2. Tritylium-Catalyzed Interrupted Povarov Reactions
2.1.3. Chlorination/Epoxidation of Biobased Glycerol
2.1.4. Retro-Claisen-Type C–C Bond Cleavage of Diketones with Tropylium Catalyst
2.1.5. Sustainable Continuous-Flow Synthesis of Allantoin
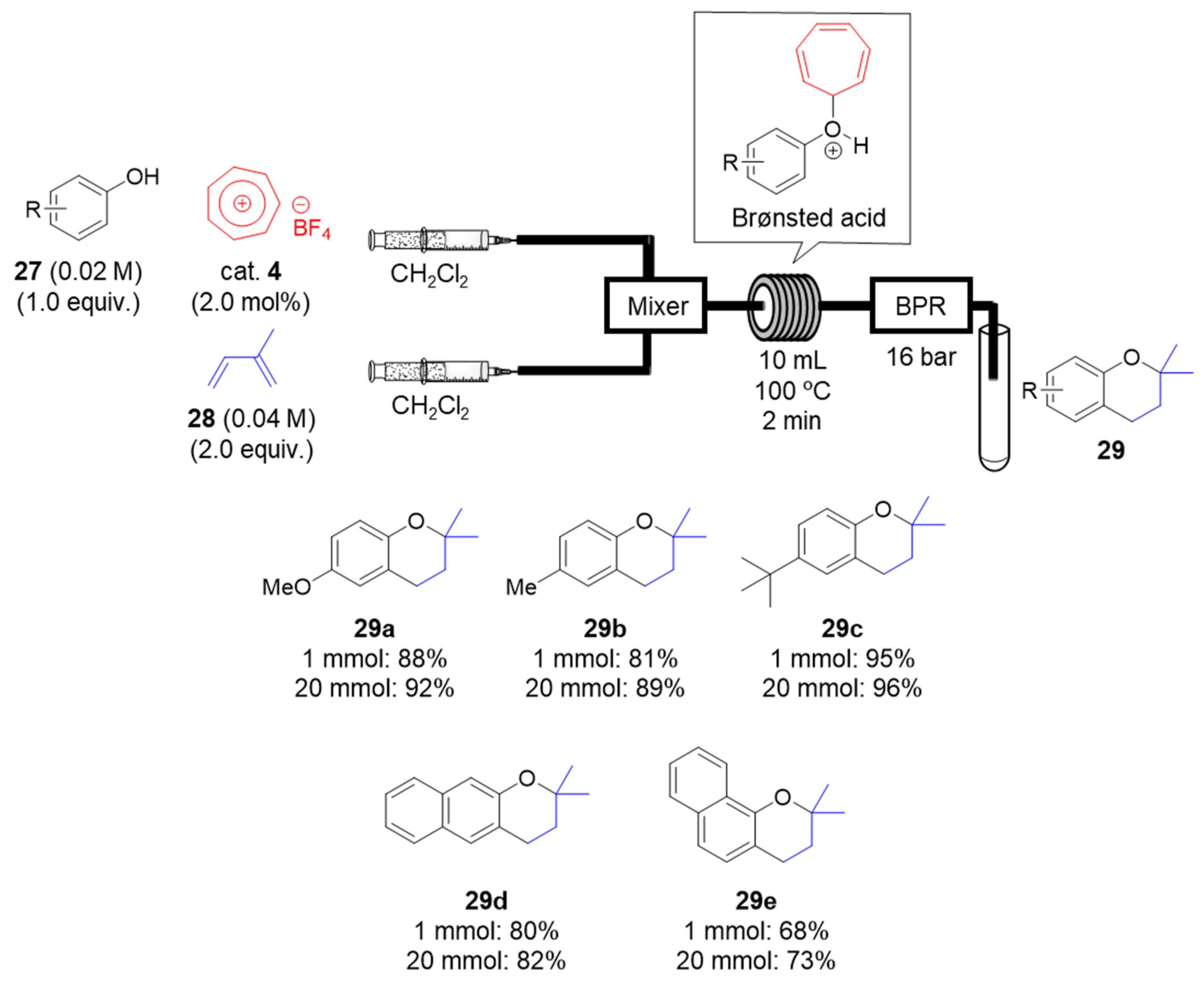
2.1.6. Tropylium-Promoted Prenylation Reactions of Phenols in Flow
2.2. Basic Catalysts
2.2.1. Organocatalytic α-Trifluoromethylthiolation of Silylenol Ethers
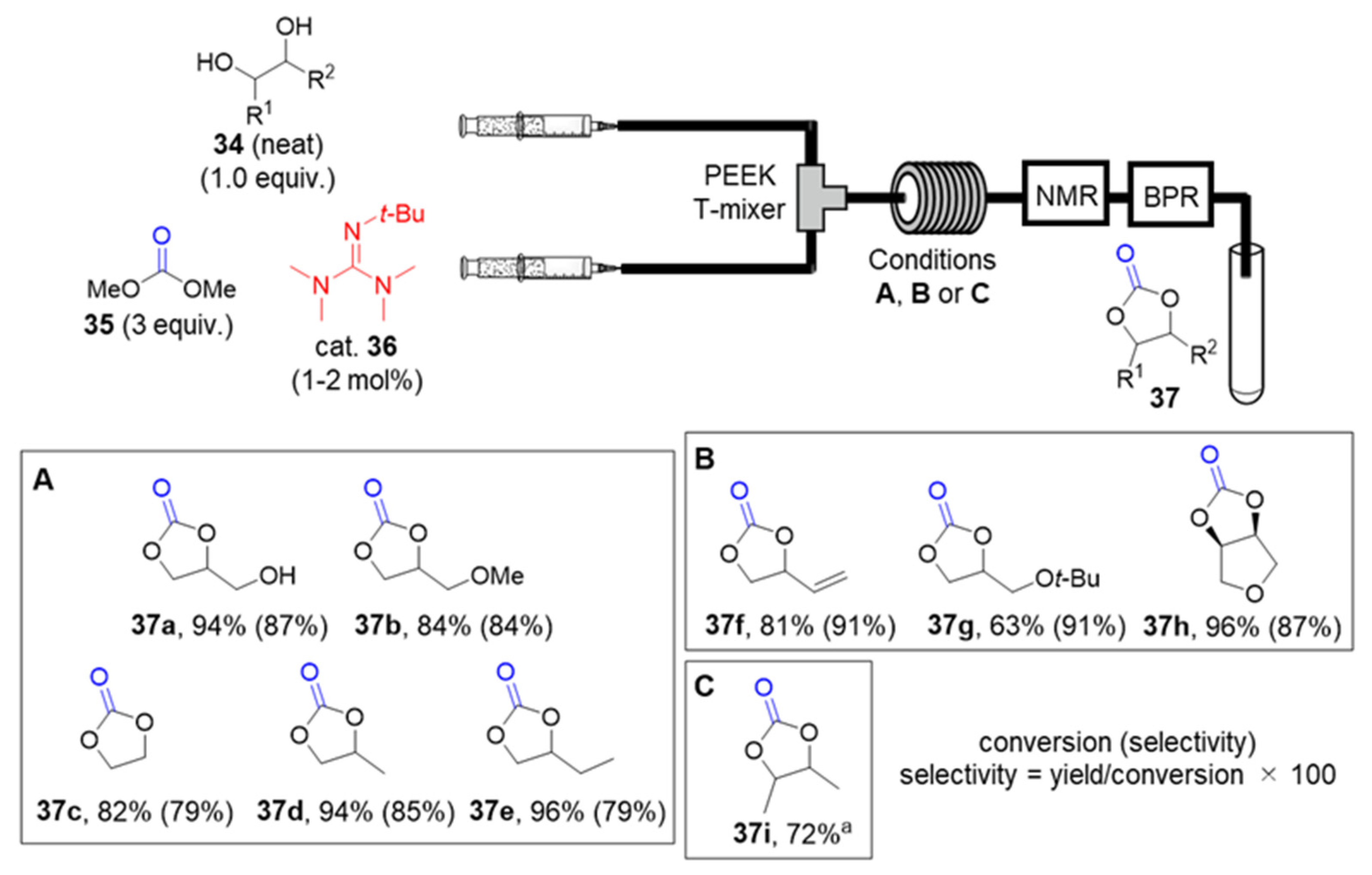
2.2.2. Solvent-Free Organocatalytic Synthesis of Cyclic Carbonates
2.2.3. Organocatalyzed Decarboxylative Trichloromethylation of Morita-Baylis-Hillman Adducts
2.2.4. Micro-Flow Synthesis of β-Amino Acid Derivatives via a Rapid Dual Activation Approach
2.3. Miscellaneous Catalysts
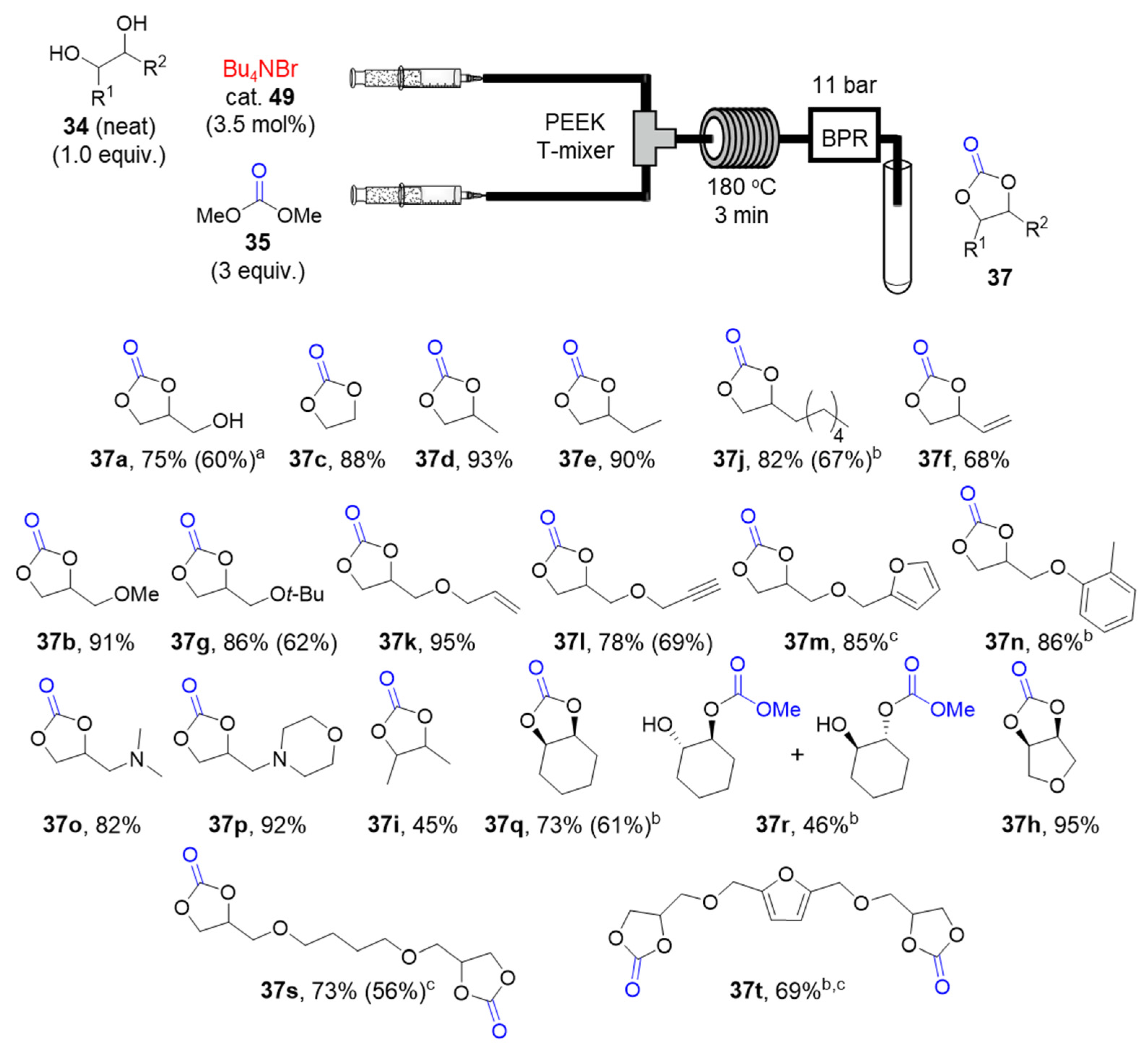
2.3.1. Organocatalytic Synthesis of Cyclic Carbonates under Continuous-Flow Conditions
2.3.2. Asymmetric Organocatalytic Aldol Reaction of a Hydrophobic Aldehyde
2.3.3. Organocatalytic Michael Addition of β-Ketoester to Nitroalkene
2.3.4. Exploration of an Enantioselective Organocatalyzed Rauhut-Currier Reaction and [3 + 2] Annulation under Flow Conditions Using Machine-Learning
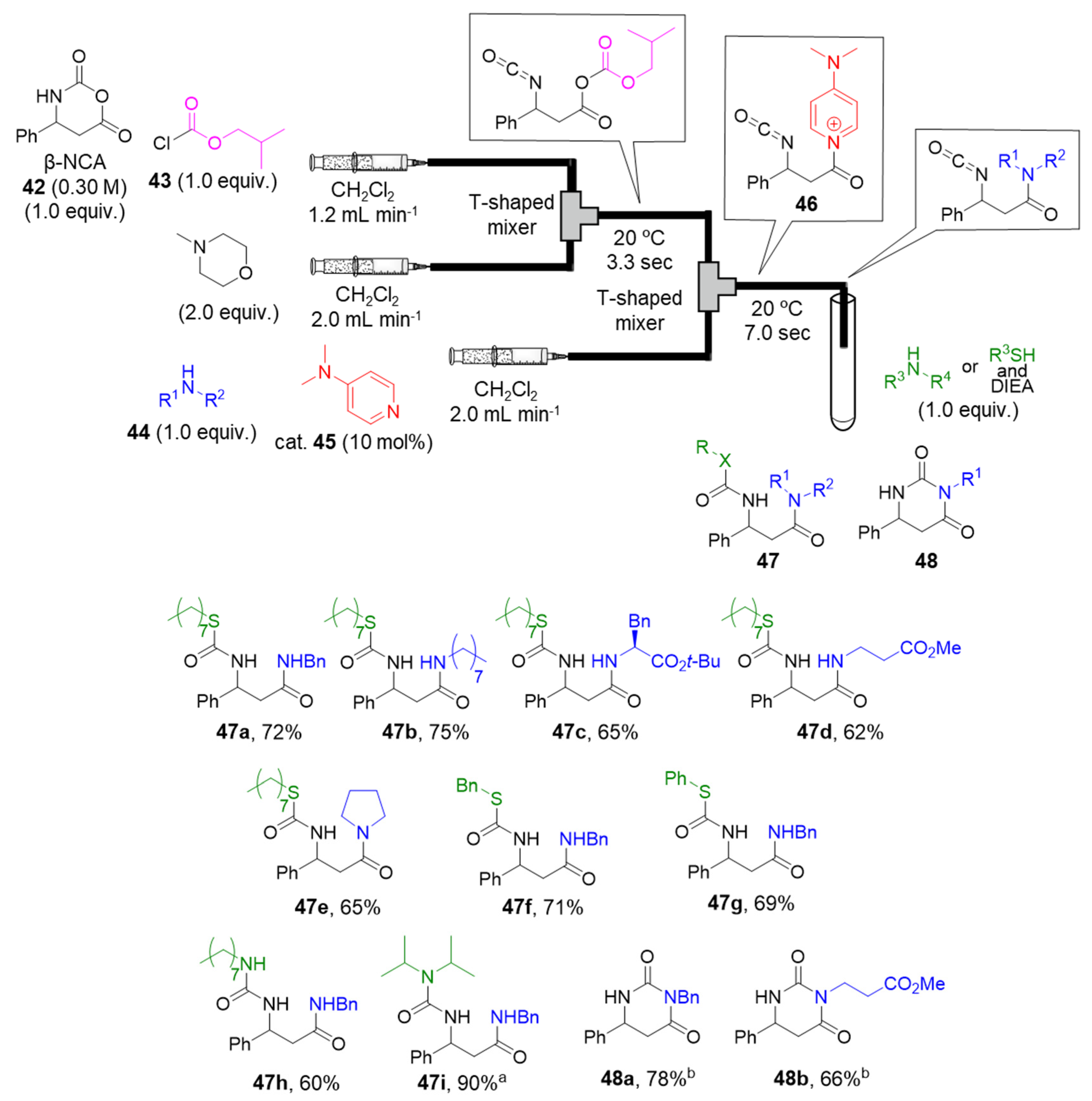
2.3.5. N-Methylated Peptide Synthesis via Acyl N-Methylimidazolium Cation Generation Accelerated by a Brønsted Acid
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yoshida, J.-I. Flash Chemistry: Fast Organic Synthesis in Micro Systems; Wiley VCH: Weinheim, Germany, 2008. [Google Scholar]
- Yoshida, J.-I.; Nagaki, A.; Yamada, T. Flash chemistry: Fast chemical synthesis by using microreactors. Chem. A Eur. J. 2008, 14, 7450–7459. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, J.-I. Flash chemistry: Flow microreactor synthesis based on high-resolution reaction time control. Chem. Rec. 2010, 10, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, J.-I.; Takahashi, Y.; Nagaki, A. Flash chemistry: Flow chemistry that cannot be done in batch. Chem. Commun. 2013, 49, 9896–9904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ley, S.V.; Fitzpatrick, D.E.; Ingham, R.; Myers, R.M. Organic synthesis: March of the machines. Angew. Chem. Int. Ed. 2015, 54, 3449–3464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumann, M.; Baxendale, I.R. The synthesis of active pharmaceutical ingredients (APIs) using continuous flow chemistry. Beilstein J. Org. Chem. 2015, 11, 1194–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutmann, B.; Cantillo, D.; Kappe, C.O. Continuous-flow technology—A tool for the safe manufacturing of active pharmaceutical ingredients. Angew. Chem. Int. Ed. 2015, 54, 6688–6728. [Google Scholar] [CrossRef]
- Fabry, D.C.; Sugiono, E.; Rueping, M. Online monitoring and analysis for autonomous continuous flow self-optimizing reactor systems. React. Chem. Eng. 2016, 1, 129–133. [Google Scholar] [CrossRef]
- Kobayashi, S. Flow “Fine” synthesis: High yielding and selective organic synthesis by flow methods. Chem. Asian J. 2016, 11, 425–436. [Google Scholar] [CrossRef]
- Rossetti, I.; Compagnoni, M. Chemical reaction engineering, process design and scale-up issues at the frontier of synthesis: Flow chemistry. Chem. Eng. J. 2016, 296, 56–70. [Google Scholar] [CrossRef]
- Fanelli, F.; Parisi, G.; DeGennaro, L.; Luisi, R. Contribution of microreactor technology and flow chemistry to the development of green and sustainable synthesis. Beilstein J. Org. Chem. 2017, 13, 520–542. [Google Scholar] [CrossRef] [Green Version]
- Shukla, C.A.; Kulkarni, A.A. Automating multistep flow synthesis: Approach and challenges in integrating chemistry, machines and logic. Beilstein J. Org. Chem. 2017, 13, 960–987. [Google Scholar] [CrossRef] [PubMed]
- Plutschack, M.B.; Pieber, B.; Gilmore, K.; Seeberger, P.H. The Hitchhiker’s guide to flow chemistry. Chem. Rev. 2017, 117, 11796–11893. [Google Scholar] [CrossRef] [PubMed]
- Britton, J.; Raston, C.L. Multi-step continuous-flow synthesis. Chem. Soc. Rev. 2017, 46, 1250–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gérardy, R.; Emmanuel, N.; Toupy, T.; Kassin, V.-E.; Tshibalonza, N.N.; Schmitz, M.; Monbaliu, J.-C.M. Continuous flow organic chemistry: Successes and pitfalls at the interface with current societal challenges. Eur. J. Org. Chem. 2018, 2018, 2301–2351. [Google Scholar] [CrossRef]
- Zhao, D.; Ding, K. Recent advances in asymmetric catalysis in flow. ACS Catal. 2013, 3, 928–944. [Google Scholar] [CrossRef]
- Finelli, F.G.; Miranda, L.S.M.; De Souza, R.O.M.A. Expanding the toolbox of asymmetric organocatalysis by continuous-flow process. Chem. Commun. 2015, 51, 3708–3722. [Google Scholar] [CrossRef] [PubMed]
- Atodiresei, I.; Vila, C.; Rueping, M. Asymmetric organocatalysis in continuous flow: Opportunities for impacting industrial catalysis. ACS Catal. 2015, 5, 1972–1985. [Google Scholar] [CrossRef]
- Len, C.; Bruniaux, S.; Delbecq, F.; Parmar, V.S. Palladium-catalyzed suzuki–miyaura cross-coupling in continuous flow. Catalysts 2017, 7, 146. [Google Scholar] [CrossRef]
- De Risi, C.; Bortolini, O.; Brandolese, A.; Di Carmine, G.; Ragno, D.; Massi, A. Recent advances in continuous-flow organocatalysis for process intensification. React. Chem. Eng. 2020, 5, 1017–1052. [Google Scholar] [CrossRef]
- Yu, T.; Ding, Z.; Nie, W.; Jiao, J.; Zhang, H.; Zhang, Q.; Xue, C.; Duan, X.-H.; Yamada, Y.M.A.; Li, P. Recent advances in continuous-flow enantioselective catalysis. Chem. Eur. J. 2020, 26, 5729–5747. [Google Scholar] [CrossRef]
- Koóš, P.; Markovič, M.; Lopatka, P.; Gracza, T. Recent applications of continuous flow in homogeneous palladium catalysis. Synthesis 2020, 52. [Google Scholar] [CrossRef]
- Lyons, D.J.M.; Crocker, R.D.; Enders, D.; Nguyen, T.V. Tropylium salts as efficient organic Lewis acid catalysts for acetalization and transacetalization reactions in batch and flow. Green Chem. 2017, 19, 3993–3996. [Google Scholar] [CrossRef]
- Lyons, D.J.M.; Crocker, R.D.; Blümel, M.; Nguyen, T.V. Promotion of organic reactions by non-benzenoid carbocyclic aromatic ions. Angew. Chem. Int. Ed. 2017, 56, 1466–1484. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, J.; Li, Z.; Huang, Y.; Wang, H.; Gao, Y.; Guo, T.-F.; Ouyang, P.; Guo, K. Carbocation organocatalysis in interrupted povarov reactions to cis-fused pyrano-and furanobenzodihydropyrans. Eur. J. Org. Chem. 2017, 2017, 3996–4003. [Google Scholar] [CrossRef]
- Horn, M.; Mayr, H. A comprehensive view on stabilities and reactivities of triarylmethyl cations (tritylium ions). J. Phys. Org. Chem. 2012, 25, 979–988. [Google Scholar] [CrossRef]
- Naidu, V.R.; Ni, S.; Franzen, J. The carbocation: A forgotten lewis acid catalyst. ChemCatChem 2015, 7, 1896–1905. [Google Scholar] [CrossRef]
- Maji, B.; Mayr, H. Nucleophilic reactivities of schiff bases. Z. Nat. B 2013, 68, 693–699. [Google Scholar] [CrossRef]
- Follet, E.; Mayer, P.; Berionni, G. Structures, lewis acidities, electrophilicities, and protecting group abilities of phenyl-fluorenylium and tritylium ions. Chem. Eur. J. 2016, 23, 623–630. [Google Scholar] [CrossRef]
- Morodo, R.; Gérardy, R.; Petit, G.; Monbaliu, J.-C.M. Continuous flow upgrading of glycerol toward oxiranes and active pharmaceutical ingredients thereof. Green Chem. 2019, 21, 4422–4433. [Google Scholar] [CrossRef]
- Santacesaria, E.; Vitiello, R.; Tesser, R.; Russo, V.; Turco, R.; Di Serio, M. Chemical and technical aspects of the synthesis of chlorohydrins from glycerol. Ind. Eng. Chem. Res. 2013, 53, 8939–8962. [Google Scholar] [CrossRef] [Green Version]
- Bell, B.M.; Briggs, J.R.; Campbell, R.M.; Chambers, S.M.; Gaarenstroom, P.D.; Hippler, J.G.; Hook, B.D.; Kearns, K.; Kenney, J.M.; Kruper, W.J.; et al. Glycerin as a renewable feedstock for epichlorohydrin production. The GTE process. CLEAN Soil Air Water 2008, 36, 657–661. [Google Scholar] [CrossRef]
- Hou, X.; Fu, Y.; Zhu, X.; Yin, H.; Wang, A. Chlorination of glycerol with HCl to 1,3-dichloro-2-propanol catalyzed by aliphatic carboxylic acids. Asia Pac. J. Chem. Eng. 2015, 10, 626–632. [Google Scholar] [CrossRef]
- Filho, C.A.D.A.; Heredia, S.; Eränen, K.; Salmi, T. Advanced millireactor technology for the kinetic investigation of very rapid reactions: Dehydrochlorination of 1,3-dichloro-2-propanol to epichlorohydrin. Chem. Eng. Sci. 2016, 149, 35–41. [Google Scholar] [CrossRef]
- Hussein, M.A.; Huynh, V.T.; Hommelsheim, R.; Koenigs, R.M.; Nguyen, T.V. An efficient method for retro-Claisen-type C–C bond cleavage of diketones with tropylium catalyst. Chem. Commun. 2018, 54, 12970–12973. [Google Scholar] [CrossRef]
- Salvadeo, E.; Monbaliu, J.-C.M. Development of a sustainable continuous flow approach toward allantoin. J. Flow Chem. 2020, 10, 251–257. [Google Scholar] [CrossRef]
- Omoregbee, K.; Luc, K.N.H.; Dinh, A.H.; Nguyen, T.V. Tropylium-promoted prenylation reactions of phenols in continuous flow. J. Flow Chem. 2020, 10, 161–166. [Google Scholar] [CrossRef]
- Dang, T.T.; Boeck, F.; Hintermann, L. Hidden brønsted acid catalysis: Pathways of accidental or deliberate generation of triflic acid from metal triflates. J. Org. Chem. 2011, 76, 9353–9361. [Google Scholar] [CrossRef]
- Abubakar, S.S.; Benaglia, M.; Rossi, S.; Annunziata, R. Organocatalytic α-trifluoromethylthiolation of silylenol ethers: Batch vs. continuous flow reactions. Catal. Today 2018, 308, 94–101. [Google Scholar] [CrossRef]
- Denmark, S.E.; Rossi, S.; Webster, M.P.; Wang, H. Catalytic, enantioselective sulfenylation of ketone-derived enoxysilanes. J. Am. Chem. Soc. 2014, 136, 13016–13028. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Gérardy, R.; Gauron, G.; Damblon, C.; Monbaliu, J.-C.M. Solvent-free organocatalytic preparation of cyclic organic carbonates under scalable continuous flow conditions. React. Chem. Eng. 2019, 4, 17–26. [Google Scholar] [CrossRef]
- Fiorani, G.; Perosa, A.; Selva, M. Dimethyl carbonate: A versatile reagent for a sustainable valorization of renewables. Green Chem. 2018, 20, 288–322. [Google Scholar] [CrossRef]
- Tundo, P.; Musolino, M.; Aricò, F. The reactions of dimethyl carbonate and its derivatives. Green Chem. 2018, 20, 28–85. [Google Scholar] [CrossRef]
- Selva, M.; Perosa, A.; Rodríguez-Padrón, D.; Luque, R. Applications of dimethyl carbonate for the chemical upgrading of biosourced platform chemicals. ACS Sustain. Chem. Eng. 2019, 7, 6471–6479. [Google Scholar] [CrossRef]
- Nogueira, D.O.; De Souza, S.P.; Leão, R.A.C.; Miranda, L.S.M.; De Souza, R.O.M.A. Process intensification for tertiary amine catalyzed glycerol carbonate production: Translating microwave irradiation to a continuous-flow process. RSC Adv. 2015, 5, 20945–20950. [Google Scholar] [CrossRef]
- Van Mileghem, S.; De Borggraeve, W.M.; Baxendale, I.R. A robust and scalable continuous flow process for glycerol carbonate. Chem. Eng. Technol. 2018, 41, 2014–2023. [Google Scholar] [CrossRef]
- Enevoldsen, M.V.; Overgaard, J.; Pedersen, M.S.; Lindhardt, A.T. Organocatalyzed decarboxylative trichloromethylation of Morita-Baylis-Hillman adducts in batch and continuous flow. Chem. Eur. J. 2018, 24, 1204–1208. [Google Scholar] [CrossRef] [PubMed]
- Sugisawa, N.; Nakamura, H.; Fuse, S. Micro-flow synthesis of β-amino acid derivatives via a rapid dual activation approach. Chem. Commun. 2020, 56, 4527–4530. [Google Scholar] [CrossRef]
- Otake, Y.; Nakamura, H.; Fuse, S. Rapid and mild synthesis of amino acid N-carboxy anhydrides: Basic-to-acidic flash switching in a microflow reactor. Angew. Chem. Int. Ed. 2018, 57, 11389–11393. [Google Scholar] [CrossRef] [PubMed]
- Sugisawa, N.; Otake, Y.; Nakamura, H.; Fuse, S. Single-step, rapid, and mild synthesis of β-amino acid N-carboxy anhydrides using micro-flow technology. Chem. Asian J. 2019, 15, 79–84. [Google Scholar] [CrossRef] [Green Version]
- Gérardy, R.; Estager, J.; Luis, P.; Debecker, D.P.; Monbaliu, J.-C.M. Versatile and scalable synthesis of cyclic organic carbonates under organocatalytic continuous flow conditions. Catal. Sci. Technol. 2019, 9, 6841–6851. [Google Scholar] [CrossRef]
- Schober, L.; Ratnam, S.; Yamashita, Y.; Adebar, N.; Pieper, M.; Berkessel, A.; Hessel, V.; Gröger, H. An asymmetric organocatalytic aldol reaction of a hydrophobic aldehyde in aqueous medium running in flow mode. Synthesis 2019, 51, 1178–1184. [Google Scholar] [CrossRef] [Green Version]
- Rulli, G.; Duangdee, N.; Hummel, W.; Berkessel, A.; Gröger, H. First tandem-type one-pot process combining asymmetric organo- and biocatalytic reactions in aqueous media exemplified for the enantioselective and diastereoselective synthesis of 1,3-diols. Eur. J. Org. Chem. 2017, 2017, 812–817. [Google Scholar] [CrossRef]
- Raj, M.; Vishnumaya, V.; Ginotra, A.S.K.; Singh, V.K. Highly enantioselective direct aldol reaction catalyzed by organic molecules. Org. Lett. 2006, 8, 4097–4099. [Google Scholar] [CrossRef] [PubMed]
- Caruso, L.; Puglisi, A.; Gillon, E.; Benaglia, M. Organocatalytic michael addition to (D)-mannitol-derived enantiopure nitroalkenes: A valuable strategy for the synthesis of densely functionalized chiral molecules. Molecules 2019, 24, 4588. [Google Scholar] [CrossRef] [Green Version]
- Okino, T.; Hoashi, Y.; Furukawa, T.; Xu, A.X.; Takemoto, Y. Enantio- and diastereoselective michael reaction of 1,3-dicarbonyl compounds to nitroolefins catalyzed by a bifunctional thiourea. J. Am. Chem. Soc. 2005, 127, 119–125. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, Q.; Li, E.; Jia, P.; Li, N.; Huang, Y. Phosphine-catalyzed intramolecular Rauhut–Currier reaction: Enantioselective synthesis of hydro-2H-indole derivatives. Org. Biomol. Chem. 2017, 15, 7097–7101. [Google Scholar] [CrossRef]
- Li, K.; Gonçalves, T.P.; Huang, K.; Lu, Y. Dearomatization of 3-nitroindoles by a phosphine-catalyzed enantioselective [3 + 2] annulation reaction. Angew. Chem. Int. Ed. 2019, 58, 5427–5431. [Google Scholar] [CrossRef]
- Kondo, M.; Wathsala, H.D.P.; Sako, M.; Hanatani, Y.; Ishikawa, K.; Hara, S.; Takaai, T.; Washio, T.; Takizawa, S.; Sasai, H. Exploration of flow reaction conditions using machine-learning for enantioselective organocatalyzed Rauhut–Currier and [3 + 2] annulation sequence. Chem. Commun. 2020, 56, 1259–1262. [Google Scholar] [CrossRef]
- Fuse, S.; Mifune, Y.; Takahashi, T. Efficient amide bond formation through a rapid and strong activation of carboxylic acids in a microflow reactor. Angew. Chem. Int. Ed. 2013, 53, 851–855. [Google Scholar] [CrossRef] [Green Version]
- Fuse, S.; Mifune, Y.; Nakamura, H.; Tanaka, H. Total synthesis of feglymycin based on a linear/convergent hybrid approach using micro-flow amide bond formation. Nat. Commun. 2016, 7, 13491. [Google Scholar] [CrossRef]
- Mifune, Y.; Nakamura, H.; Fuse, S. A rapid and clean synthetic approach to cyclic peptides via micro-flow peptide chain elongation and photochemical cyclization: Synthesis of a cyclic RGD peptide. Org. Biomol. Chem. 2016, 14, 11244–11249. [Google Scholar] [CrossRef] [PubMed]
- Fuse, S.; Masuda, K.; Otake, Y.; Nakamura, H. Peptide-chain elongation using unprotected amino acids in a micro-flow reactor. Chem. Eur. J. 2019, 25, 15091–15097. [Google Scholar] [CrossRef] [PubMed]
- Fuse, S.; Otake, Y.; Nakamura, H. Peptide synthesis utilizing micro-flow technology. Chem. Asian J. 2018, 13, 3818–3832. [Google Scholar] [CrossRef] [PubMed]
- Otake, Y.; Shibata, Y.; Hayashi, Y.; Kawauchi, S.; Nakamura, H.; Fuse, S. N-Methylated peptide synthesis via generation of an acyl N-methylimidazolium cation accelerated by a brønsted acid. Angew. Chem. Int. Ed. 2020, 59, 12925–12930. [Google Scholar] [CrossRef] [PubMed]
- Ueki, H.; Ellis, T.K.; Martin, C.H.; Boettiger, T.U.; Bolene, S.B.; Soloshonok, V.A. Improved synthesis of proline-derived Ni(II) complexes of glycine: Versatile chiral equivalents of nucleophilic glycine for general asymmetric synthesis of α-amino acids. J. Org. Chem. 2003, 68, 7104–7107. [Google Scholar] [CrossRef]
- Wakasugi, K.; Iida, A.; Misaki, T.; Nishii, Y.; Tanabe, Y. Simple, mild, and practical esterification, thioesterification, and amide formation utilizingp-toluenesulfonyl chloride and N-methylimidazole. Adv. Synth. Catal. 2003, 345, 1209–1214. [Google Scholar] [CrossRef]












| Entry | Reactor | Concentration/M | Catalyst Loading/ mol% | Reaction Time | Temperature/°C | Product | Yield/% |
|---|---|---|---|---|---|---|---|
| 1 | batch | 0.83 | 5 | 5 h | 70 | 5a | 92 |
| 2 | flow | 0.08 | 1 | 45 min | 90 | 5a | 99 |
| 3 | batch | 0.83 | 5 | 5 h | 70 | 5b | 91 |
| 4 | flow | 0.08 | 1 | 45 min | 90 | 5b | 96 |
| 5 | batch | 0.83 | 5 | 5 h | 70 | 5c | 99 |
| 6 | flow | 0.08 | 1 | 45 min | 90 | 5c | 98 |
| 7 | batch | 0.83 | 5 | 5 h | 70 | 5d | 90 |
| 8 | flow | 0.08 | 1 | 45 min | 90 | 5d | 94 |
| 9 | batch | 0.83 | 5 | 5 h | 70 | 5e | 95 |
| 10 | flow | 0.08 | 1 | 45 min | 90 | 5e | 95 |
| 11 | batch | 0.83 | 5 | 5 h | 70 | 5f | 75 |
| 12 | flow | 0.08 | 1 | 45 min | 90 | 5f | 86 |
| 13 | batch | 0.83 | 5 | 5 h | 70 | 5g | 36 |
| 14 | flow | 0.08 | 1 | 45 min | 90 | 5g | 80 |
| Entry | Reactor | Catalyst Loading/mol% | Reaction Time/min | Temperature/°C | Yield/% | cis:trans |
|---|---|---|---|---|---|---|
| 1 | batch | 1 | 10 a | r.t.d | 60 | 70:30 |
| 2 | flow | 1 | 2 b, 1 c | 60, 25 | 88 | 90:10 |
| Entry | Reactor | Concentration/M | Catalyst Loading/mol% | Reaction Time | Temperature/°C | Product | Yield/% |
|---|---|---|---|---|---|---|---|
| 1 | batch | neat | 10 | 16 h | 100 | 23a | 94 |
| 2 | batch | 1.67 | 10 | 24 h | r.t. a | 23a | 89 |
| 3 | flow | 0.25 | 5 | 30 min | 150 | 23a | 93 |
| 4 | batch | neat | 10 | 16 h | 100 | 23b | 97 |
| 5 | batch | 1.67 | 10 | 24 h | r.t. a | 23b | 85 |
| 6 | flow | 0.25 | 5 | 30 min | 150 | 23b | 91 |
| 7 | batch | neat | 10 | 16 h | 100 | 23c | 82 |
| 8 | flow | 0.25 | 5 | 30 min | 150 | 23c | 84 |
| 9 | batch | neat | 10 | 16 h | 100 | 23d | 85 |
| 10 | flow | 0.25 | 5 | 30 min | 150 | 23d | 84 |
| 11 | batch | neat | 10 | 16 h | 100 | 23e | 76 |
| 12 | batch | 1.67 | 10 | 24 h | r.t. a | 23e | 80 |
| 13 | flow | 0.25 | 5 | 30 min | 150 | 23e | 81 |
| 14 | batch | neat | 10 | 16 h | 100 | 23f | 70 |
| 15 | flow | 0.25 | 5 | 30 min | 150 | 23f | 78 |
| Entry | Reactor | Catalyst Loading/mol% | Reaction Time | Temperature/°C | Product | Yield/% |
|---|---|---|---|---|---|---|
| 1 | batch | 10 | 24 h | 60 | 29a | 60 |
| 2 | flow | 2 | 2 min | 100 | 29a | 88 |
| Entry | Reactor | Catalyst Loading/mol% | Reaction Time | Temperature/°C | Product | Conversion/% |
|---|---|---|---|---|---|---|
| 1 | batch | 10 | 5 h | 80 | 33a | 74 |
| 2 | flow | 10 | 10 min | 60 | 33a | 52 |
| Entry | Reactor | Catalyst Loading/mol% | Reaction Time/min | Temperature | Yield/% | ee/% |
|---|---|---|---|---|---|---|
| 1 | batch | 3.6 | 60 | r.t. a | 67 | 91 |
| 2 | flow | 3.6 | 60 | r.t. a | 74 | 89 |
| Entry | Reactor | Catalyst Loading/mol% | Reaction Time | Temperature/°C | Product | Yield/% a | ee/% |
|---|---|---|---|---|---|---|---|
| 1 | batch | 20 | <0.5 h | 80 | 61a | 65 | 92 |
| 2 | flow | 20 | <26 s | 80 | 61a | 78 | 94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugisawa, N.; Nakamura, H.; Fuse, S. Recent Advances in Continuous-Flow Reactions Using Metal-Free Homogeneous Catalysts. Catalysts 2020, 10, 1321. https://doi.org/10.3390/catal10111321
Sugisawa N, Nakamura H, Fuse S. Recent Advances in Continuous-Flow Reactions Using Metal-Free Homogeneous Catalysts. Catalysts. 2020; 10(11):1321. https://doi.org/10.3390/catal10111321
Chicago/Turabian StyleSugisawa, Naoto, Hiroyuki Nakamura, and Shinichiro Fuse. 2020. "Recent Advances in Continuous-Flow Reactions Using Metal-Free Homogeneous Catalysts" Catalysts 10, no. 11: 1321. https://doi.org/10.3390/catal10111321
APA StyleSugisawa, N., Nakamura, H., & Fuse, S. (2020). Recent Advances in Continuous-Flow Reactions Using Metal-Free Homogeneous Catalysts. Catalysts, 10(11), 1321. https://doi.org/10.3390/catal10111321