Synergistic Effect on Photocatalytic Activity of Co-Doped NiTiO3/g-C3N4 Composites under Visible Light Irradiation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphological and Structural Properties
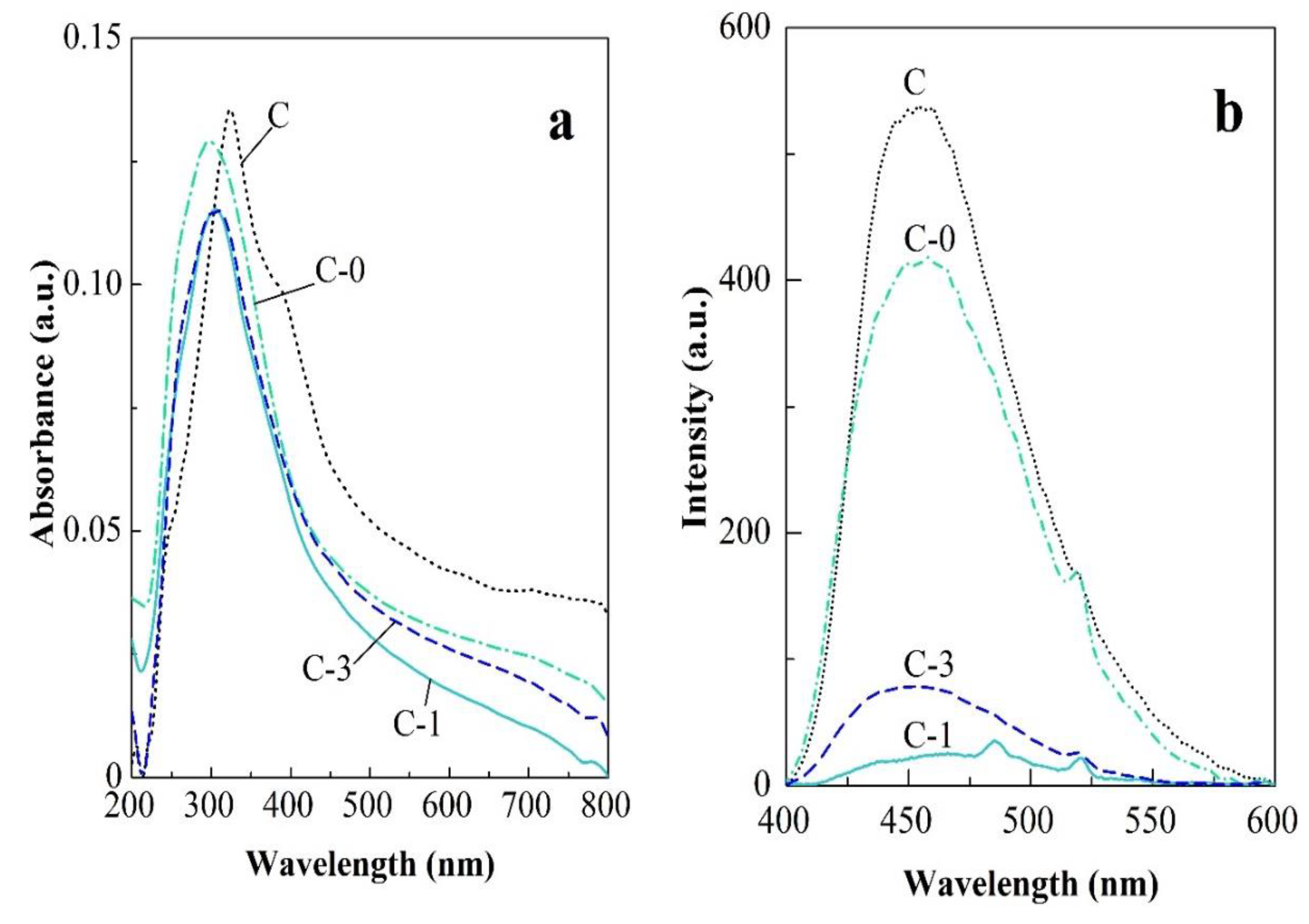
2.2. Electrical and Optical Properties
2.3. Photocatalytic Performance
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Photocatalysts
3.3. Characterization Techniques
3.4. Photocatalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bandyopadhyay, A.; Ghosh, D.; Kaley, N.M.; Pati, S.K. Photocatalytic activity of g-C3N4 quantum dots in visible light: Effect of physicochemical modifications. J. Phys. Chem. C 2017, 121, 1982–1989. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, M.; Li, X.; Li, Q.; Yang, J. Effect of the calcination temperature on the visible light photocatalytic activity of direct contact Z-scheme g-C3N4-TiO2 heterojunction. Appl. Catal. B Environ. 2017, 212, 106–114. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, B.; Baruah, A.; Shanker, V. Synthesis of magnetically separable and recyclable g-C3N4–Fe3O4 hybrid nanocomposites with enhanced photocatalytic performance under visible-light irradiation. J. Phys. Chem. C 2013, 117, 26135–26143. [Google Scholar] [CrossRef]
- Wang, J.; Huang, J.; Xie, H.; Qu, A. Synthesis of g-C3N4/TiO2 with enhanced photocatalytic activity for H2 evolution by a simple method. Int. J. Hydrogen Energy 2014, 39, 6354–6363. [Google Scholar] [CrossRef]
- Qu, A.; Xu, X.; Xie, H.; Zhang, Y.; Li, Y.; Wang, J. Effects of calcining temperature on photocatalysis of g-C3N4/TiO2 composites for hydrogen evolution from water. Mater. Res. Bull. 2016, 80, 167–176. [Google Scholar] [CrossRef]
- Jung, H.; Pham, T.-T.; Shin, E.W. Interactions between ZnO nanoparticles and amorphous g-C3N4 nanosheets in thermal formation of g-C3N4/ZnO composite materials: The annealing temperature effect. Appl. Surf. Sci. 2018, 458, 369–381. [Google Scholar] [CrossRef]
- Jung, H.; Pham, T.-T.; Shin, E.W. Effect of g-C3N4 precursors on the morphological structures of g-C3N4/ZnO composite photocatalysts. J. Alloys Compd. 2019, 788, 1084–1092. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, Y.; Chen, J.; Jiang, Y.; Kiani, M.; Li, B.; Wang, R. Fabrication of high-activity hybrid NiTiO3/g-C3N4 heterostructured photocatalysts for water splitting to enhanced hydrogen production. Ceram. Int. 2016, 42, 12297–12305. [Google Scholar] [CrossRef]
- Qu, Y.; Zhou, W.; Ren, Z.; Du, S.; Meng, X.; Tian, G.; Pan, K.; Wang, G.; Fu, H. Facile preparation of porous NiTiO3 nanorods with enhanced visible-light-driven photocatalytic performance. J. Mater. Chem. 2012, 22, 16471–16476. [Google Scholar] [CrossRef]
- Pham, T.-T.; Shin, E.W. Thermal formation effect of g-C3N4 structure on the visible light driven photocatalysis of g-C3N4/NiTiO3 Z-scheme composite photocatalysts. Appl. Surf. Sci. 2018, 447, 757–766. [Google Scholar] [CrossRef]
- Pham, T.-T.; Shin, E.W. Inhibition of charge recombination of NiTiO3 photocatalyst by the combination of Mo-doped impurity state and Z-scheme charge transfer. Appl. Surf. Sci. 2020, 501, 143992. [Google Scholar] [CrossRef]
- Pham, T.-T.; Shin, E.W. Influence of g-C3N4 Precursors in g-C3N4/NiTiO3 Composites on Photocatalytic Behavior and the Interconnection between g-C3N4 and NiTiO3. Langmuir 2018, 34, 13144–13154. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yuan, X.; Wang, H.; Chen, X.; Wu, Z.; Jiang, L.; Xiong, W.; Zhang, Y.; Zeng, G. One-step calcination method for synthesis of mesoporous gC3N4/NiTiO3 heterostructure photocatalyst with improved visible light photoactivity. RSC Adv. 2015, 5, 95643–95648. [Google Scholar] [CrossRef]
- Jiang, K.; Pham, T.-T.; Kang, S.G.; Men, Y.; Shin, E.W. Modification of the structural properties of NiTiO3 materials by transition metal dopants: The dopant size effect. J. Alloys Compd. 2018, 739, 393–400. [Google Scholar] [CrossRef]
- Pham, T.-T.; Kang, S.G.; Shin, E.W. Optical and structural properties of Mo-doped NiTiO3 materials synthesized via modified Pechini methods. Appl. Surf. Sci. 2017, 411, 18–26. [Google Scholar] [CrossRef]
- Lenin, N.; Karthik, A.; Sridharpanday, M.; Selvam, M.; Srither, S.R.; Arunmetha, S.; Paramasivam, P.; Rajendran, V. Electrical and magnetic behavior of iron doped nickel titanate (Fe3+/NiTiO3) magnetic nanoparticles. J. Magn. Magn. Mater. 2016, 397, 281–286. [Google Scholar] [CrossRef]
- Carter, C.B.; Norton, M.G. Ceramic Materials: Science and Engineering; Springer: New York, NY, USA, 2007. [Google Scholar]
- Hu, S.; Ma, L.; You, J.; Li, F.; Fan, Z.; Lu, G.; Liu, D.; Gui, J. Enhanced visible light photocatalytic performance of g-C3N4 photocatalysts co-doped with iron and phosphorus. Appl. Surf. Sci. 2014, 311, 164–171. [Google Scholar] [CrossRef]
- Gao, D.; Xu, Q.; Zhang, J.; Yang, Z.; Si, M.; Yan, Z.; Xue, D. Defect-related ferromagnetism in ultrathin metal-free g-C3N4 nanosheets. Nanoscale 2014, 6, 2577–2581. [Google Scholar] [CrossRef]
- Tian, N.; Huang, H.; Liu, C.; Dong, F.; Zhang, T.; Du, X.; Yu, S.; Zhang, Y. In situ co-pyrolysis fabrication of CeO2/g-C3N4 n–n type heterojunction for synchronously promoting photo-induced oxidation and reduction properties. J. Mater. Chem. A 2015, 3, 17120–17129. [Google Scholar] [CrossRef]
- Nguyen, T.K.A.; Pham, T.-T.; Nguyen-Phu, H.; Shin, E.W. The effect of graphitic carbon nitride precursors on the photocatalytic dye degradation of water-dispersible graphitic carbon nitride photocatalysts. Appl. Surf. Sci. 2020, 537, 148027. [Google Scholar] [CrossRef]
- Inceesungvorn, B.; Teeranunpong, T.; Nunkaew, J.; Suntalelat, S.; Tantraviwat, D. Novel NiTiO3/Ag3VO4 composite with enhanced photocatalytic performance under visible light. Catal. Commun. 2014, 54, 35–38. [Google Scholar] [CrossRef]
- Bellam, J.B.; Ruiz-Preciado, M.A.; Edely, M.; Szade, J.; Jouanneaux, A.; Kassiba, A.H. Visible-light photocatalytic activity of nitrogen-doped NiTiO3 thin films prepared by a co-sputtering process. RSC Adv. 2015, 5, 10551–10559. [Google Scholar] [CrossRef]
- Radecka, M.; Rekas, M.; Trenczek-Zajac, A.; Zakrzewska, K. Importance of the band gap energy and flat band potential for application of modified TiO2 photoanodes in water photolysis. J. Power Sources 2008, 181, 46–55. [Google Scholar] [CrossRef]
- Phan, T.T.N.; Nikoloski, A.N.; Bahri, P.A.; Li, D. Heterogeneous photo-Fenton degradation of organics using highly efficient Cu-doped LaFeO3 under visible light. J. Ind. Eng. Chem. 2018, 61, 53–64. [Google Scholar] [CrossRef]





| Sample | Description | d (nm) a | Eg (eV) b | kapp × 103 (min−1) c | R2 |
|---|---|---|---|---|---|
| N-0 | Pure NiTiO3 | 32.39 | 2.97 | 0.34 | 0.989 |
| N-1 | Co-doped NiTiO3 (Co = 1%) | 35.06 | 2.76 | 3.17 | 0.990 |
| N-3 | Co-doped NiTiO3 (Co = 3%) | 36.54 | 2.91 | 1.95 | 0.995 |
| C | Pure g-C3N4 | - | 2.65 | 0.44 | 0.983 |
| C-0 | g-C3N4/N-0 composite | 21.62 | 2.67 | 0.79 | 0.984 |
| C-1 | g-C3N4/N-1 composite | 27.32 | 2.53 | 7.15 | 0.992 |
| C-3 | g-C3N4/N-3 composite | 33.83 | 2.45 | 5.66 | 0.987 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dao, D.Q.; Nguyen, T.K.A.; Pham, T.-T.; Shin, E.W. Synergistic Effect on Photocatalytic Activity of Co-Doped NiTiO3/g-C3N4 Composites under Visible Light Irradiation. Catalysts 2020, 10, 1332. https://doi.org/10.3390/catal10111332
Dao DQ, Nguyen TKA, Pham T-T, Shin EW. Synergistic Effect on Photocatalytic Activity of Co-Doped NiTiO3/g-C3N4 Composites under Visible Light Irradiation. Catalysts. 2020; 10(11):1332. https://doi.org/10.3390/catal10111332
Chicago/Turabian StyleDao, Duc Quang, Thi Kim Anh Nguyen, Thanh-Truc Pham, and Eun Woo Shin. 2020. "Synergistic Effect on Photocatalytic Activity of Co-Doped NiTiO3/g-C3N4 Composites under Visible Light Irradiation" Catalysts 10, no. 11: 1332. https://doi.org/10.3390/catal10111332
APA StyleDao, D. Q., Nguyen, T. K. A., Pham, T.-T., & Shin, E. W. (2020). Synergistic Effect on Photocatalytic Activity of Co-Doped NiTiO3/g-C3N4 Composites under Visible Light Irradiation. Catalysts, 10(11), 1332. https://doi.org/10.3390/catal10111332






