Abstract
Under the guidance of the idea of “treating waste with waste”, copper-loaded carbon-based catalysts were prepared in situ using waste chelating resin with adsorbed copper. The effect of the catalyst activation temperature on dye brilliant red (X-3B) degradation was investigated and the characterization of the catalysts was analyzed. The results show that a catalyst activated at 800 °C (Cu-AC-800) has the largest specific surface area and abundant pore structure and the highest proportion of Cu under low valence states, which leads to the best performance in adsorbing and degrading X-3B. The influences of operation conditions and inorganic salt anions on persulfate (PS) activation were also investigated. Moreover, the degradation mechanism was preliminarily explored by quenching reactions. The main active free radicals in the system were determined as sulfate radicals (•SO4−). Given its use in solid waste recycling, copper-loaded carbon-based catalyst may provide some new insights for the remediation of wastewater.
1. Introduction
As one of the four main kinds of industrial wastewater, dye wastewater has been widely concerning due to its extremely complex composition and high proportion of organic pollutants [1,2]. It is difficult to meet discharge standards using traditional biodegradation and physical removal methods. Therefore, it is necessary to apply chemical methods for advanced treatment of dye wastewater.
Owing to rapid and thorough oxidation, adaptability of wide pH range, and easy storage and transportation of oxidants, activated persulfate (PS) advanced oxidation technology has now attracted researchers’ attention [3,4,5,6]. It mainly relies on sulfate radicals (•SO4−) to degrade organic pollutants. With its strong oxidizing property, •SO4− can destroy most of the organic pollutants non-selectively to produce simple organic compounds which can be easily biodegradable, thereby realizing the degradation of pollutants. Common persulfate activation technologies include thermal activation [7,8], light activation [5,9,10], activated carbon activation [11,12], and transition metal activation [8,13].
The transition metal activation method has been widely used because of its advantages of high catalytic ability, simple operation, low energy consumption, and moderate reaction conditions [14,15]. Among them, copper oxide is one of the effective catalysts, and different forms of copper oxides can activate persulfate and produce radicals [16,17,18,19]. It has been confirmed that cuprous oxide (Cu2O) has strong capability to activate PS owing to the high activity of Cu+ [20]. Cu0 can also activate PS through reducing Cu2+ to Cu+ [21]. However, Cu+ can be easily oxidized to Cu2+, which could decrease its catalytic stability. It has been reported that loading Cu on the surface of high specific surface area materials such as activated carbon fiber, activated carbon, and zeolite could improve the dispersion of copper oxides and greatly enhance the catalytic ability of persulfate activation owing to the abundant active sites [22,23,24].
In recent years, chelating resins have been successfully employed for heavy metal removal due to their high uptake capacity for the target species, fast reaction kinetics, and efficient elution [25,26]. However, as is well known, heavy metals are difficult to degrade and easily accumulate toxicity. Waste chelating resin adsorbed heavy metals can not only deteriorate the urban soil ecological environment, but also seriously harm the health of urban residents through a groundwater food chain polluted by surface dust and runoff. In this paper, under the guidance of the idea of “treating waste with waste”, copper-loaded carbon-based catalysts were prepared in situ using simulative waste chelating resin adsorbed copper. The polyporous structure of the resin and its excellent chelation performance for metal ions were supposed to increase the specific surface area of activated carbon and improve the dispersion of metals. The waste chelating resin was carbonized and activated to obtain the copper-loaded carbon-based catalyst. Using dye brilliant red (X-3B) as the target pollutant, the performance of the copper-loaded carbon-based catalyst in activating PS to degrade X-3B was studied. The effect of operation conditions on catalyst performance was investigated and the mechanism of persulfate activation and radical formation was further discussed.
2. Results and Discussion
2.1. Catalyst Characteristics
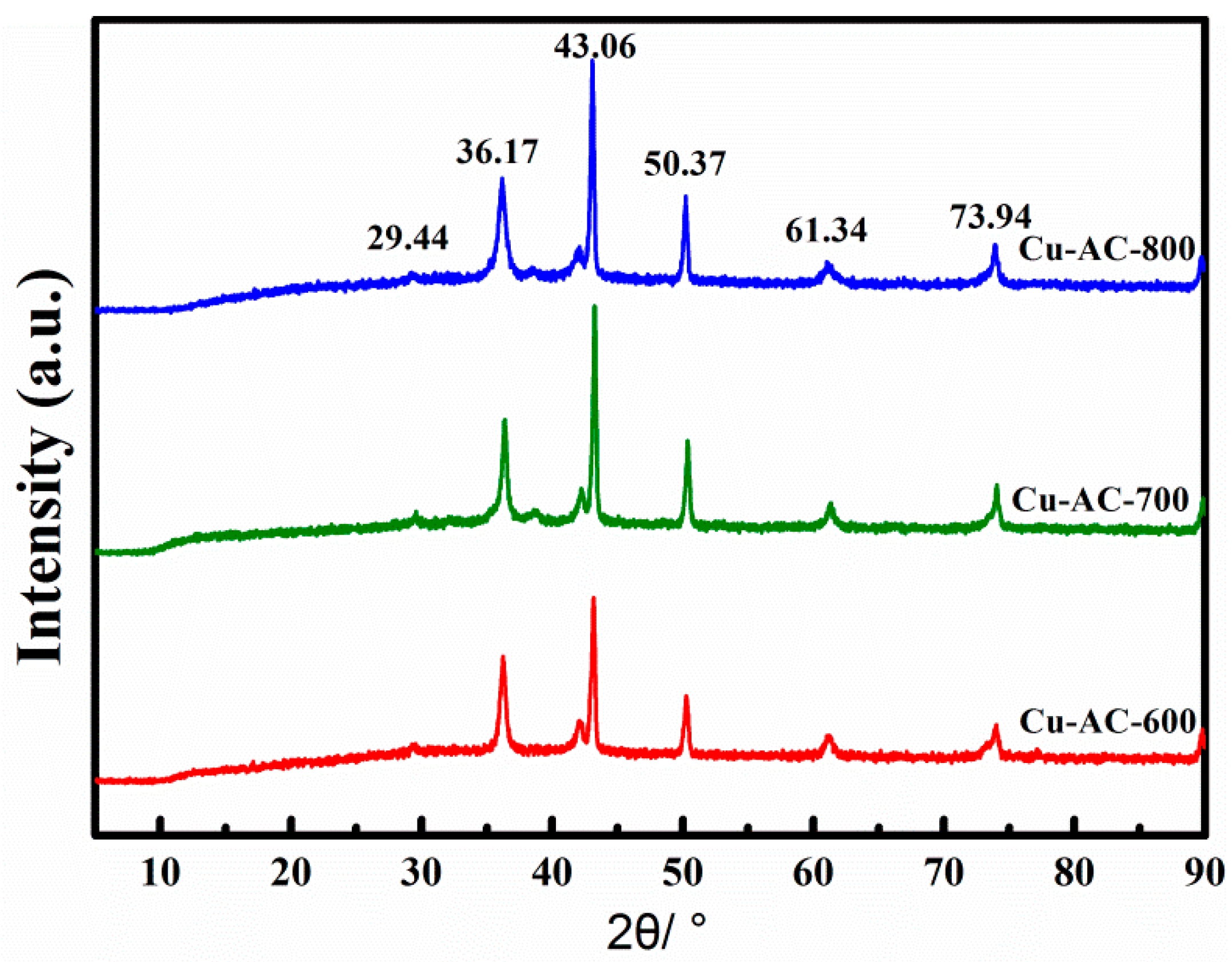
The samples activated at 600, 700, and 800 °C were labeled as Cu-AC-600, Cu-AC-700, and Cu-AC-800, respectively. Figure 1 presents the XRD patterns of the Cu-AC samples prepared at different activation temperatures. The sharp diffraction peaks located at 43.06 and 50.37 indicated the existence of Cu0 (JCPDS 04-0836) in the prepared samples. With the increase of activation temperature, the characteristic diffraction peak of Cu0 was enhanced. Further, weak diffraction peaks were observed at 2θ of 29.44°, 36.17°, and 61.34°, which were the characteristic peaks of Cu2O (JCPDS 05-0667) [27]. It is worth noting that there was no obvious diffraction peak of CuO.
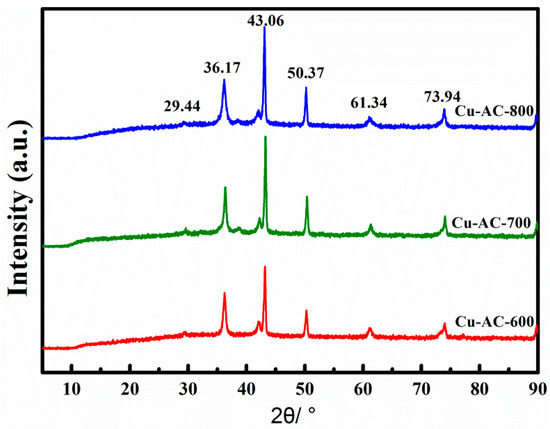
Figure 1.
XRD patterns of the Cu-AC samples at different activation temperatures.
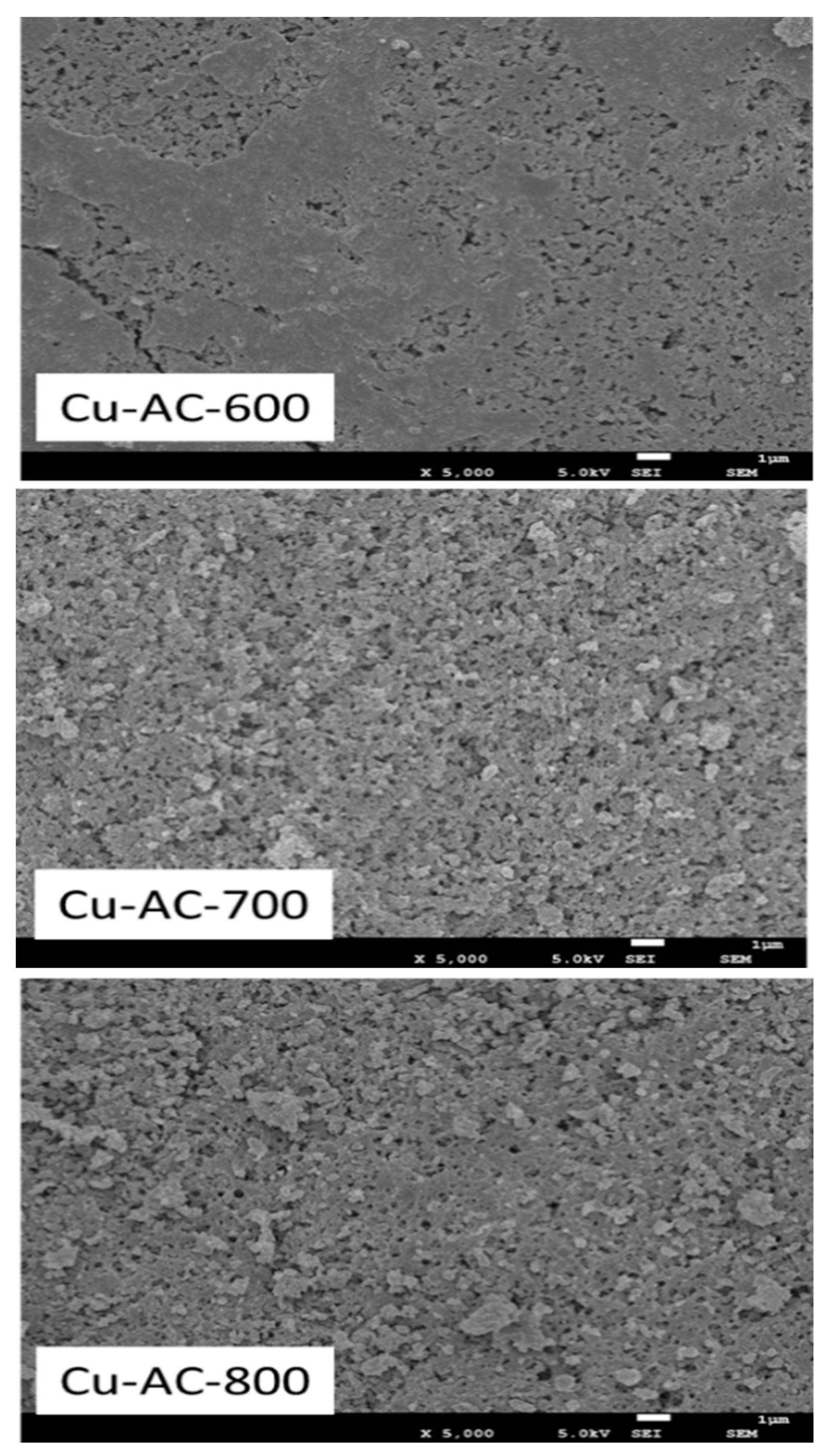
The SEM images of the Cu-AC samples are shown in Figure 2. It can be found that the surface of Cu-AC-600 was relatively smooth. With the increase of activation temperature, the pore structure increased obviously originating from the thermal cracking and corrosive effect of KOH. A partially aggregated and crinkled structure could be observed on Cu-AC-800. The corresponding data of pore structure and specific surface area are illustrated in Table 1. Remarkably, with the increase of the activation temperature, the specific surface area and micropore volume of the catalyst increased. It has been reported that a large specific surface area offers more active sites and a large number of micropores can make the reactants and products adsorb and react rapidly in the channel, so as to improve the reaction rate. In this study, Cu-AC-800 possesses a well-developed porous structure and high amount of defective edges.
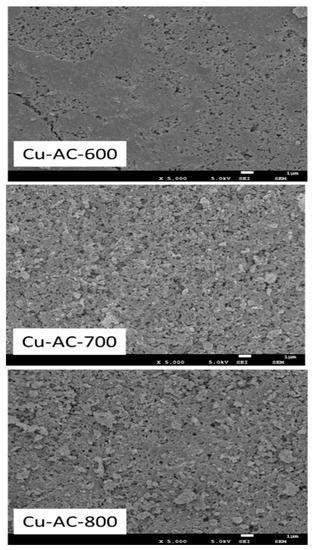
Figure 2.
SEM images of the Cu-AC samples at different activation temperatures.

Table 1.
Specific surface area and pore size distribution of samples.
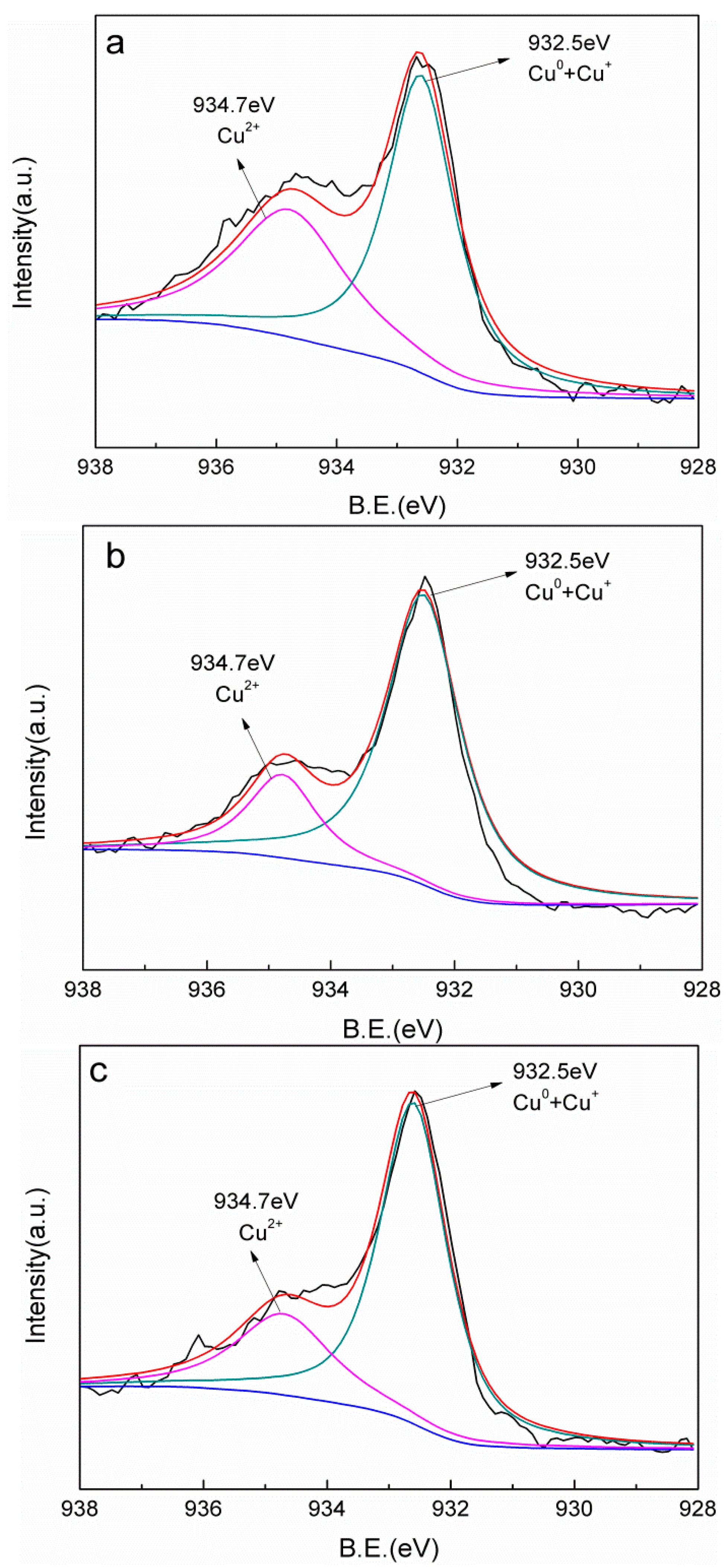
In order to analyze the surface valence state of copper, XPS analysis was performed as shown in Figure 3. It can be found that the Cu 2p spectra can be divided into two components. The main peak at 932.5 eV was assigned to Cu0 and Cu+, while the other weak peak located at 934.7 eV could be attributed to Cu2+ [28,29]. Remarkably, Cu0 and Cu+ species played dominant roles in all of the Cu-AC samples. Unfortunately, as the binding energy of Cu0 and Cu+ is relatively close, it is difficult to distinguish them. It is worth noting that with the increase of the annealing temperature, the proportion of Cu0 and Cu+ increased. The percentage of Cu0 and Cu+ for Cu-AC-800 can reach 73.1% (Table 2), indicating that a better reductive degree was obtained for Cu-AC-800.
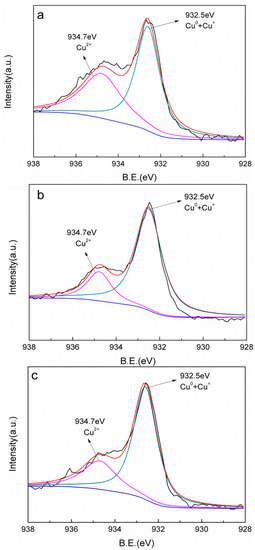
Figure 3.
XPS survey spectra of Cu-AC-600 (a), Cu-AC-700 (b), and Cu-AC-800 (c).

Table 2.
Proportion of Cu species for different Cu-AC samples.
2.2. Catalytic Activity of Catalysts
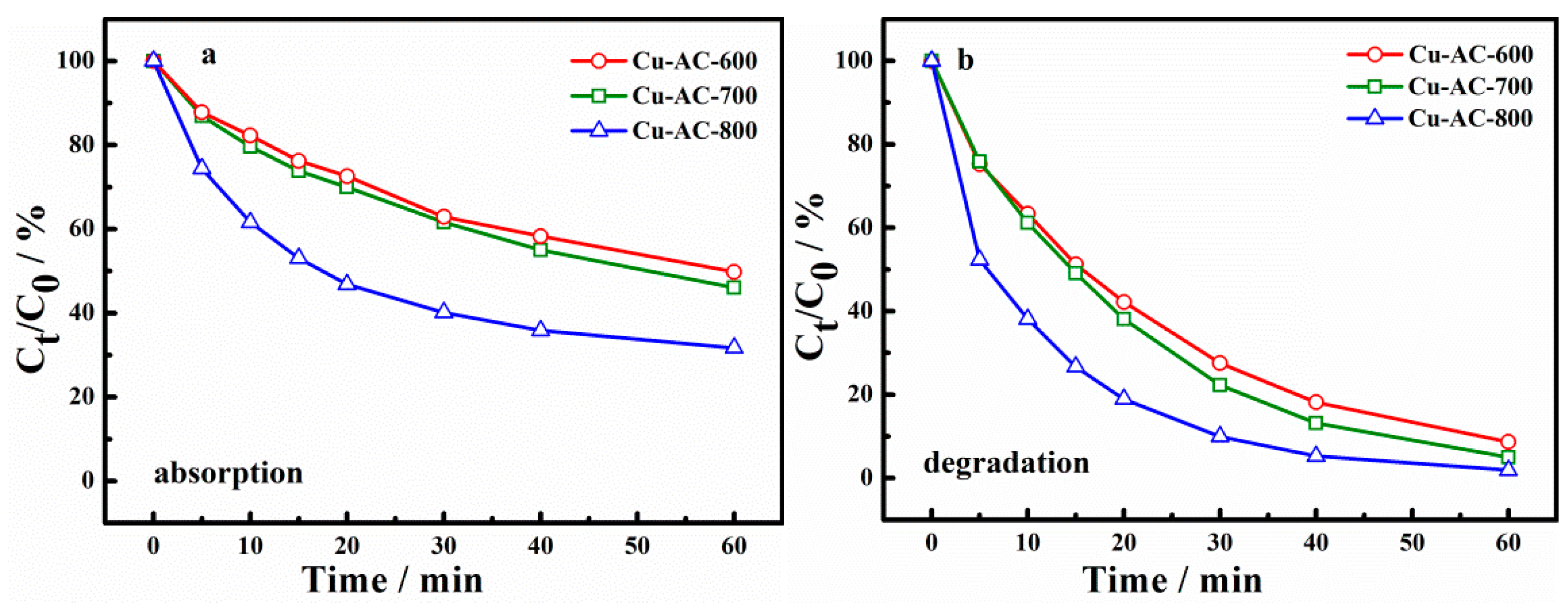
Considering that the activation temperature could affect not only the surface physical properties of catalysts, but also the morphology of the metal, the catalytic activity and adsorption ability of catalysts at different activation temperatures were explored, as shown in Figure 4. Figure 4a,b shows the adsorption and degradation of X-3B using different catalysts. It can be seen from Figure 4a that the X-3B adsorption ratios of Cu-AC-600, Cu-AC-700, and Cu-AC-800 were 50.2%, 53.9%, and 68.3%, respectively, at 60 min. It is indicated that increasing the activation temperature has conduced to the improvement of the adsorption performance of the catalysts. It also can be concluded that the degradation rate of X-3B has been significantly improved after adding PS. The removal ratio of Cu-AC-600, Cu-AC-700, and Cu-AC-800 reached 91.3%, 95.0%, and 98.1%, respectively, at 60 min (Figure 4b). This shows that the catalyst can achieve a higher oxidative degradation rate of X-3B by activating persulfate.
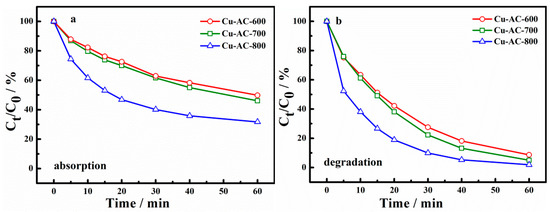
Figure 4.
The absorption (a) and degradation (b) of dye brilliant red (X-3B) with different catalysts.
Moreover, it can be seen that the catalytic activity of the catalyst on X-3B degradation was greatly affected by the activation temperature. With the increase of the activation temperature, the removal rate of X-3B in both adsorption and degradation systems was gradually improved. Cu-AC-800 shows the highest degradation and adsorption performance. The reasons may be listed as follows. On one hand, the large specific surface area and abundant pore structure of Cu-AC-800 were more conducive to the adsorption and degradation of X-3B. On the other hand, a high proportion of Cu+ and Cu0 on the surface of Cu-AC-800 could activate persulfate rapidly to generate •SO4− and •OH (Equations (1)–(4)) promoting the degradation of X-3B [30,31,32]. Accordingly, Cu-AC-800 was selected as the catalyst in the subsequent investigation of reaction conditions.
Cu+ + S2O82− → Cu2+ + SO42− + •SO4−
Cu0 + Cu2+ → 2Cu+
•SO4− + H2O → SO42− + •OH + H+
•SO4− + OH− → SO42− + •OH
2.3. Influence of the Operation Conditions on X-3B Degradation
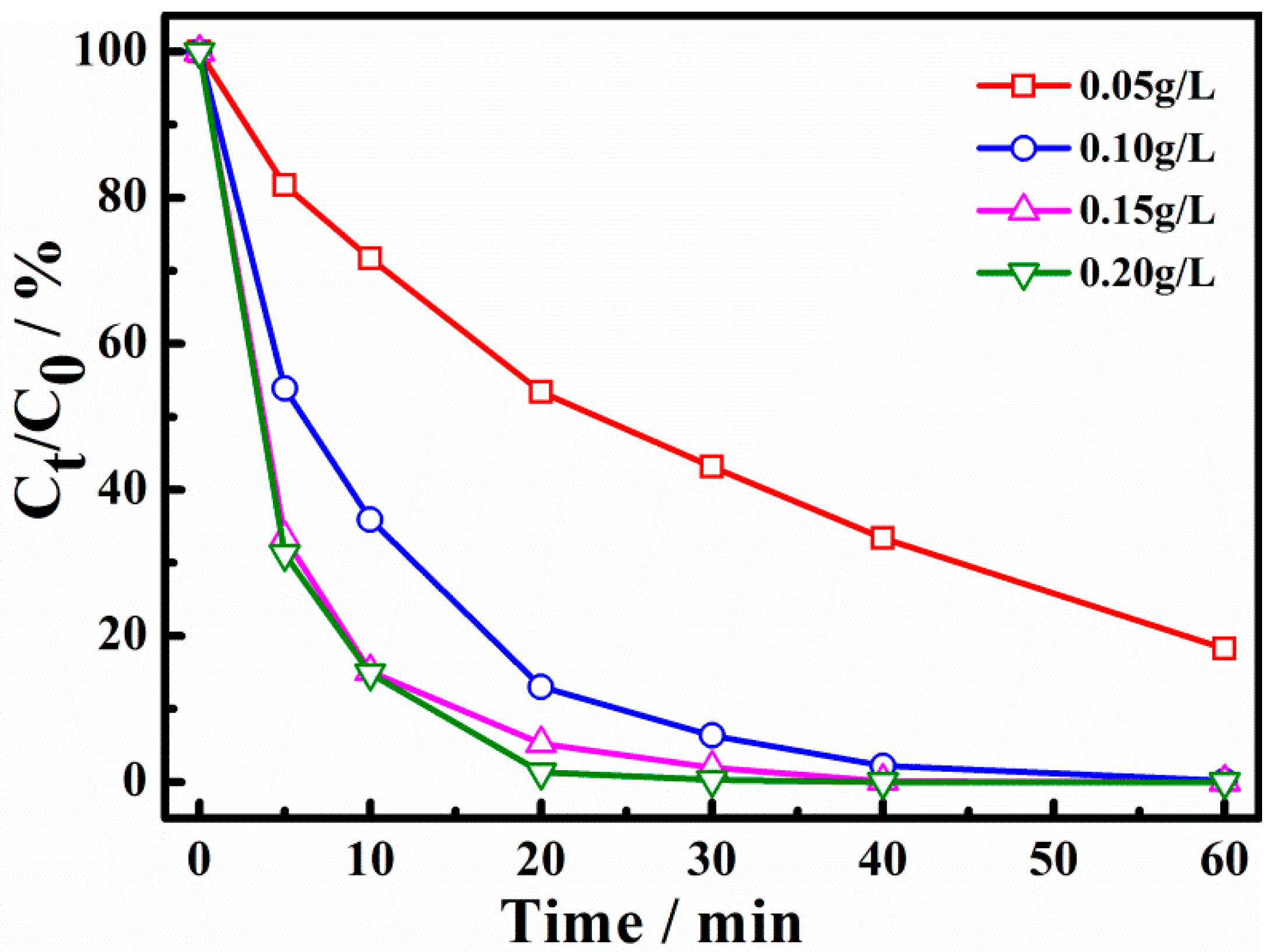
The effect of catalyst dosage on the degradation of X-3B is shown in Figure 5. It can be seen that when the catalyst dosage was 0.05, 0.10, 0.15, and 0.20 g/L, the degradation ratio of X-3B for 30 min was 58%, 93.4%, 97.7%, and 100%, respectively. The data show that the degradation of X-3B was significantly enhanced when the amount of catalyst was increased. The Cu-AC samples acted as both the adsorbent and the catalysts in the X-3B removal process. With the increase of the catalyst dosage, the adsorption of X-3B was obviously enhanced. A higher amount of catalysts could also offer more catalytic active sites for persulfate activation and X-3B degradation, which can activate and generate more active free radicals, thus significantly increasing the degradation rate. At the same time, it was observed that the degradation curves of 0.15 g/L and 0.20 g/L were basically coincident. It is indicated that when the amount of catalyst was larger than 0.15g /L, PS concentration may become the key factor for X-3B removal. When the amount of catalyst was further increased, the degradation rate would not be improved.
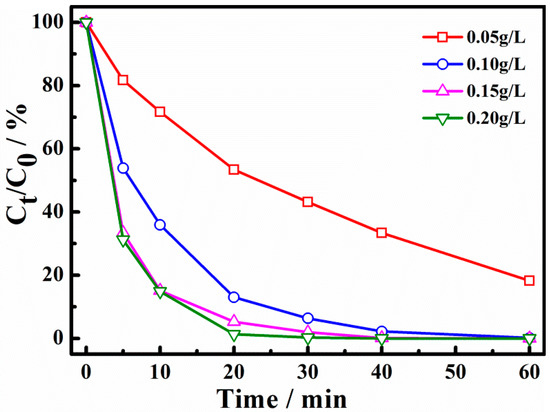
Figure 5.
Effect of catalyst dosage on degradation of X-3B.
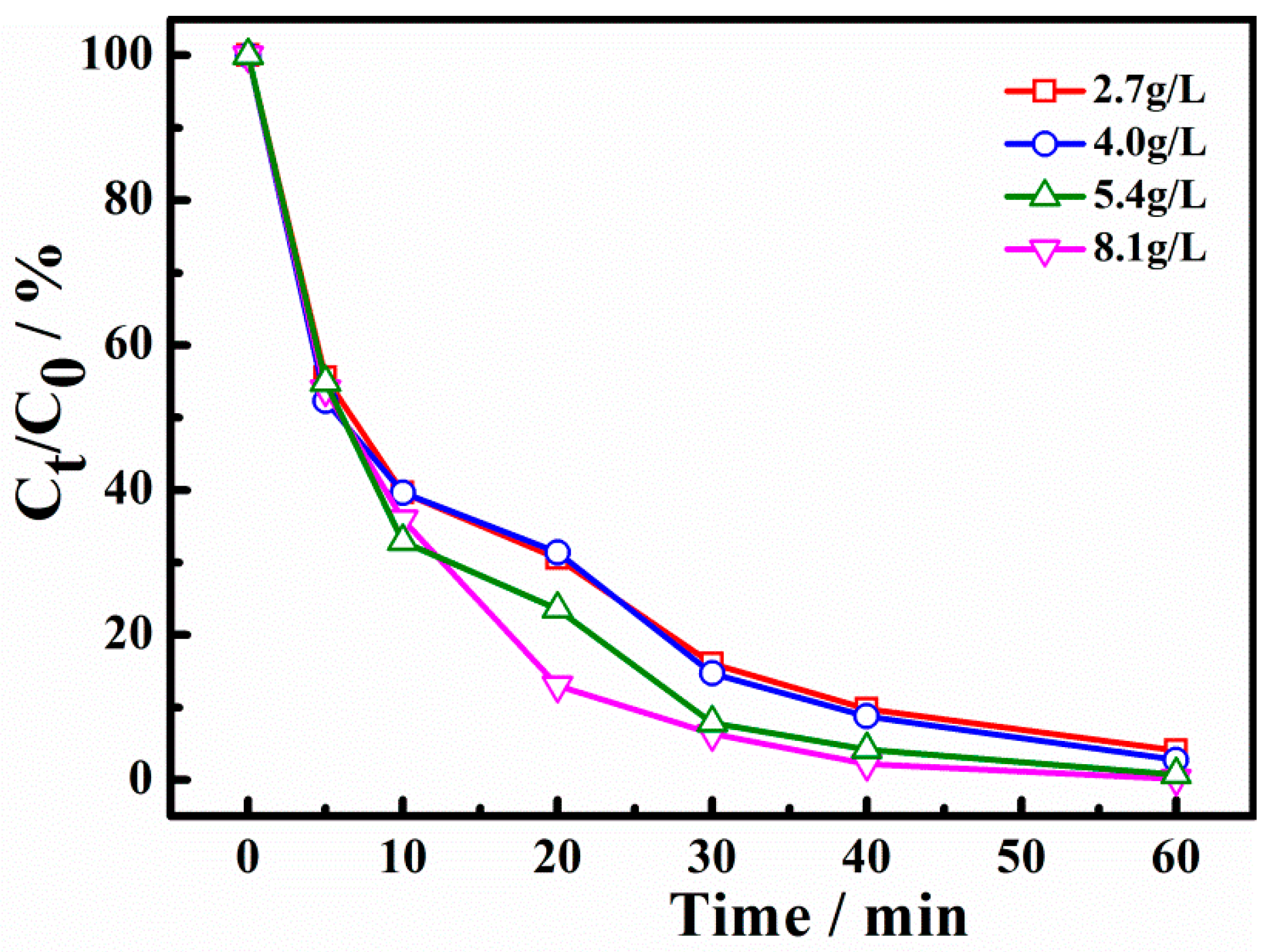
The effect of PS dosage on the removal efficiency of X-3B is shown in Figure 6. When PS dosage was 2.7, 4.0, 5.4, and 8.1 g/L, the degradation ratio of X-3B in the system for 30 min was 83.4%, 85.53%, 92.55%, and 93.87%, respectively. It can be seen that when PS dosage increased from 2.7 g/L to 5.4 g/L, the degradation ratio of X-3B significantly increased. However, when the PS dosage was higher than 5.4 g/L, the X-3B removal could not be further enhanced. The reason may be that when the PS concentration is lower than 5.4 g/L, increasing the PS dosage can increase the amount of •SO4− with strong oxidizing property. However, the active sites of the catalyst are limited. When the additional amount of PS is more than 5.4 g/L, it will not have a significant impact on the degradation rate of X-3B. In addition, the excess S2O82− can also react with •SO4− to generate •S2O8− with lower reactivity (Equation (5)) [31,32]. Therefore, PS can promote the reaction within a certain concentration range.
S2O82− + •SO4−→SO42− + • S2O82−
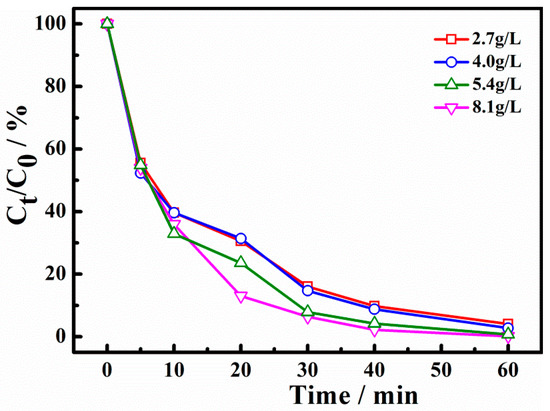
Figure 6.
Effect of persulfate (PS) dosage on degradation of X-3B.
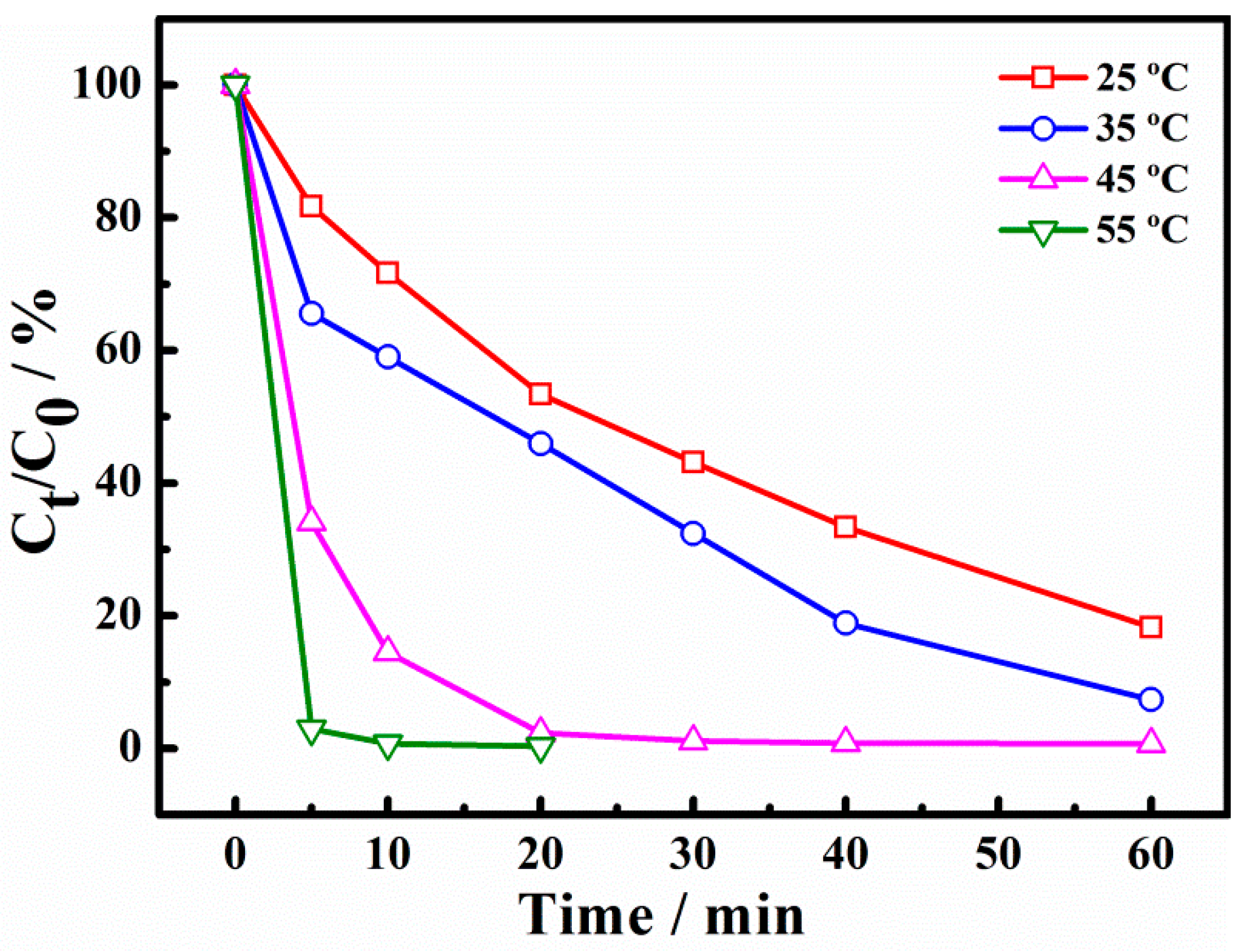
The removal rates of X-3B catalysts at different reaction temperatures are presented in Figure 7. It can be seen that after 30 min, the degradation rates of X-3B were 50.9%, 65.8%, 100%, and 100%, respectively, when the reaction temperature was 25, 35, 45, and 55 °C. The data showed that with the increase of reaction temperature, the degradation rate of X-3B was also increased, which may be attributed to the acceleration of the PS activation and the production of more •SO4−. Moreover, the reaction rate of the active radical with X-3B was also accelerated. When the temperature of the system was 5 °C, the degradation rate increased sharply, possibly owing to the thermal activation of PS [7,8]. Under the combined action of catalytic activation and thermal activation, the system accelerates the generation of active radicals, thus promoting the degradation rate of X-3B.
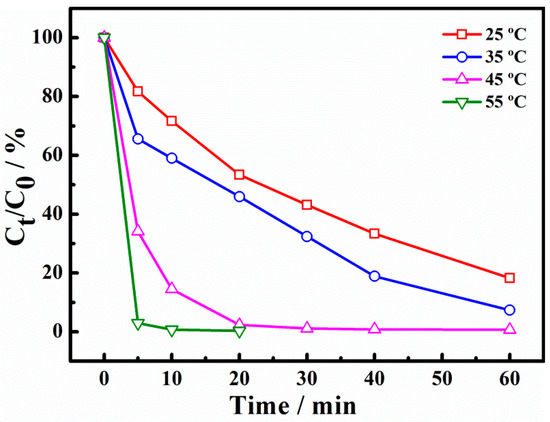
Figure 7.
Effect of temperature on degradation of X-3B.
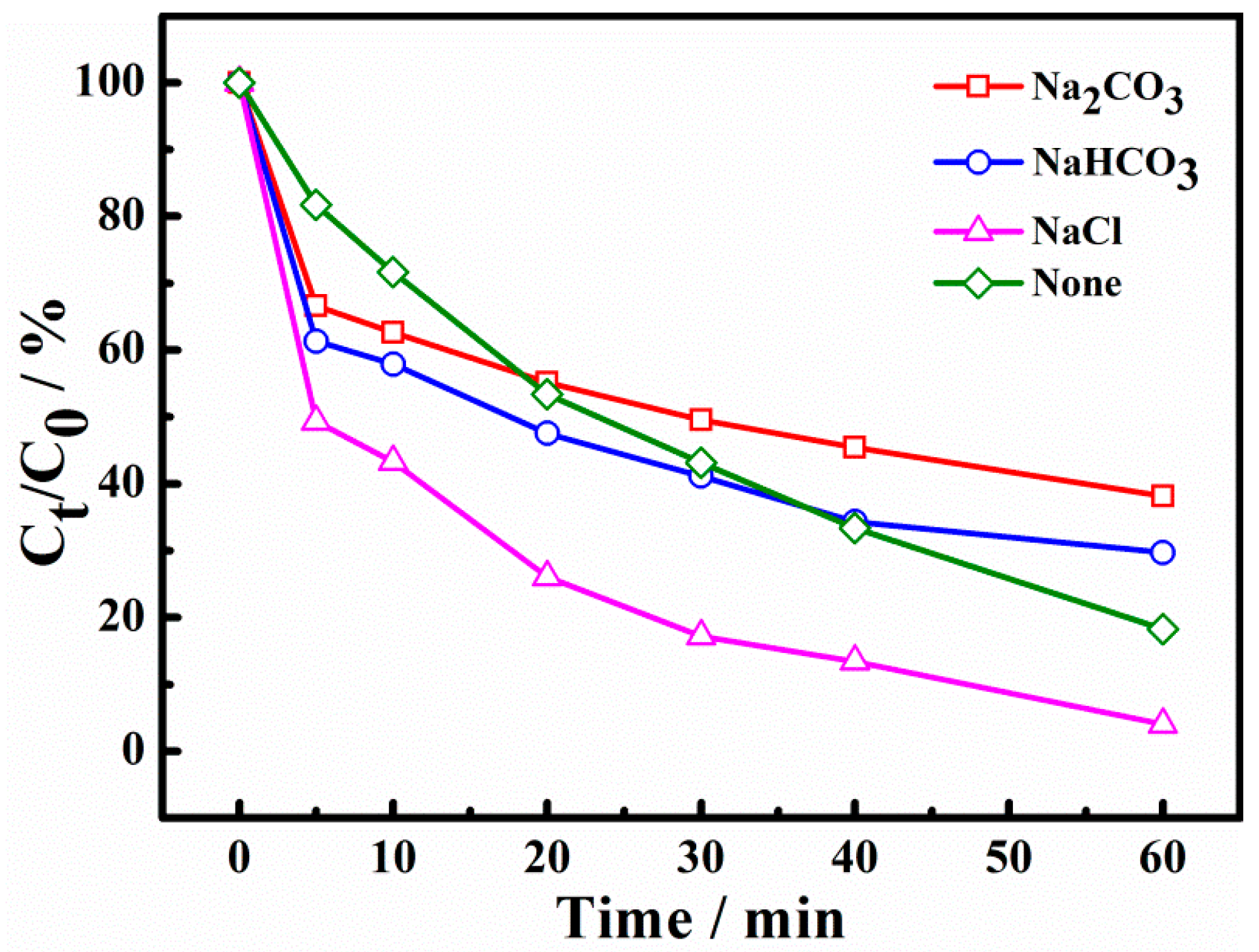
As is well known, a lot of inorganic salts are left in dyeing wastewater. In this experiment, sodium carbonate, sodium bicarbonate, and sodium chloride were selected to investigate the effect of different inorganic salt anions on dye degradation. As illustrated in Figure 8, it can be seen that the X-3B degradation ratio reached 77.05% at 60 min without inorganic salt anions. The degradation ratios became 58.10%, 65.59%, and 94.46% with the addition of CO32−, HCO3− and Cl−, respectively, indicating that CO32− and HCO3− had a certain inhibitory effect on the removal of X-3B, while Cl− showed a promoting effect. The reason for this is the powerful free radical scavenging effect of CO32− and HCO3−, which can react with •SO4− to produce low-activity free radicals (Equations (6) and (7)) [33,34]. The scavenging of radicals would reduce the oxidation capacity and affect the degradation of X-3B. Although Cl− can be transformed into •Cl and •Cl2− with weak oxidation ability (Equations (8) and (9)), it still has a strong reactivity with electron-rich groups such as X-3B [14,35]. Therefore, Cl− promoted the removal of X-3B.
HCO3− + •SO4− → SO42− + •HCO3
CO32− + •SO4− → SO42− + •CO3−
Cl− + •SO4− → SO42− + •Cl
Cl− + •Cl →•Cl2−
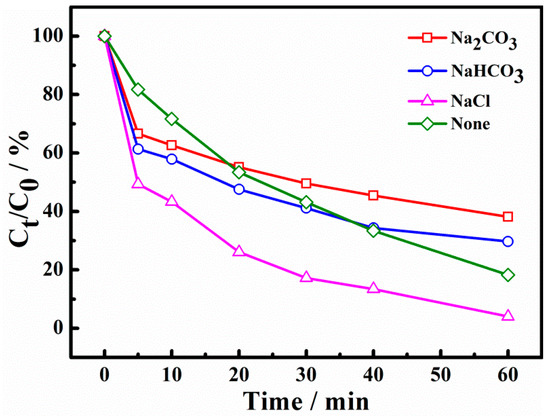
Figure 8.
Effect of inorganic salt anions on degradation of X-3B.
2.4. Reusability of the Catalyst
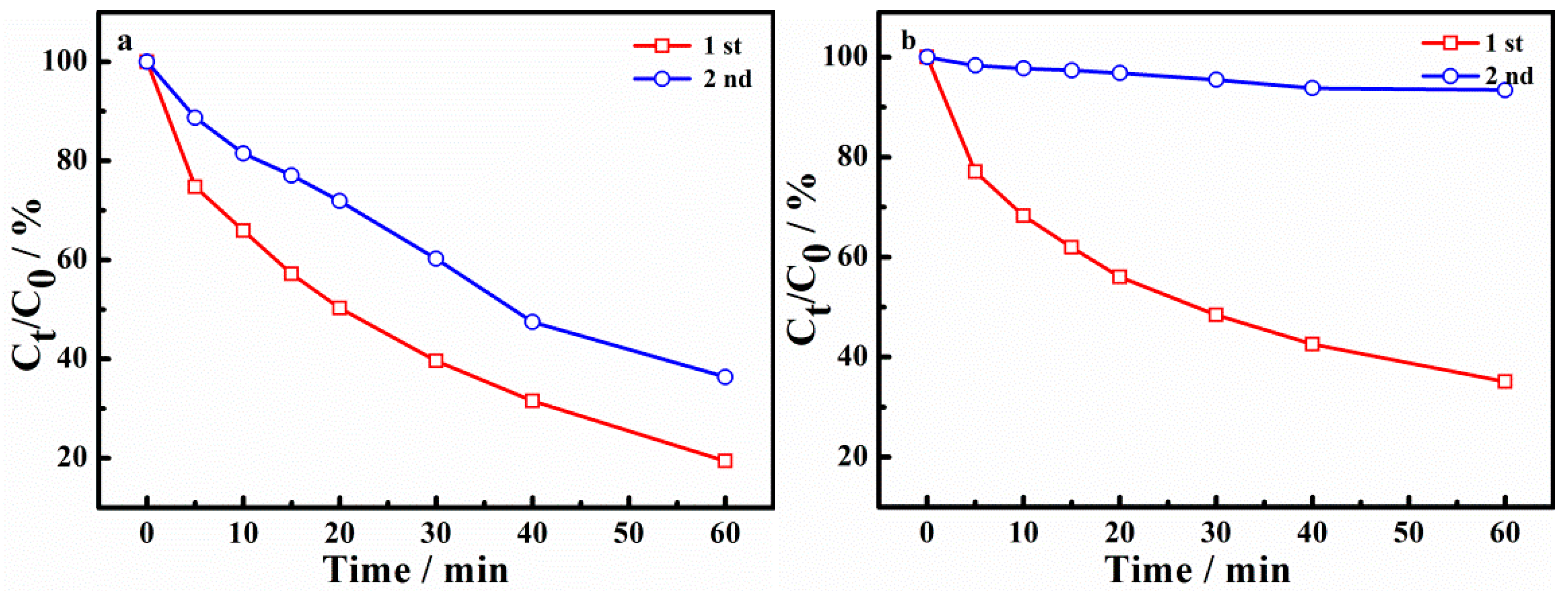
In order to investigate the recycling ability of the catalyst, the used Cu-AC-800 sample was washed and dried and then reused. The experimental conditions were consistent with the original experimental conditions. The impact on degradation and adsorption is shown in Figure 9. As can be seen in Figure 9a, the degradation ratio of X-3B at 60 min was reduced to 80.93% of the first recycling, and 63.8% of the second recycling. It can be seen from Figure 9b that the adsorption of X-3B decreased from 64.93% of the first recycling to 6.6% of the second recycling. It can be concluded that after the first recycling, the adsorption of X-3B on the catalyst reached the saturation state, and washing could not remove the adsorbed X-3B and intermediates in the micropores. Therefore, the adsorbed X-3B and intermediates occupied the pore structure of micropores, leading to a greatly reduced adsorption during the second use. The removal of X-3B of the second recycling mainly contributed to degradation with •SO4−.

Figure 9.
Reuse experiments. (The degradation of X-3B (a) and the absorption of X-3B (b).)
2.5. Radical Quenching Experiment
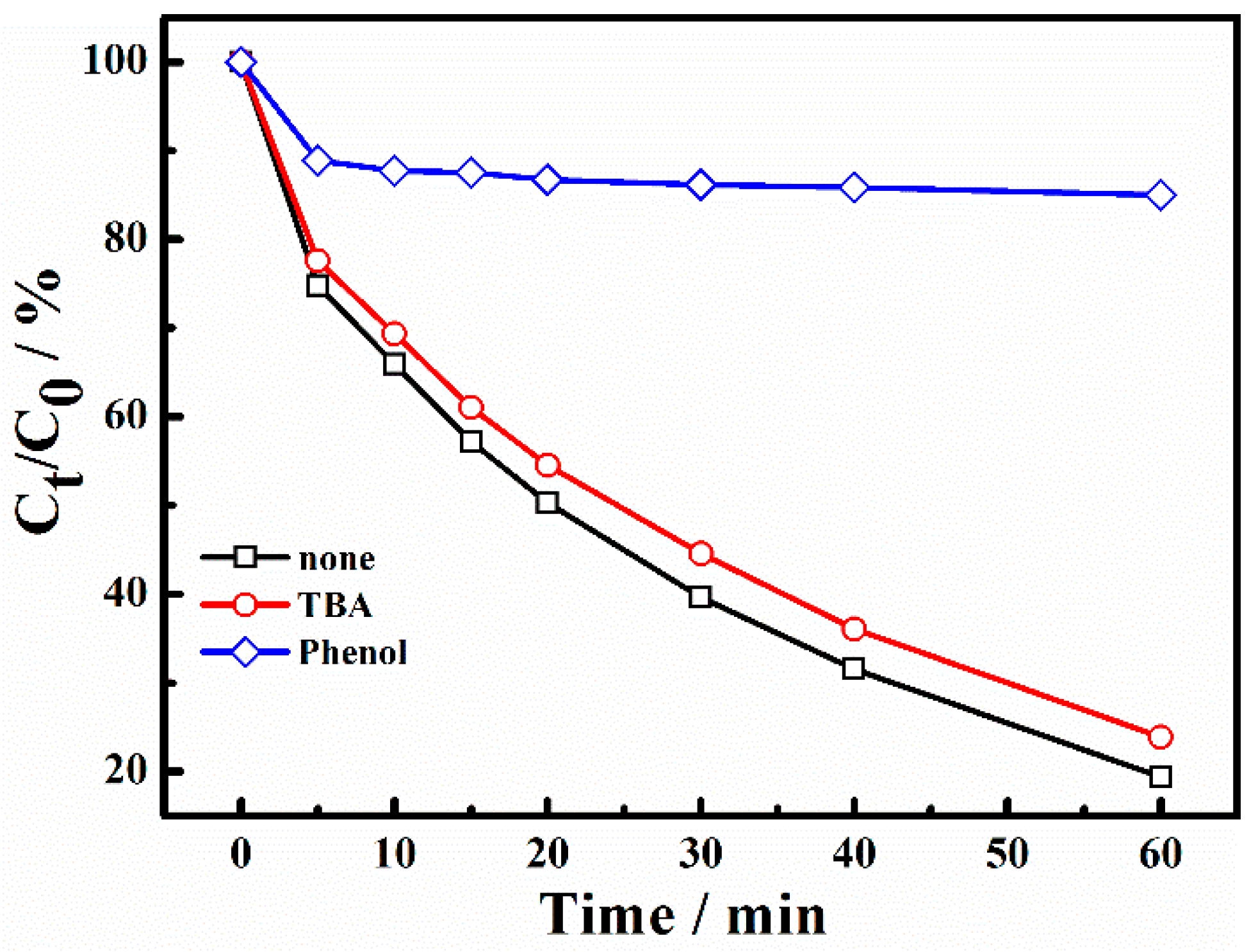
To identify the dominating species in X-3B degradation, the process was performed using different radical scavengers. Based on the literature, Tert-butanol (TBA) can be used as a scavenging agent for •OH (k•OH/TBA = 3.8 × 108 − 7.6 × 108 M−1s−1, k•SO4−/TBA = 4 × 105 − 9.1 × 105 M−1s−1) and phenol as a scavenging agent for both •SO4− and •OH (k•SO4−/phenol = 8.8 × 109 M−1s−1, k•OH/phenol = 6.6 × 109 M−1s−1) [4,12,36,37]. Experimental results of free radical capture are shown in Figure 10. As can be seen from the figure, when the molar ratio of TBA and reactants reached 1000/1, the degradation rate for 60 min was 74.4%. By contrast, the degradation rate was only 18.4% with the addition of phenol under the same molar ratio. It can be concluded that the inhibitory effect of TBA was not obvious. This means there was a small number of •OH in the system. The addition of phenol can inhibit the reaction sharply. The main active free radical in the system was •SO4−.
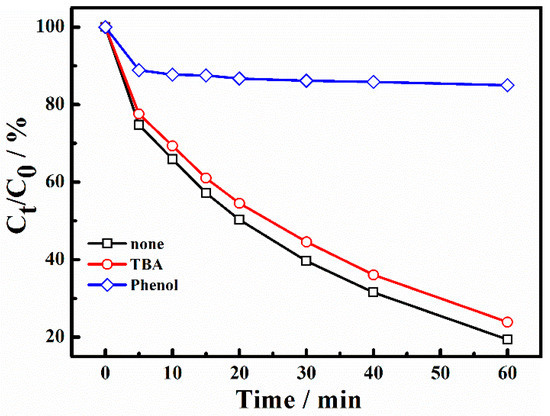
Figure 10.
The effect of radical scavengers on the degradation of X-3B. ([TBA]0 = 20 M, [Phenol]0 = 20 M).
3. Experimental Materials and Methods
3.1. Chemicals
X-3B was purchased from Lianyungang Shuangdie Dye Chemical Reagent Co. LTD, Lianyungang, China. K2S2O8, CuNO3∙3H2O and KOH were obtained from Tianjin Tianli Chemical Reagent Co. LTD, Tianjin, China. Chelate resin was purchased from Zhengzhou Qinshi Technology Co. LTD, Zhengzhou, China. All chemicals employed were used without further purification. All the solutions were prepared with deionized water.
3.2. Catalyst Preparation
In situ copper-loaded carbon-based catalysts (Cu-AC) were synthesized by chemical activation of copper adsorbed chelate resin with KOH as an activator under different annealing temperatures. Briefly, 10 g of dried resin and 3.6 g of copper nitrate hydrate were mixed with stirring for 24 h at room temperature. The resulting product was filtrated and washed several times, then dried at 120 °C overnight and carbonized at 600 °C for 2 h to obtain the carbonized material. Thereafter, the carbonized material and KOH in the mass ratio of 1:3 were activated under a nitrogen atmosphere at certain temperatures for 2 h. The obtained products were washed until neutral pH and then dried in an oven at 110 °C. The samples activated at 600, 700, and 800 °C were labeled as Cu-AC-600, Cu-AC-700, and Cu-AC-800, respectively.
3.3. Catalysis and Adsorption Experiments
The typical catalysis experiments were performed in 250 mL conical flasks containing 100 mL of X-3B solution at 25 °C without pH adjustment. The flasks were shaken in a constant temperature shaker. Unless otherwise specified, all experiments were conducted at initial X-3B concentration ([X-3B]0) of 100 mg/L, initial PS concentration ([PS]0) of 5.4 g/L, and catalyst dosage of 0.05 g/L at 25 °C. A control experiment with PS alone was also carried out. The adsorption experiments were conducted under the same conditions as the catalysis experiments only without the addition of PS. The experiments examining the effects of inorganic salt anions on degradation of X-3B were conducted at an initial Na2CO3, NaHCO3, and NaCl concentration of 1 mM. At given time intervals, a certain amount of solution was sampled and filtered. X-3B concentration was determined with a UV/VIS spectrophotometer at 540 nm.
3.4. Analytical Methods
X-ray diffraction (XRD) patterns were obtained on a Bruker AXS-D8 Advance model system using Cu target (λ = 1.5418 Å). The microstructure of the prepared samples was observed on a thermal field emission scanning electron microscope (SEM, JSM-7500F). X-ray photoelectron spectroscopic (XPS) analyses were performed via a specs spectrometer (AXIS ULTRA DLD) using an Al-Kα excitation source (1486.6 eV) to detect the elemental composition and the surface chemistry of the as-prepared samples. The specific surface area and pore structure of the material were determined by N2 adsorption/desorption analysis at 77 K using a Micromeritics ASAP 2020 system.
4. Conclusions
The as-prepared copper-loaded carbon-based catalysts using waste chelating resin adsorbed copper could effectively activate PS to degrade X-3B. With an increase in the activation temperature, the removal rate of X-3B in both adsorption and degradation systems was gradually improved owing to its large specific surface area and abundant reductive Cu+ and Cu0 on the surface. Both •SO4− and •OH promoted the degradation of X-3B, in which •SO4− dominated. CO32− and HCO3− had a certain inhibitory effect on the removal rate of X-3B attributed to its powerful ability to capture free radicals, while Cl− with strong reactivity with electron-rich groups showed a promotional effect. In summary, the guidance of the idea of “treating waste with waste” might provide some new insights in activating PS to degrade dye wastewater.
Author Contributions
Y.C. and Z.Z. designed the study and experiments; X.R. performed the experiments; Y.C. and X.R. analyzed results and drafted the paper; Z.Z. and Z.H. edited the paper. Y.C. and J.Y. administrated the funds. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Youth Scientific and Technological Foundation of Shanxi Province (201701D221232) and the Youth Scientific and Technological Foundation of Shanxi Province (201901D211580).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kang, X.; Cheng, Y.; Wen, Y.; Qi, J.; Li, X. Bio-inspired co-deposited preparation of GO composite loose nanofiltration membrane for dye contaminated wastewater sustainable treatment. J. Hazard. Mater. 2020, 400, 123121. [Google Scholar] [CrossRef] [PubMed]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef]
- Dominguez, J.R.; Beltran, J.; Rodriguez, O. Vis and UV photocatalytic detoxification methods (using TiO2, TiO2/H2O2, TiO2/O3, TiO2/S2O82−, O3, H2O2, S2O82−, Fe3+/H2O2 and Fe3+/H2O2/C2O42−) for dyes treatment. Catal. Today 2005, 101, 389–395. [Google Scholar] [CrossRef]
- Li, X.; Liao, F.; Ye, L.; Yeh, L. Controlled pyrolysis of MIL-88A to prepare iron/carbon composites for synergistic persulfate oxidation of phenol: Catalytic performance and mechanism. J. Hazard. Mater. 2020, 398, 122938. [Google Scholar] [CrossRef]
- Hazime, R.; Nguyen, Q.H.; Ferronato, C.; Salvador, A.; Jaber, F.; Chovelon, J.M. Comparative study of imazalil degradation in three systems: UV/TiO2, UV/K2S2O8 and UV/TiO2/K2S2O8. Appl. Catal. B Environ. 2014, 144, 286–291. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, Z.; Huang, Z.; Cui, Y. Acceleration of Persulfate Activation by MIL-101(Fe) with Vacuum Thermal Activation: Effect of FeII/FeIII Mixed-Valence Center. Catalysts 2019, 9, 906. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Du, X.Z.; Huang, W.L. Temperature effect on the kinetics of persulfate oxidation of p-chloroaniline. Chin. Chem. Lett. 2011, 22, 358–361. [Google Scholar] [CrossRef]
- Liang, C.J.; Bruell, C.J.; Marley, M.C.; Sperry, K.L. Persulfate oxidation for in situ remediation of TCE. I. Activated by ferrous ion with and without a persulfate-thiosulfate redox couple. Chemosphere 2004, 55, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Hori, H.; Yamamoto, A.; Hayakawa, E.; Taniyasu, S.; Yamashita, N.; Kutsuna, S. Efficient decomposition of environmentally persistent perfluorocarboxylic acids by use of persulfate as a photochemical oxidant. Environ. Sci. Technol. 2005, 39, 2383–2388. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.R.; Wang, S.B.; Ang, H.M.; Tade, M.O. Photocatalytic oxidation of phenolic compounds using zinc oxide and sulphate radicals under artificial solar light. Sep. Purif. Technol. 2010, 70, 338–344. [Google Scholar] [CrossRef]
- Xing, B.; Dong, J.W.; Yang, G.; Jiang, N.; Liu, X.Y.; Yuan, J.G. An insight into N, S-codoped activated carbon for the catalytic persulfate oxidation of organic pollutions in water: Effect of surface functionalization. Appl. Catal. A Gen. 2020, 602, 9. [Google Scholar] [CrossRef]
- Yang, S.; Yang, X.; Shao, X.; Niu, R.; Wang, L. Activated carbon catalyzed persulfate oxidation of Azo dye acid orange 7 at ambient temperature. J. Hazard. Mater. 2011, 186, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Akbari, A.; Arsalani, N.; Eftekhari-Sis, B.; Amini, M.; Gohari, G.; Jabbari, E. Cube-octameric silsesquioxane (POSS)-capped magnetic iron oxide nanoparticles for the efficient removal of methylene blue. Front. Chem. Sci. Eng. 2019, 13, 563–573. [Google Scholar] [CrossRef]
- Guo, R.; Chen, Y.; Nengzi, L.-C.; Meng, L.; Song, Q.; Gou, J.; Cheng, X. In situ preparation of carbon-based Cu-Fe oxide nanoparticles from CuFe Prussian blue analogues for the photo-assisted heterogeneous peroxymonosulfate activation process to remove lomefloxacin. Chem. Eng. J. 2020, 398, 125556. [Google Scholar] [CrossRef]
- Cao, Z.Q.; Zuo, C.Y.; Wu, H.Y. One step for synthesis of magnetic CuFe2O4 composites as photo-fenton catalyst for degradation organics. Mater. Chem. Phys. 2019, 237, 6. [Google Scholar] [CrossRef]
- Deng, J.; Xu, M.; Chen, Y.; Li, J.; Qiu, C.; Li, X.; Zhou, S. Highly-efficient removal of norfloxacin with nanoscale zero-valent copper activated persulfate at mild temperature. Chem. Eng. J. 2019, 366, 491–503. [Google Scholar] [CrossRef]
- Ghorbanian, Z.; Asgari, G.; Samadi, M.T.; Seid-mohammadi, A. Removal of 2,4 dichlorophenol using microwave assisted nanoscale zero-valent copper activated persulfate from aqueous solutions: Mineralization, kinetics, and degradation pathways. J. Mol. Liq. 2019, 296, 111873. [Google Scholar] [CrossRef]
- Ni, Y.-J.; Cheng, Y.-Q.; Xu, M.-Y.; Qiu, C.-G.; Ma, X.-Y.; Li, J.; Deng, J. Nanoscale Zero-valent Copper-Activated Molecular Oxygen for the Degradation of Enrofloxacin in Water. Huan Jing Ke Xue = Huanjing Kexue 2019, 40, 293–299. [Google Scholar]
- Qu, G.; Chu, R.; Wang, H.; Wang, T.; Zhang, Z.; Qiang, H.; Liang, D.; Hu, S. Simultaneous removal of chromium(VI) and tetracycline hydrochloride from simulated wastewater by nanoscale zero-valent iron/copper-activated persulfate. Environ. Sci. Pollut. Res. Int. 2020, 27, 40826–40836. [Google Scholar] [CrossRef]
- Ferreira de Sousa, P.V.; de Oliveira, A.F.; da Silva, A.A.; Vaz, B.G.; Lopes, R.P. Study of ciprofloxacin degradation by zero-valent copper nanoparticles. Chem. Pap. 2019, 73, 249–260. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, J.; Zhang, Y.; Zhang, G.; Li, W.; Wei, C.; Liang, J.; Liu, Y.; Shu, S. Degradation of 2,4-dichlorophenol by activating persulfate and peroxomonosulfate using micron or nanoscale zero-valent copper. J. Hazard. Mater. 2018, 344, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhang, G.Q.; Yan, L.F.; Kong, L.Q.; Zheng, H.Y.; Mi, J.; Li, Z. Carbon nanotube-supported Cu-based catalysts for oxidative carbonylation of methanol to methyl carbonate: Effect of nanotube pore size. Catal. Sci. Technol. 2020, 10, 2615–2626. [Google Scholar] [CrossRef]
- Ren, J.; Wang, W.; Wang, D.L.; Zuo, Z.J.; Lin, J.Y.; Li, Z. A theoretical investigation on the mechanism of dimethyl carbonate formation on Cu/AC catalyst. Appl. Catal. A Gen. 2014, 472, 47–52. [Google Scholar] [CrossRef]
- Liu, L.; Bao, R.; Yi, J.H. Mono-dispersed and homogeneous CNT/Cu composite powder preparation through forming Cu2O intermediates. Powder Technol. 2018, 328, 430–435. [Google Scholar] [CrossRef]
- Ulloa, L.; Martínez-Minchero, M.; Bringas, E.; Cobo, A.; San-Román, M.F. Split regeneration of chelating resins for the selective recovery of nickel and copper. Sep. Purif. Technol. 2020, 253, 117516. [Google Scholar] [CrossRef]
- Sirola, K.; Laatikainen, M.; Lahtinen, M.; Paatero, E. Removal of copper and nickel from concentrated ZnSO4 solutions with silica-supported chelating adsorbents. Sep. Purif. Technol. 2008, 64, 88–100. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Z.; Liu, Y.B.; Liu, Y. Fenton-like degradation of sulfamerazine at nearly neutral pH using Fe-Cu-CNTs and Al-0-CNTs for in-situ generation of H2O2/(OH)-O-center dot/O-2(center dot-). Chem. Eng. J. 2020, 396, 11. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Gu, P.; Li, X.Y.; Zhang, G.H. Efficient adsorption of radioactive iodide ion from simulated wastewater by nano Cu2O/Cu modified activated carbon. Chem. Eng. J. 2017, 322, 129–139. [Google Scholar] [CrossRef]
- Wu, X.; Meng, H.; Du, Y.L.; Liu, J.N.; Hou, B.H.; Xie, X.M. Insight into Cu2O/CuO collaboration in the selective catalytic reduction of NO with NH3: Enhanced activity and synergistic mechanism. J. Catal. 2020, 384, 72–87. [Google Scholar] [CrossRef]
- Wang, B.Q.; Fu, T.; An, B.H.; Liu, Y. UV light-assisted persulfate activation by Cu-0-Cu2O for the degradation of sulfamerazine. Sep. Purif. Technol. 2020, 251, 11. [Google Scholar] [CrossRef]
- Guo, Y.; Zeng, Z.; Liu, Y.; Huang, Z.; Cui, Y.; Yang, J. One-pot synthesis of sulfur doped activated carbon as a superior metal-free catalyst for the adsorption and catalytic oxidation of aqueous organics. J. Mater. Chem. A 2018, 6, 4055–4067. [Google Scholar] [CrossRef]
- Guo, Y.; Zeng, Z.; Zhu, Y.; Huang, Z.; Cui, Y.; Yang, J. Catalytic oxidation of aqueous organic contaminants by persulfate activated with sulfur-doped hierarchically porous carbon derived from thiophene. Appl. Catal. B Environ. 2018, 220, 635–644. [Google Scholar] [CrossRef]
- Medellin-Castillo, N.A.; Ocampo-Perez, R.; Leyva-Ramos, R.; Sanchez-Polo, M.; Rivera-Utrilla, J.; Mendez-Diaz, J.D. Removal of diethyl phthalate from water solution by adsorption, photo-oxidation, ozonation and advanced oxidation process (UV/H2O2, O3/H2O2 and O3/activated carbon). Sci. Total. Environ. 2013, 442, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Lutze, H.V.; Kerlin, N.; Schmidt, T.C. Sulfate radical-based water treatment in presence of chloride: Formation of chlorate, inter-conversion of sulfate radicals into hydroxyl radicals and influence of bicarbonate. Water Res. 2015, 72, 349–360. [Google Scholar] [CrossRef]
- Huang, Z.F.; Lin, Q.T.; Luo, H.Y.; Guo, P.R.; Weng, Q.S.; Lei, Y.Q.; Cheng, S.L.; Liu, S.S. Degradation of progesterone by coexisting free radical and nonradical pathways in the CuO/HNTs-PS system. Chem. Eng. J. 2020, 398, 10. [Google Scholar] [CrossRef]
- Ji, F.; Li, C.; Deng, L. Performance of CuO/Oxone system: Heterogeneous catalytic oxidation of phenol at ambient conditions. Chem. Eng. J. 2011, 178, 239–243. [Google Scholar] [CrossRef]
- Duan, X.G.; Sun, H.Q.; Wang, Y.X.; Kang, J.; Wang, S.B. N-doping-induced nonradical reaction on single-walled carbon nanotubes for catalytic phenol oxidation. ACS Catal. 2015, 5, 553–559. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).