Catalytic Combustion of Propane over Pt-Mo/ZSM-5 Catalyst: The Promotional Effects of Molybdenum
Abstract
:1. Introduction
2. Results and Discussion
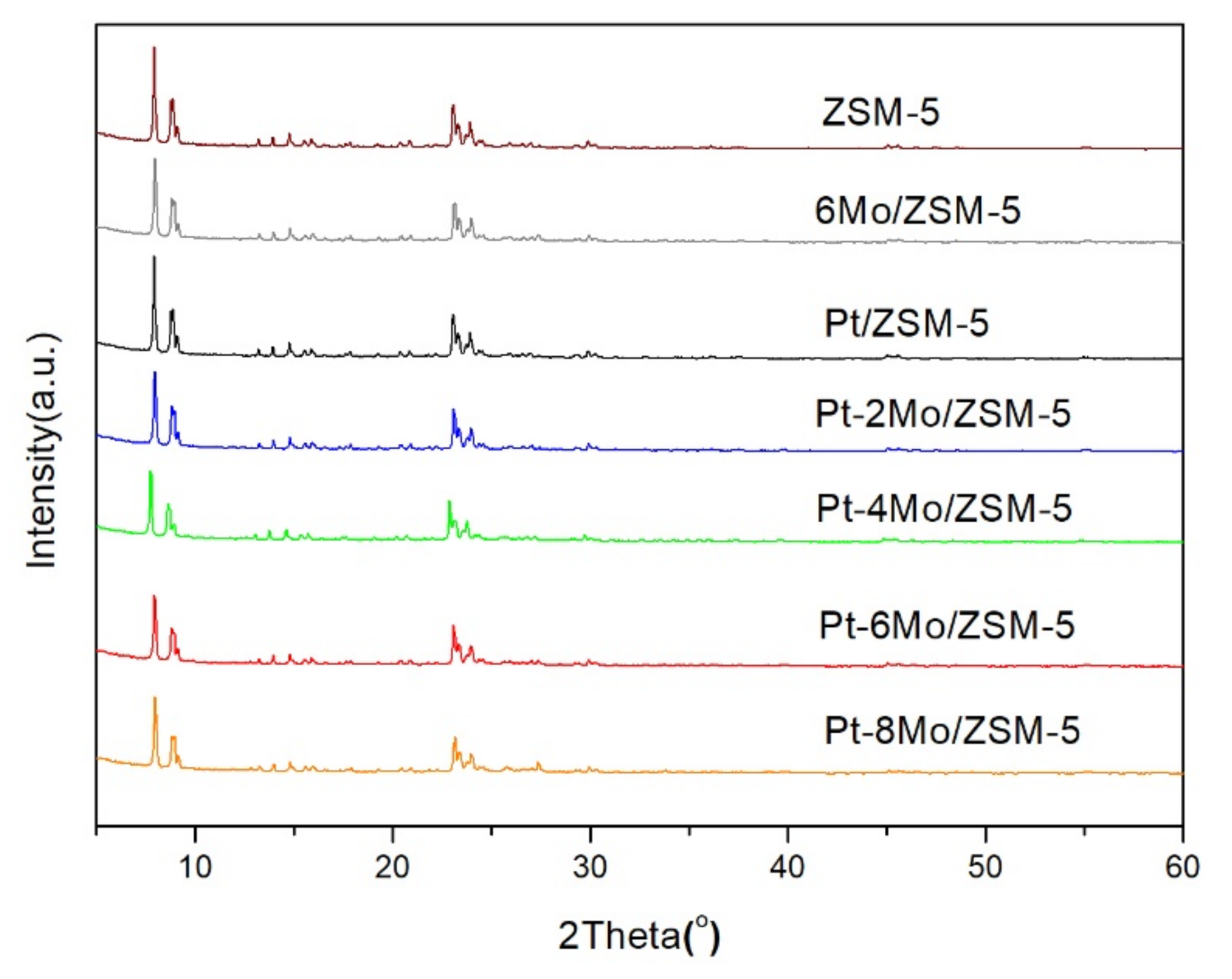
2.1. Textural Properties
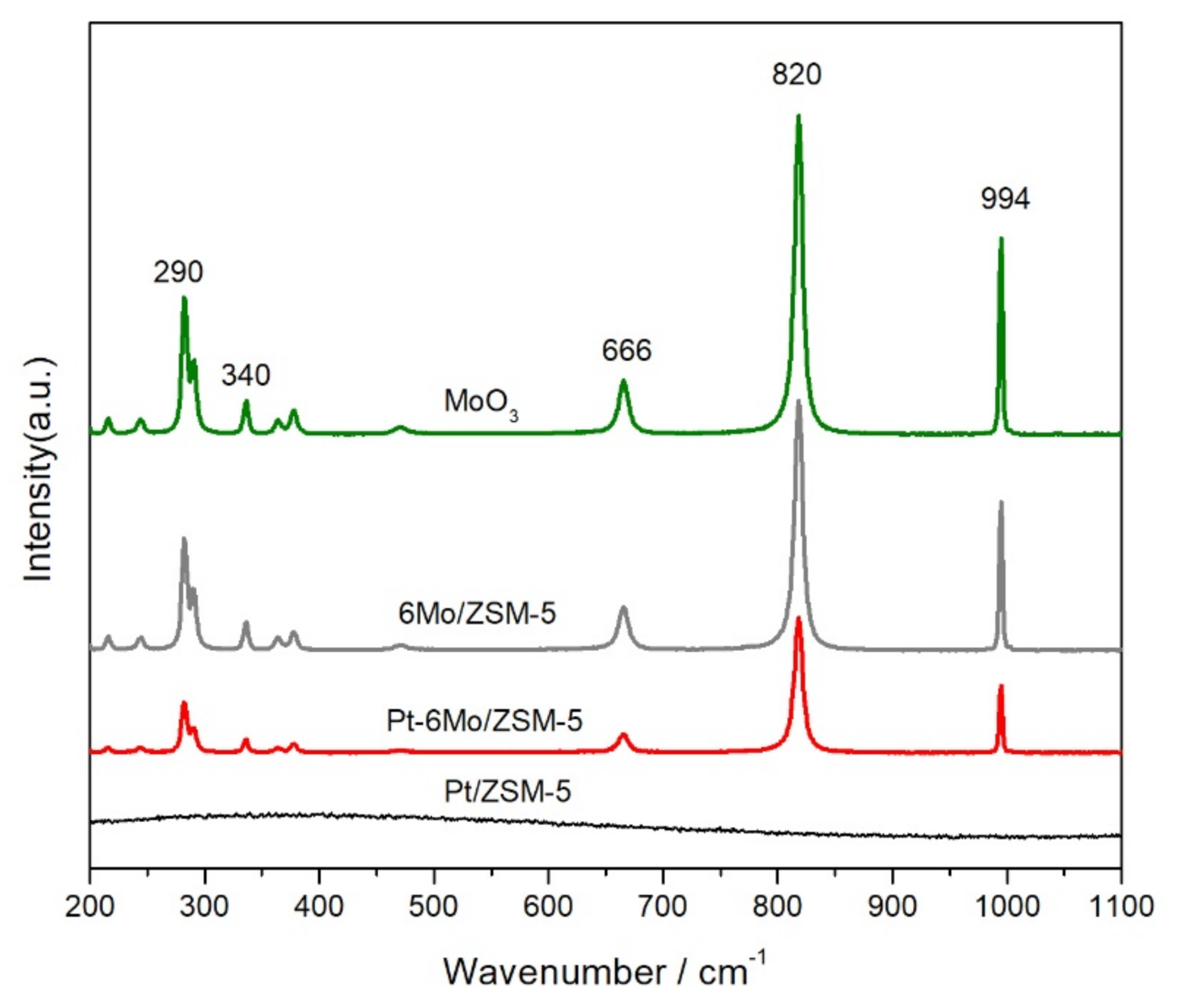
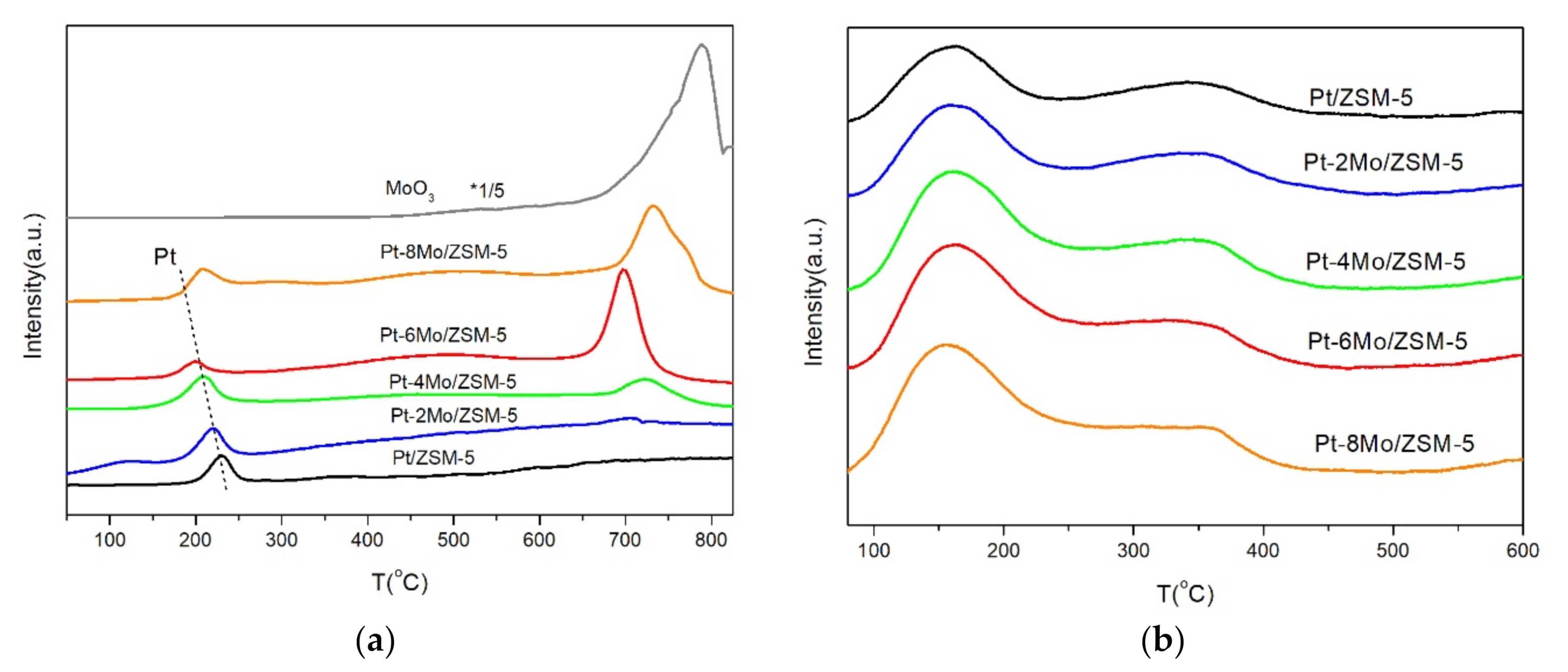
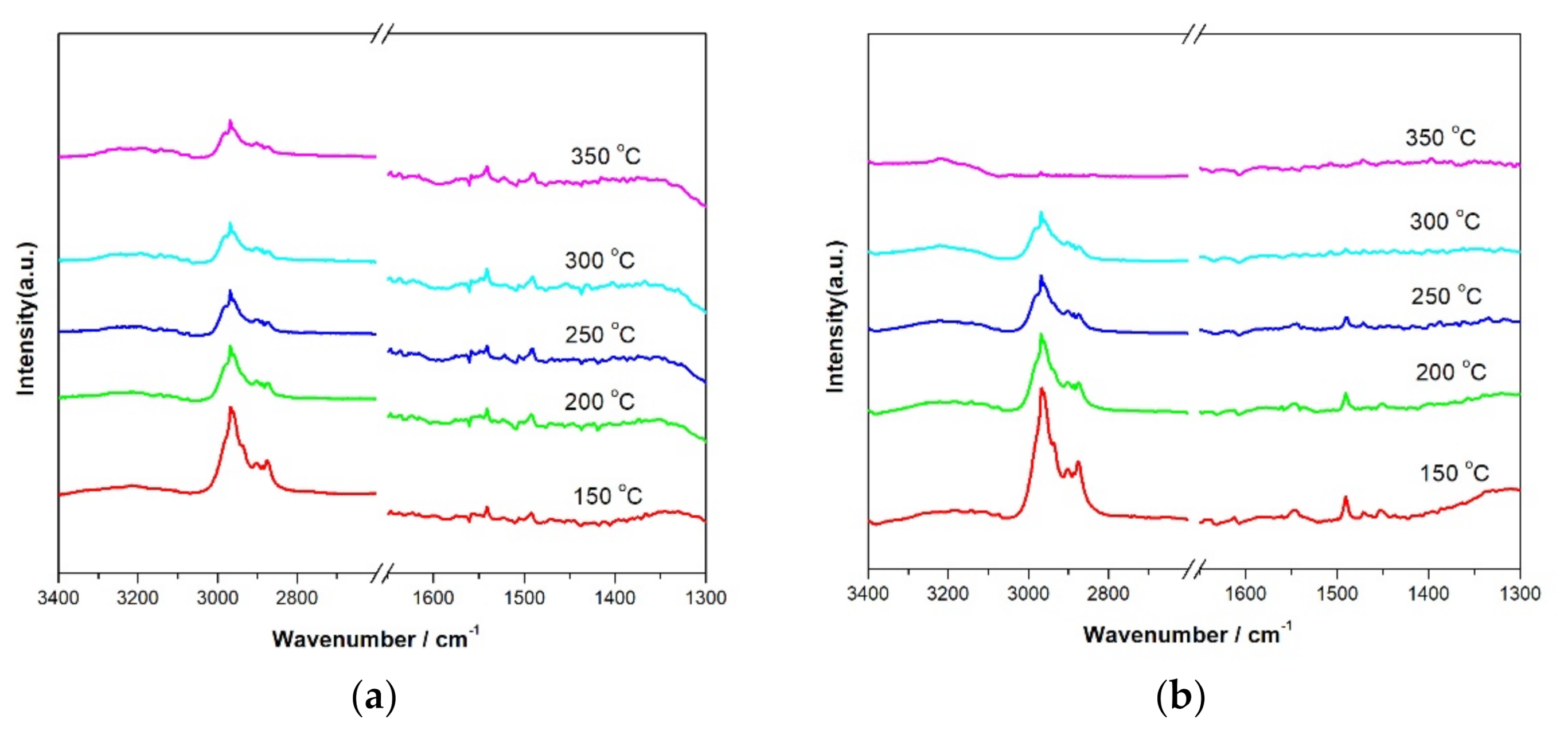
2.2. Redox and Acid Properties
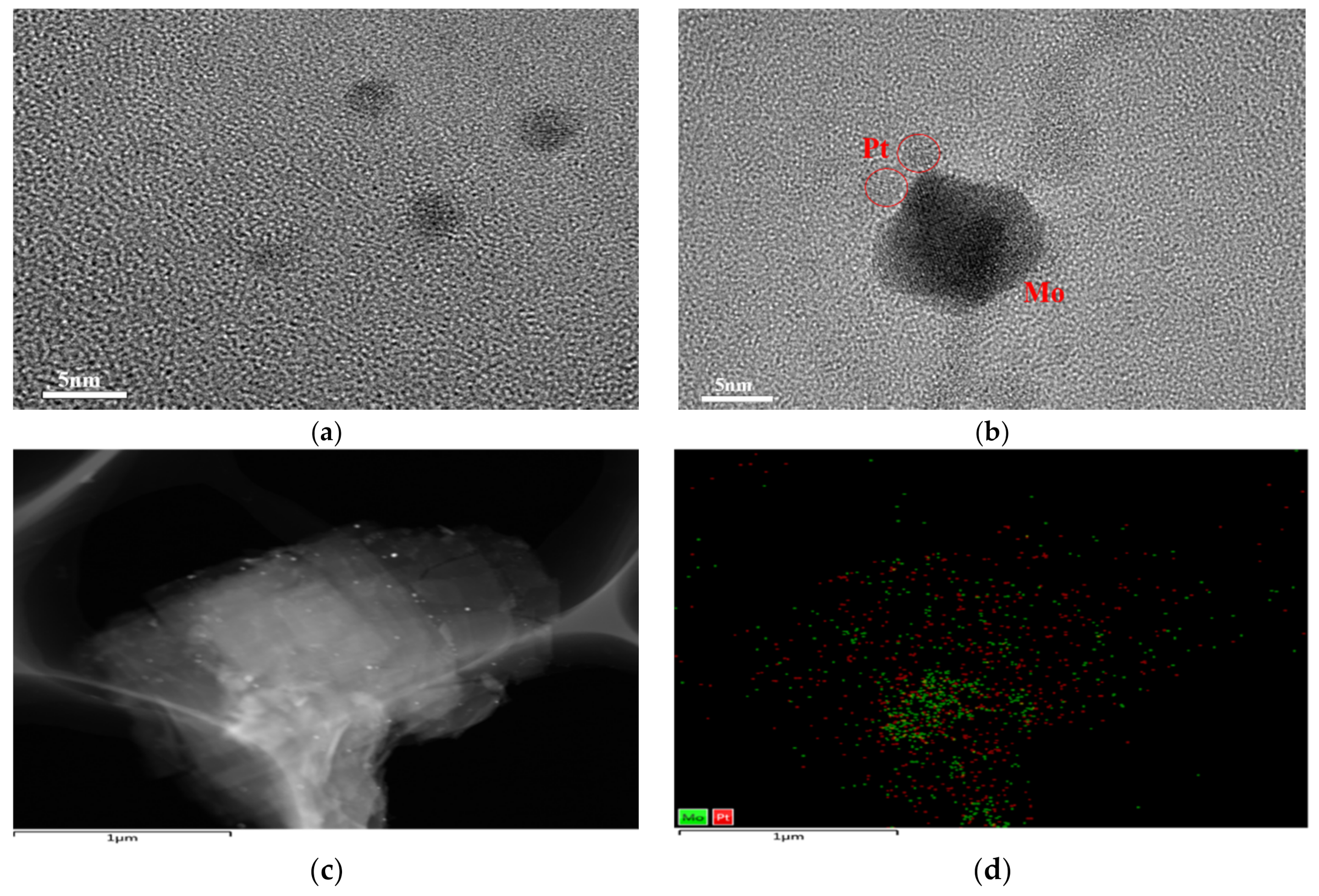
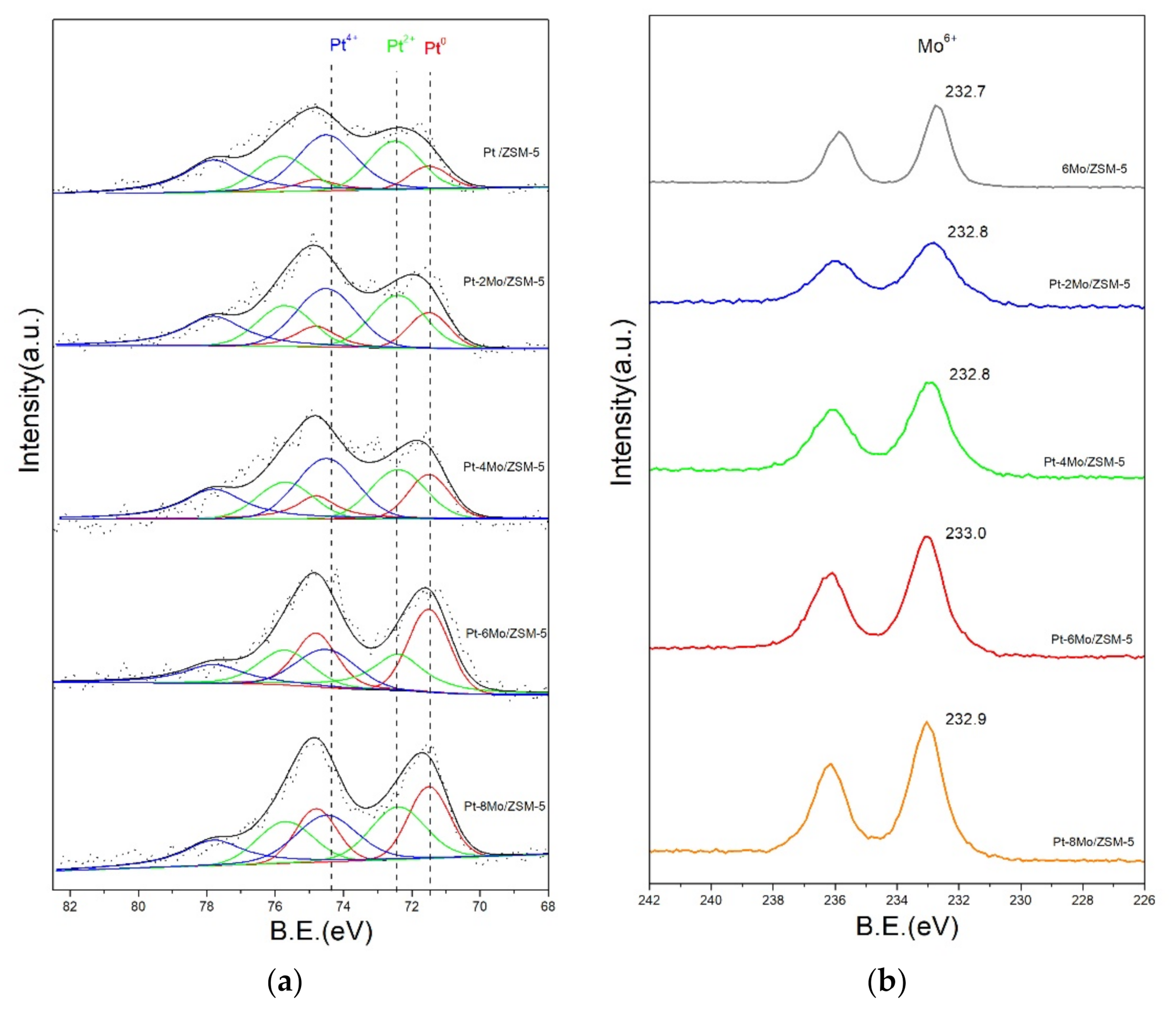
2.3. Surface Element Analysis
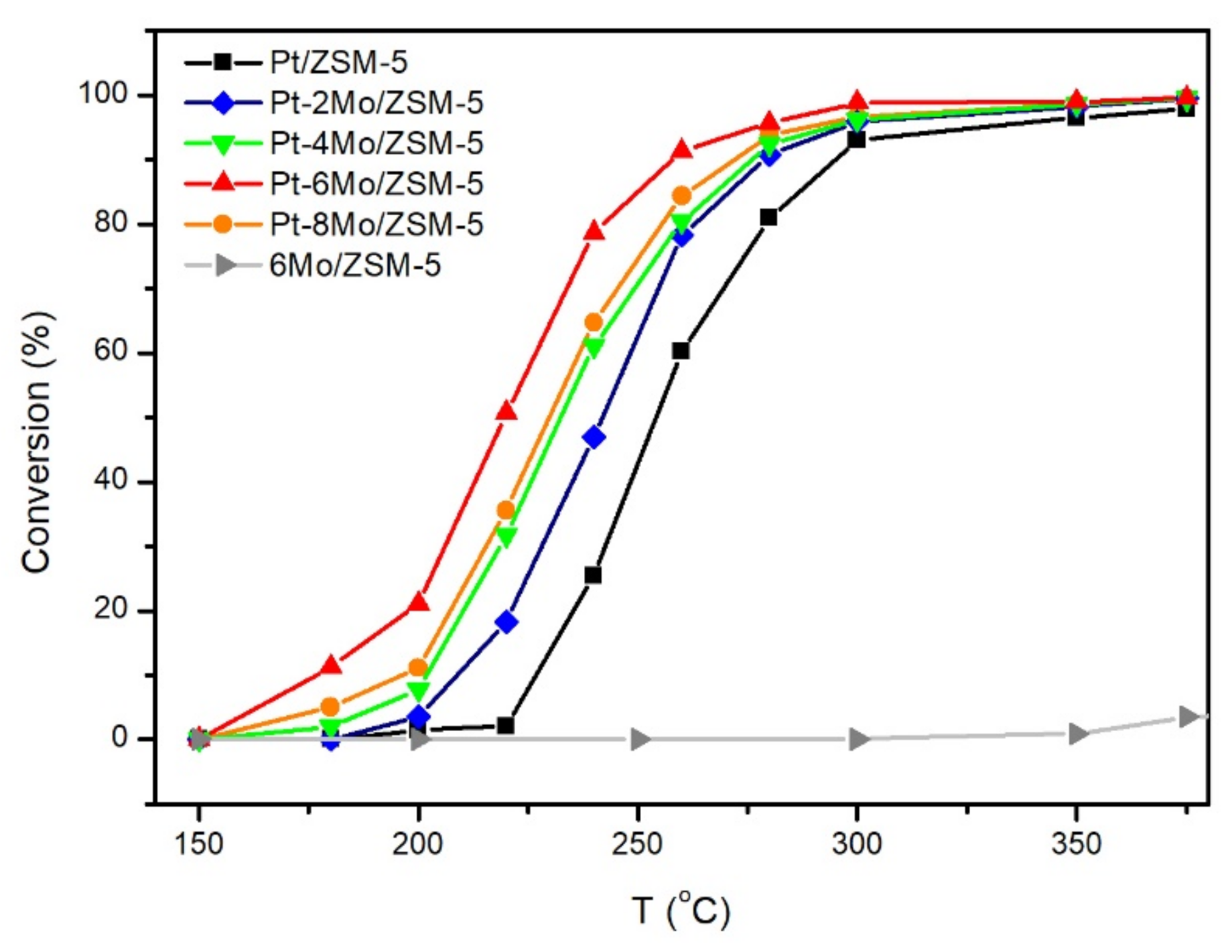
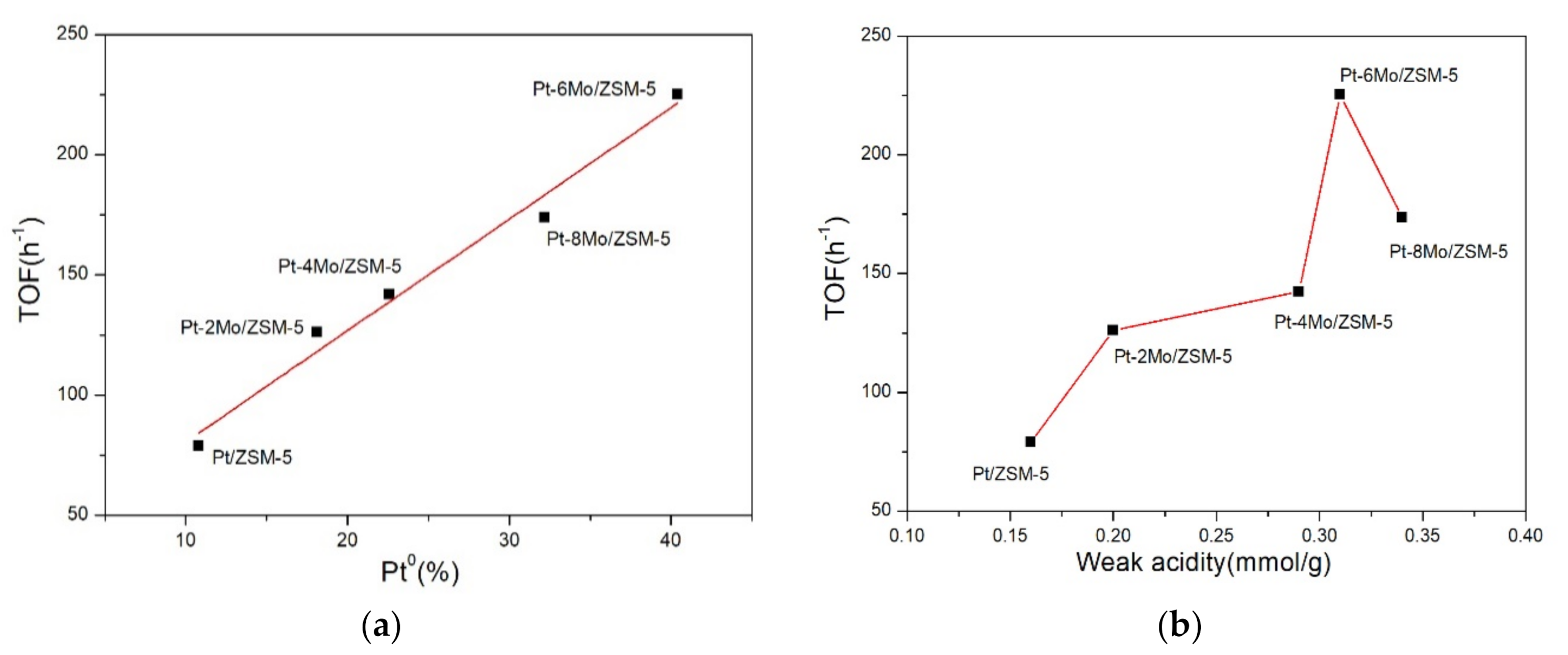
2.4. Catalytic Tests of Propane Catalytic Combustion
2.4.1. Activity of Propane Catalytic Combustion
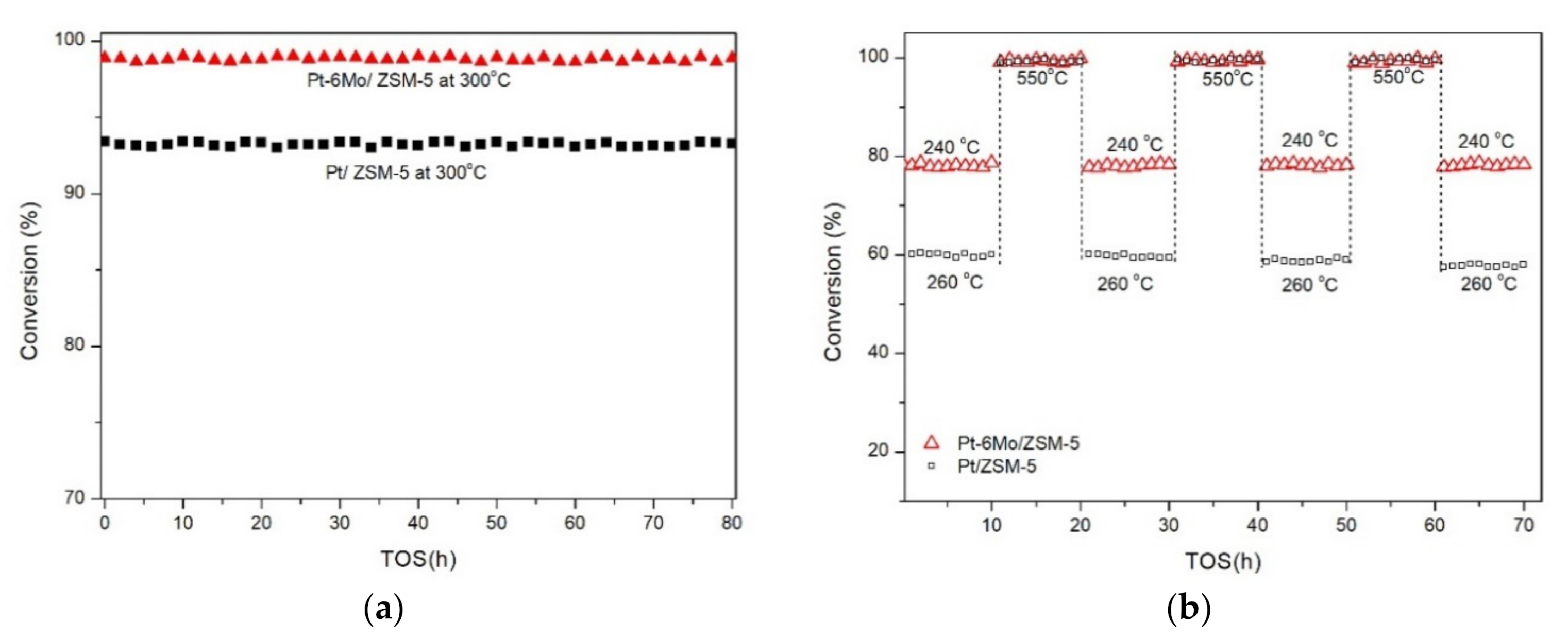
2.4.2. Stability of Propane Catalytic Combustion
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Activity Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zheng, B.; Tong, D.; Li, M.; Liu, F.; Hong, C.; Geng, G.; Li, H.; Li, X.; Peng, L.; Qi, J.; et al. Trends in China’s anthropogenic emissions since 2010 as the consequence of clean air actions. Atmos. Chem. Phys. Discuss. 2018, 18, 14095–14111. [Google Scholar] [CrossRef] [Green Version]
- Cetin, E.; Odabasi, M.; Seyfioglu, R. Ambient volatile organic compound (VOC) concentrations around a petrochemical complex and a petroleum refinery. Sci. Total Environ. 2003, 312, 103–112. [Google Scholar] [CrossRef]
- Klimont, Z.; Streets, D.G.; Gupta, S.; Cofala, J.; Lixin, F.; Ichikawa, Y. Anthropogenic emissions of non-methane volatile organic compounds in China. Atmos. Environ. 2002, 36, 1309–1322. [Google Scholar] [CrossRef]
- Huang, H.; Xu, Y.; Feng, Q.; Leung, D.Y.C. Low temperature catalytic oxidation of volatile organic compounds: A review. Catal. Sci. Technol. 2015, 5, 2649–2669. [Google Scholar] [CrossRef]
- Kołodziej, A.; Łojewska, J. Optimization of structured catalyst carriers for VOC combustion. Catal. Today 2005, 105, 378–384. [Google Scholar] [CrossRef]
- Khan, F.I.; Kr. Ghoshal, A. Removal of Volatile Organic Compounds from polluted air. J. Loss Prev. Process Ind. 2000, 13, 527–545. [Google Scholar] [CrossRef]
- Garcia, T.; Solsona, B.; Taylor, S.H. The Oxidative Destruction of Hydrocarbon Volatile Organic Compounds Using Palladium–Vanadia–Titania Catalysts. Catal. Lett. 2004, 97, 99–103. [Google Scholar] [CrossRef]
- Aryafar, M.; Zaera, F. Kinetic study of the catalytic oxidation of alkanes over nickel, palladium, and platinum foils. Catal. Lett. 1997, 48, 173–183. [Google Scholar] [CrossRef]
- Yazawa, Y.; Yoshida, H.; Takagi, N.; Komai, S.-I.; Satsuma, A.; Hattori, T. Acid Strength of Support Materials as a Factor Controlling Oxidation State of Palladium Catalyst for Propane Combustion. J. Catal. 1999, 187, 15–23. [Google Scholar] [CrossRef]
- Ivanova, S.; Petit, C.; Pitchon, V. Application of heterogeneous gold catalysis with increased durability: Oxidation of CO and hydrocarbons at low temperature. Gold Bull. 2006, 39, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Ruth, K.; Hayes, M.; Burch, R.; Tsubota, S.; Haruta, M. The effects of SO2 on the oxidation of CO and propane on supported Pt and Au catalysts. Appl. Catal. B Environ. 2000, 24, L133–L138. [Google Scholar] [CrossRef]
- Okal, J.; Zawadzki, M. Influence of Catalyst Pretreatments on Propane Oxidation Over Ru/γ-Al2O3. Catal. Lett. 2009, 132, 225–234. [Google Scholar] [CrossRef]
- Enterkin, J.A.; Setthapun, W.; Elam, J.W.; Christensen, S.T.; Rabuffetti, F.A.; Marks, L.D.; Stair, P.C.; Poeppelmeier, K.R.; Marshall, C.L. Propane Oxidation over Pt/SrTiO3 Nanocuboids. Acs Catal. 2011, 1, 629–635. [Google Scholar] [CrossRef]
- Yazawa, Y.; Yoshida, H.; Takagi, N.; Komai, S.-I.; Satsuma, A.; Hattori, T. Oxidation state of palladium as a factor controlling catalytic activity of Pd/SiO2–Al2O3 in propane combustion. Appl. Catal. B Environ. 1998, 19, 261–266. [Google Scholar] [CrossRef]
- Avila, M.S.; Vignatti, C.I.; Apesteguía, C.R.; Garetto, T.F. Effect of support on the deep oxidation of propane and propylene on Pt-based catalysts. Chem. Eng. J. 2014, 241, 52–59. [Google Scholar] [CrossRef]
- Taylor, M.N.; Zhou, W.; Garcia, T.; Solsona, B.; Carley, A.F.; Kiely, C.J.; Taylor, S.H. Synergy between tungsten and palladium supported on titania for the catalytic total oxidation of propane. J. Catal. 2012, 285, 103–114. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, X.; Meng, D.; Guo, Y.; Guo, Y.; Lu, G. Effect of Ceria Crystal Plane on the Physicochemical and Catalytic Properties of Pd/Ceria for CO and Propane Oxidation. ACS Catal. 2016, 6, 2265–2279. [Google Scholar] [CrossRef]
- Yazawa, Y.; Takagi, N.; Yoshida, H.; Komai, S.-i.; Satsuma, A.; Tanaka, T.; Yoshida, S.; Hattori, T. The support effect on propane combustion over platinum catalyst: Control of the oxidation-resistance of platinum by the acid strength of support materials. Appl. Catal. A Gen. 2002, 233, 103–112. [Google Scholar] [CrossRef]
- Garetto, T.F.; Rincón, E.; Apesteguía, C.R. The origin of the enhanced activity of Pt/zeolites for combustion of C2–C4 alkanes. Appl. Catal. B Environ. 2007, 73, 65–72. [Google Scholar] [CrossRef]
- Garetto, T.F.; Rincón, E.; Apesteguıa, C.R. Deep oxidation of propane on Pt-supported catalysts: Drastic turnover rate enhancement using zeolite supports. Appl. Catal. B Environ. 2004, 48, 167–174. [Google Scholar] [CrossRef]
- Yazawa, Y.; Yoshida, H.; Komai, S.-i.; Hattori, T. The additive effect on propane combustion over platinum catalyst: Control of the oxidation-resistance of platinum by the electronegativity of additives. Appl. Catal. A Gen. 2002, 233, 113–124. [Google Scholar] [CrossRef]
- Avila, M.S.; Vignatti, C.I.; Apesteguía, C.R.; Venkat Rao, V.; Chary, K.; Garetto, T.F. Effect of V2O5 Loading on Propane Combustion over Pt/V2O5–Al2O3 Catalysts. Catal. Lett. 2009, 134, 118–123. [Google Scholar] [CrossRef]
- Garcia, T.; Agouram, S.; Taylor, S.H.; Morgan, D.; Dejoz, A.; Vázquez, I.; Solsona, B. Total oxidation of propane in vanadia-promoted platinum-alumina catalysts: Influence of the order of impregnation. Catal. Today 2015, 254, 12–20. [Google Scholar] [CrossRef]
- Zhu, Z.; Lu, G.; Guo, Y.; Guo, Y.; Zhang, Z.; Wang, Y.; Gong, X.-Q. High Performance and Stability of the Pt-W/ZSM-5 Catalyst for the Total Oxidation of Propane: The Role of Tungsten. ChemCatChem 2013, 5, 2495–2503. [Google Scholar] [CrossRef]
- Zhao, P.; Li, X.; Liao, W.; Wang, Y.; Chen, J.; Lu, J.; Luo, M. Understanding the Role of NbOx on Pt/Al2O3 for Effective Catalytic Propane Oxidation. Ind. Eng. Chem. Res. 2019, 58, 21945–21952. [Google Scholar] [CrossRef]
- Adamska, K.; Okal, J.; Tylus, W. Stable bimetallic Ru-Mo/Al2O3 catalysts for the light alkane combustion: Effect of the Mo addition. Appl. Catal. B Environ. 2019, 246, 180–194. [Google Scholar] [CrossRef]
- Ou, J.Z.; Campbell, J.L.; Yao, D.; Wlodarski, W.; Kalantar-zadeh, K. In Situ Raman Spectroscopy of H2 Gas Interaction with Layered MoO3. J. Phys. Chem. C 2011, 115, 10757–10763. [Google Scholar] [CrossRef]
- Chempath, S.; Zhang, Y.; Bell, A.T. DFT Studies of the Structure and Vibrational Spectra of Isolated Molybdena Species Supported on Silica. J. Phys. Chem. C 2007, 111, 1291–1298. [Google Scholar] [CrossRef]
- Cheng, C.P.; Schrader, G.L. Characterization of supported molybdate catalysts during preparation using laser Raman spectroscopy. J. Catal. 1979, 60, 276–294. [Google Scholar] [CrossRef]
- Lieske, H.; Lietz, G.; Spindler, H.; Völter, J. Reactions of platinum in oxygen- and hydrogen-treated Pt γ-Al2O3 catalysts. I. Temperature-programmed reduction, adsorption, and redispersion of platinum. J. Catal. 1983, 81, 8–16. [Google Scholar] [CrossRef]
- López Cordero, R.; López Agudo, A. Effect of water extraction on the surface properties of Mo/Al2O3 and NiMo/Al2O3 hydrotreating catalysts. Appl. Catal. A Gen. 2000, 202, 23–35. [Google Scholar] [CrossRef]
- Ballesteros-Plata, D.; Infantes-Molina, A.; Rodríguez-Cuadrado, M.; Rodríguez-Aguado, E.; Braos-García, P.; Rodríguez-Castellón, E. Incorporation of molybdenum into Pd and Pt catalysts supported on commercial silica for hydrodeoxygenation reaction of dibenzofuran. Appl. Catal. A Gen. 2017, 547, 86–95. [Google Scholar] [CrossRef]
- Boufaden, N.; Akkari, R.; Pawelec, B.; Fierro, J.L.G.; Zina, M.S.; Ghorbel, A. Dehydrogenation of methylcyclohexane to toluene over partially reduced silica-supported Pt-Mo catalysts. J. Mol. Catal. A Chem. 2016, 420, 96–106. [Google Scholar] [CrossRef]
- Kobayashi, M.; Morita, A.; Ikeda, M. The support effect in oxidizing atmosphere on propane combustion over platinum supported on TiO2, TiO2–SiO2 and TiO2–SiO2–WO3. Appl. Catal. B Environ. 2007, 71, 94–100. [Google Scholar] [CrossRef]
- Carlsson, P.-A.; Mollner, S.; Arnby, K.; Skoglundh, M. Effect of periodic operation on the low-temperature activity for propane oxidation over Pt/Al2O3 catalysts. Chem. Eng. Sci. 2004, 59, 4313–4323. [Google Scholar] [CrossRef]
- Kazansky, V.B.; Subbotina, I.R.; Jentoft, F.C.; Schlögl, R. Intensities of C−H IR Stretching Bands of Ethane and Propane Adsorbed by Zeolites as a New Spectral Criterion of Their Chemical Activation via Polarization Resulting from Stretching of Chemical Bonds. J. Phys. Chem. B 2006, 110, 17468–17477. [Google Scholar] [CrossRef]
- Yao, J.; Shi, H.; Sun, D.; Lu, H.; Hou, B.; Jia, L.; Xiao, Y.; Li, D. Facet-Dependent Activity of Co3O4 Catalyst for C3H8 Combustion. ChemCatChem 2019, 11, 5570–5579. [Google Scholar] [CrossRef]

| Sample | Pt (wt.%) a | Mo (wt.%) a | Dispersion of Pt (%) b | SBET (m2/g) | Volume of Pores (cm3/g) | dPt (nm) c |
|---|---|---|---|---|---|---|
| Pt/ZSM-5 | 0.98 | — | 31.4 | 299.2 | 0.134 | 2.75 |
| Pt-2Mo/ZSM-5 | 0.99 | 1.95 | 33.1 | 283.8 | 0.116 | 2.50 |
| Pt-4Mo/ZSM-5 | 0.97 | 3.86 | 36.7 | 266.1 | 0.115 | 1.91 |
| Pt-6Mo/ZSM-5 | 0.95 | 5.93 | 34.2 | 265.8 | 0.113 | 2.15 |
| Pt-8Mo/ZSM-5 | 0.97 | 7.75 | 37.8 | 248.0 | 0.107 | 1.82 |
| Sample | Weak Acidity (mmol/g) | Strong Acidity (mmol/g) | Total Acidity (mmol/g) |
|---|---|---|---|
| Pt/ZSM-5 | 0.16 | 0.13 | 0.29 |
| Pt-2Mo/ZSM-5 | 0.20 | 0.14 | 0.34 |
| Pt-4Mo/ZSM-5 | 0.29 | 0.14 | 0.43 |
| Pt-6Mo/ZSM-5 | 0.31 | 0.15 | 0.45 |
| Pt-8Mo/ZSM-5 | 0.34 | 0.13 | 0.47 |
| Sample | Pt0 (%) | Pt2+ (%) | Pt4+ (%) |
|---|---|---|---|
| Pt/ZSM-5 | 10.8 | 35.9 | 53.3 |
| Pt-2Mo/ZSM-5 | 18.1 | 37.7 | 44.2 |
| Pt-4Mo/ZSM-5 | 22.6 | 33.1 | 44.3 |
| Pt-6Mo/ZSM-5 | 40.4 | 32.8 | 26.8 |
| Pt-8Mo/ZSM-5 | 32.2 | 33.6 | 34.2 |
| Sample | T10 (°C) | T50 (°C) | T90 (°C) | TOF (h−1) a |
|---|---|---|---|---|
| Pt/ZSM-5 | 230 | 255 | 295 | 79 |
| Pt-2Mo/ZSM-5 | 210 | 240 | 280 | 126 |
| Pt-4Mo/ZSM-5 | 202 | 228 | 275 | 142 |
| Pt-6Mo/ZSM-5 | 180 | 215 | 255 | 225 |
| Pt-8Mo/ZSM-5 | 200 | 225 | 270 | 174 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, Z.; Zha, K.; Sun, W.; Huang, Z.; Xu, H.; Shen, W. Catalytic Combustion of Propane over Pt-Mo/ZSM-5 Catalyst: The Promotional Effects of Molybdenum. Catalysts 2020, 10, 1377. https://doi.org/10.3390/catal10121377
Liao Z, Zha K, Sun W, Huang Z, Xu H, Shen W. Catalytic Combustion of Propane over Pt-Mo/ZSM-5 Catalyst: The Promotional Effects of Molybdenum. Catalysts. 2020; 10(12):1377. https://doi.org/10.3390/catal10121377
Chicago/Turabian StyleLiao, Zhenan, Kaiwen Zha, Wenjie Sun, Zhen Huang, Hualong Xu, and Wei Shen. 2020. "Catalytic Combustion of Propane over Pt-Mo/ZSM-5 Catalyst: The Promotional Effects of Molybdenum" Catalysts 10, no. 12: 1377. https://doi.org/10.3390/catal10121377






