Synthesis, Characterization and Photocatalytic Activity of MoS2/ZnSe Heterostructures for the Degradation of Levofloxacin
Abstract
:1. Introduction
2. Results and Discussion
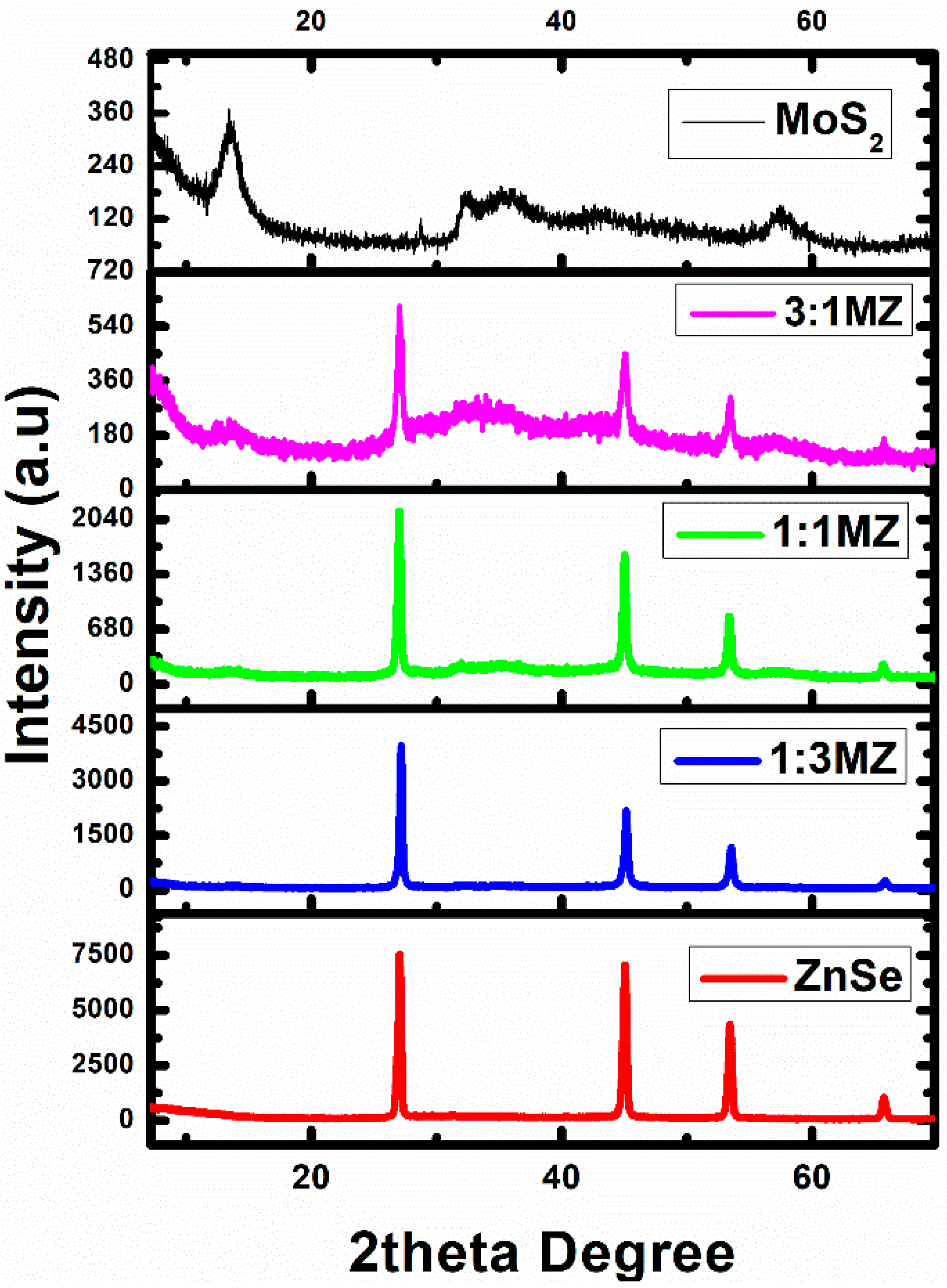
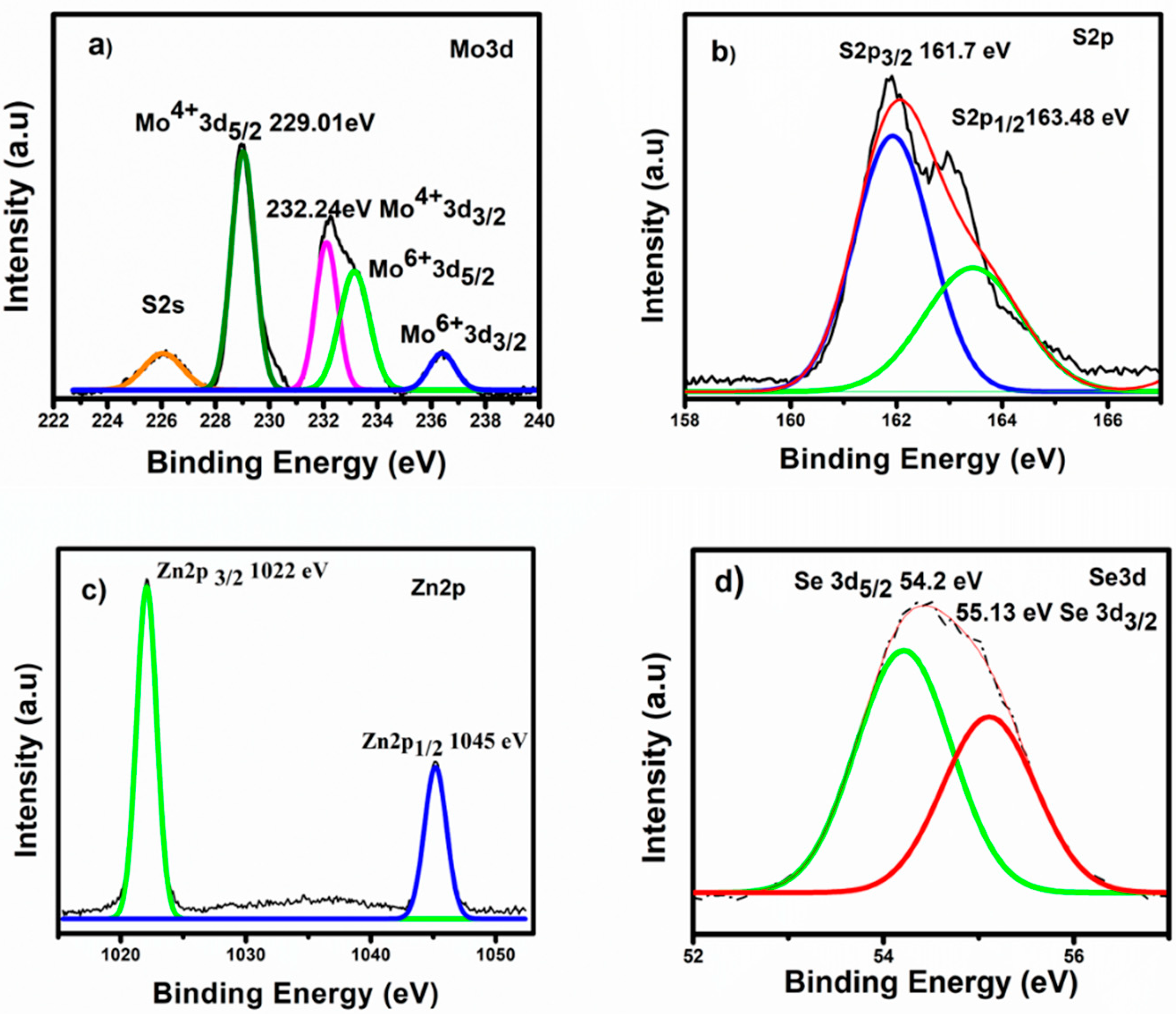
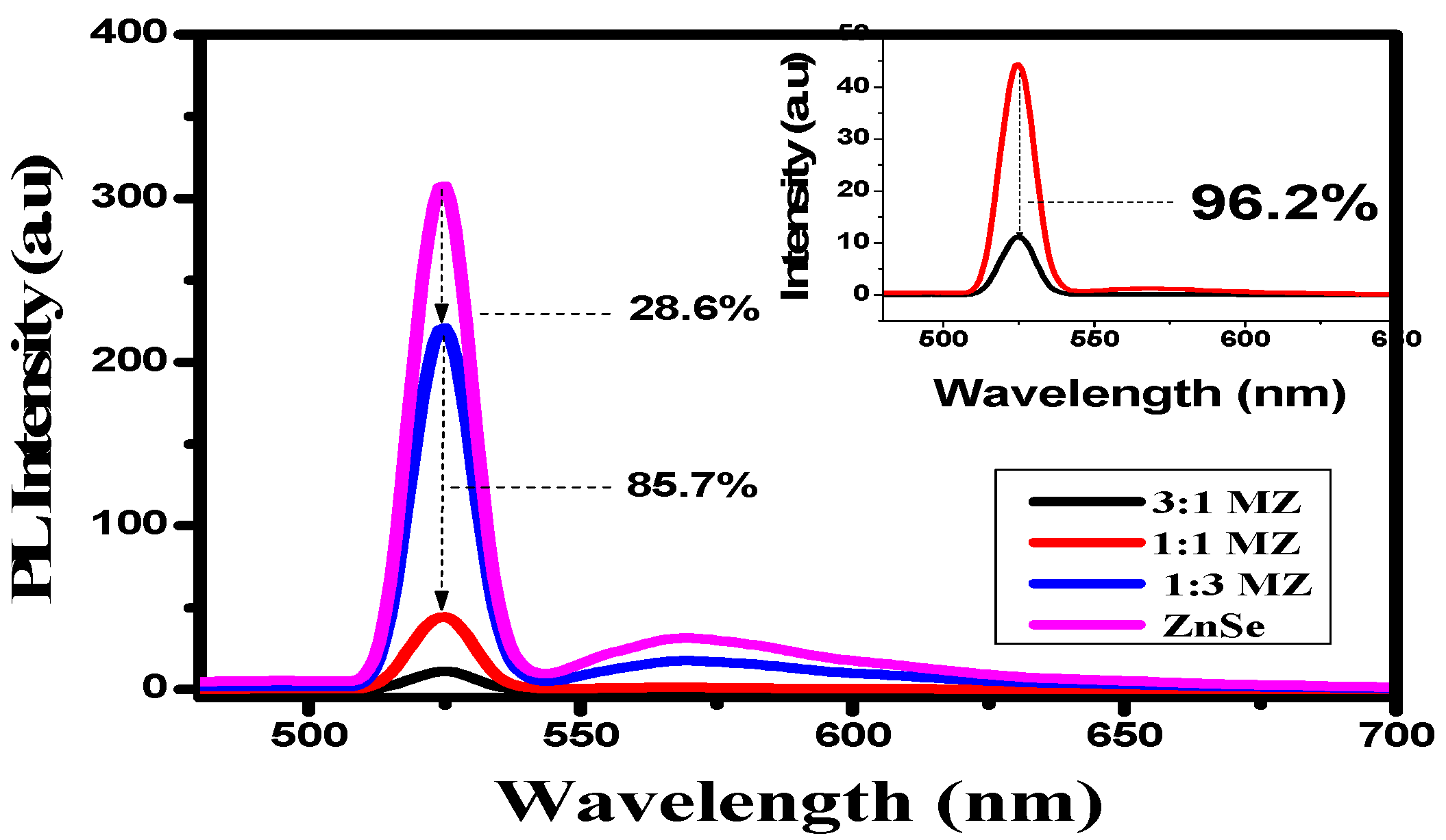
2.1. Phase and Microstructural Analysis
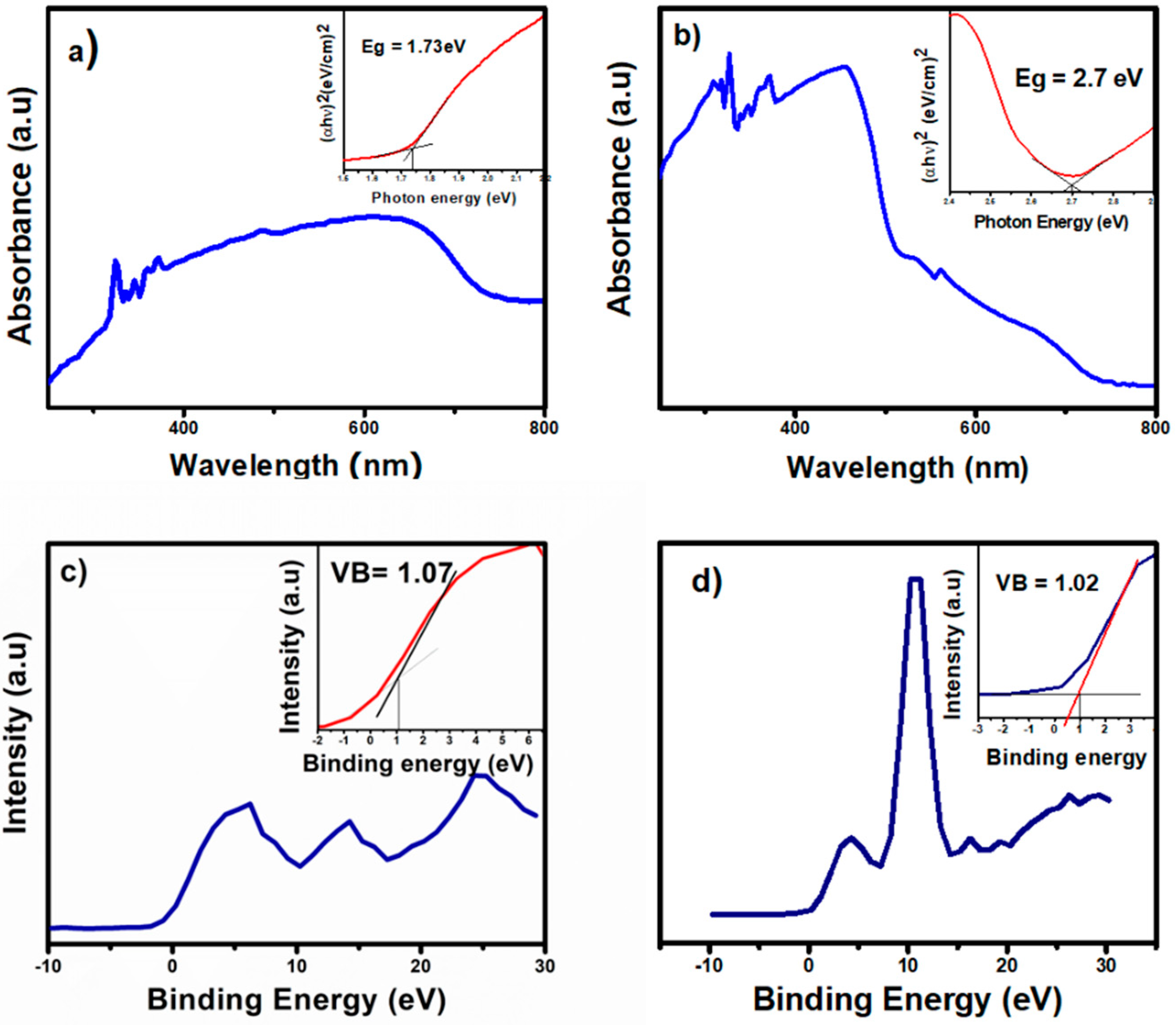
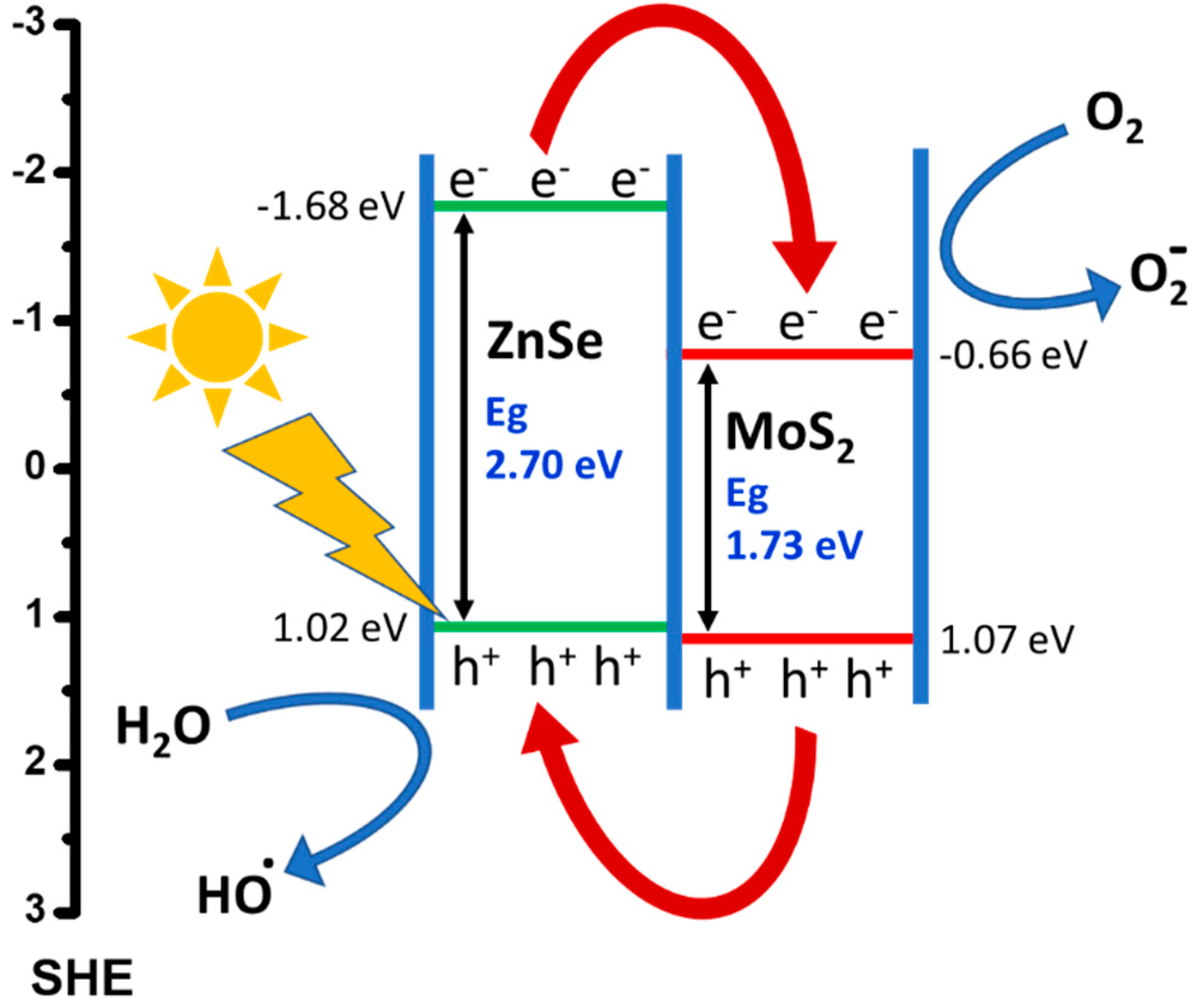
2.2. Alignment of Energy Level
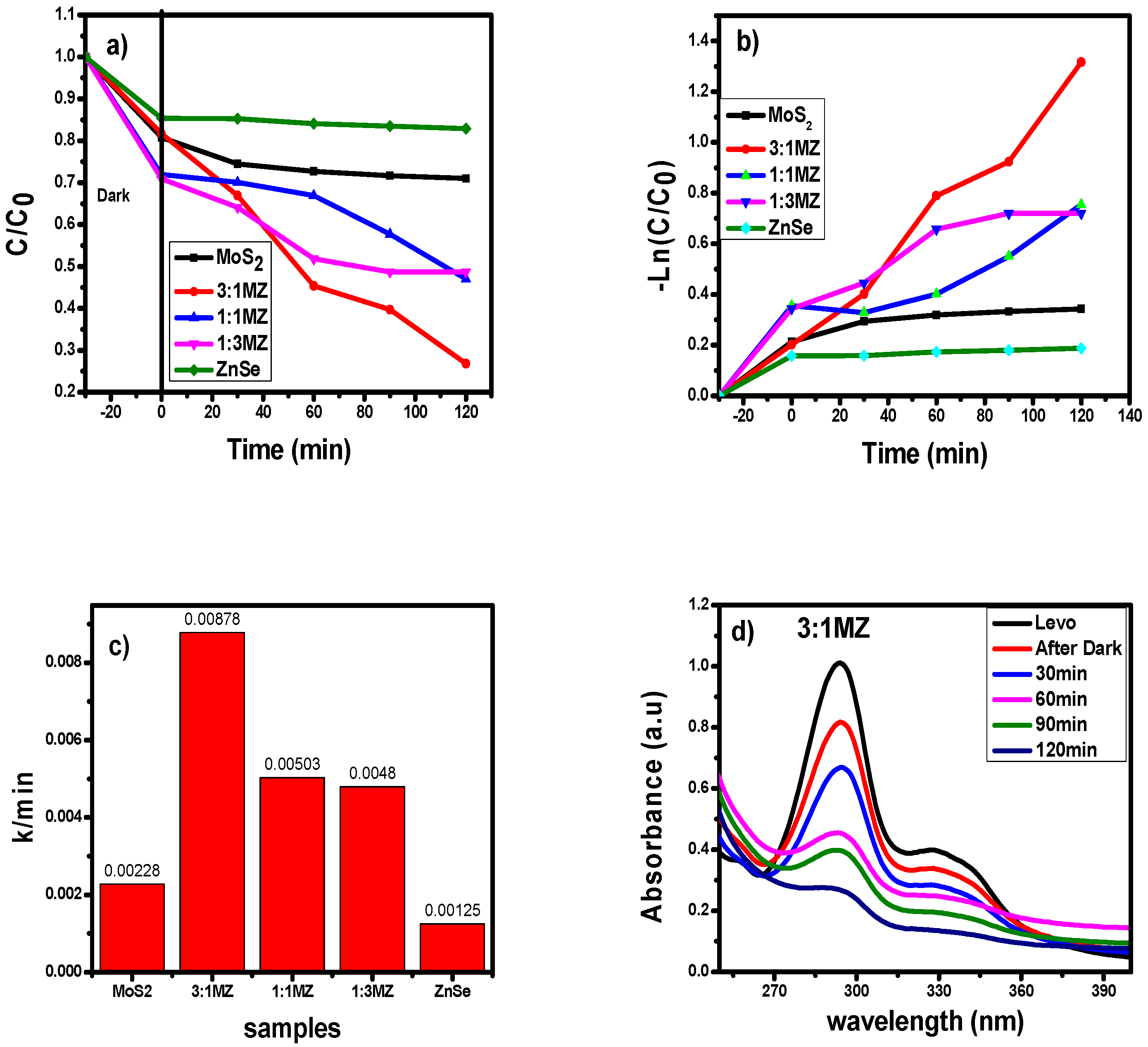
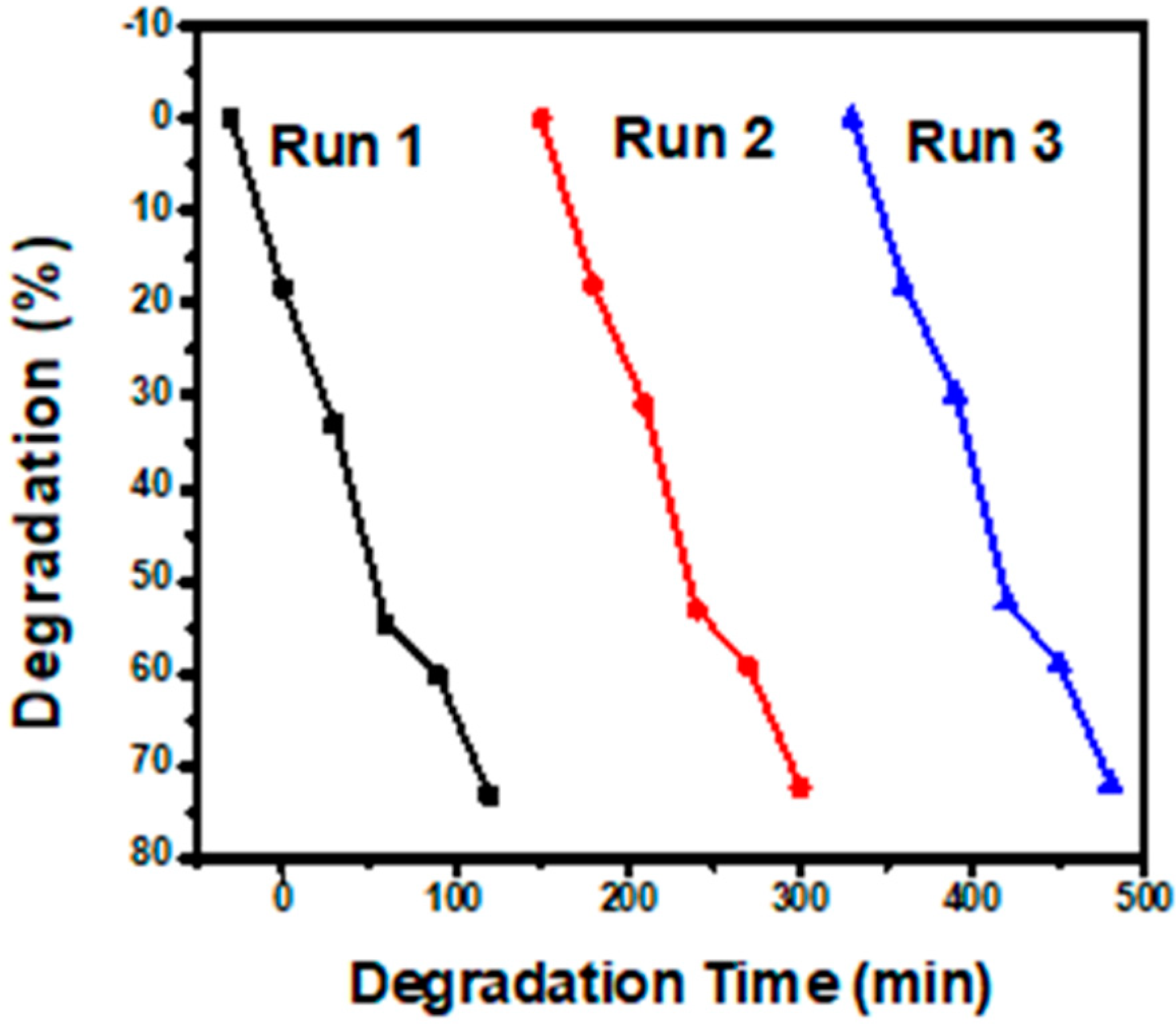
2.3. Photocatalytic Degradation of Levofloxacin
3. Materials and Methods
3.1. Synthesis of MoS2
3.2. Synthesis of ZnSe
3.3. Synthesis of MoS2/ZnSe Heterostructures
3.4. Photodegradation of Levofloxacin
3.5. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meng, F.Q.; Ma, W.; Duan, C.Y.; Liu, X.; Chen, Z.; Wang, M.Y.; Gao, J.; Zhang, Z. High efficient degradation of levofloxacin by edge-selectively Fe@3D-WS2: Self-renewing behavior and Degradation mechanism study. Appl. Catal. B 2019, 252, 187–197. [Google Scholar] [CrossRef]
- Klaus, K. Antibiotics in the aquatic environment—A review—Part II. Chemosphere 2009, 75, 435–441. [Google Scholar]
- Chow, D.N.F.T. The Clinical Pharmacokinetics of Levofloxacin. Clin. Pharmacokinet. 1997, 32, 101–119. [Google Scholar]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef]
- Ding, C.; Fu, K.; Pan, Y.; Liu, J.; Deng, H.; Shi, J. Comparison of Ag and AgI-Modified ZnO as Heterogeneous Photocatalysts for Simulated Sunlight Driven Photodegradation of Metronidazole. Catalysts 2020, 10, 1097. [Google Scholar] [CrossRef]
- Ghosh, M.; Lohrasbi, M.; Chuang, S.S.C.; Jana, S.C. Mesoporous Titanium Dioxide Nanofibers with a Significantly Enhanced Photocatalytic Activity. ChemCatChem 2016, 8, 2525–2535. [Google Scholar] [CrossRef]
- Wen, X.-J.; Niu, C.-G.; Guo, H.; Zhang, L.; Liang, C.; Zeng, G.-M. Photocatalytic degradation of levofloxacin by ternary Ag2CO3/CeO2/AgBr photocatalyst under visible-light irradiation: Degradation pathways, mineralization ability, and an accelerated interfacial charge transfer process study. J. Catal. 2018, 358, 211–223. [Google Scholar] [CrossRef]
- Brillas, E. A Review on the Degradation of Organic Pollutants in Waters by UV Photoelectro-Fenton and Solar Photoelectro-Fenton. J. Braz. Chem. Soc. 2014, 25, 393–417. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, P.; Wei, N.; Wang, D. Enhanced Photoelectrochemical Water-Splitting Property on TiO2 Nanotubes by Surface Chemical Modification and Wettability Control. ACS Appl. Mater. Interfaces 2020, 12, 20110–20118. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yin, S.; Wu, T.; Di, J.; Ji, M.X.; Wang, B.; Chen, Y.; Xia, J.X.; Li, H.M. Controlled preparation of MoS2/PbBiO2I hybrid microspheres with enhanced visible-light photocatalytic behaviour. J. Colloid Interface Sci. 2018, 517, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Theerthagiri, J.; Senthil, R.A.; Senthilkumar, B.; Reddy Polu, A.; Madhavan, J.; Ashokkumar, M. Recent advances in MoS2 nanostructured materials for energy and environmental applications—A review. J. Solid State Chem. 2017, 252, 43–71. [Google Scholar] [CrossRef]
- Li, Z.; Meng, X.; Zhang, Z. Recent development on MoS2 -based photocatalysis: A review. J. Photochem. Photobiol. C 2018, 35, 39–55. [Google Scholar] [CrossRef]
- Sabarinathan, M.; Harish, S.; Archana, J.; Navaneethan, M.; Ikeda, H.; Hayakawa, Y. Highly efficient visible-light photocatalytic activity of MoS2–TiO2 mixtures hybrid photocatalyst and functional properties. RSC Adv. 2017, 7, 24754–24763. [Google Scholar] [CrossRef] [Green Version]
- Bandow, S.; Maruyama, Y.; Bi, X.X.; Ochoa, R.; Holden, J.M.; Lee, W.T.; Eklund, P.C. Electronic and vibrational properties of Rb-intercalated MoS2 nanoparticles. Mat. Sci. Eng. A 1995, 204, 222–226. [Google Scholar] [CrossRef]
- Ge, L.; Han, C.; Xiao, X.; Guo, L. Synthesis and characterization of composite visible light active photocatalysts MoS2–g-C3N4 with enhanced hydrogen evolution activity. Int. J. Hydrog. Energy 2013, 38, 6960–6969. [Google Scholar] [CrossRef]
- Islam, S.E.; Hang, D.R.; Chen, C.H.; Sharma, K.H. Facile and Cost-Efficient Synthesis of Quasi-0D/2D ZnO/MoS2 Nanocomposites for Highly Enhanced Visible-Light-Driven Photocatalytic Degradation of Organic Pollutants and Antibiotics. Chemistry 2018, 24, 9305–9315. [Google Scholar] [CrossRef]
- Wang, C.; Lin, H.; Xu, Z.; Cheng, H.; Zhang, C. One-step hydrothermal synthesis of flowerlike MoS2/CdS heterostructures for enhanced visible-light photocatalytic activities. RSC Adv. 2015, 5, 15621–15626. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Zaen, R.; Oktiani, R. Correlation between crystallite size and photocatalytic performance of micrometer-sized monoclinic WO3 particles. Arab. J. Chem. 2020, 13, 1283–1296. [Google Scholar] [CrossRef]
- Li, Y.; Xiang, F.; Lou, W.; Zhang, X. MoS2 with structure tuned photocatalytic ability for degradation of methylene blue. IOP Conf. Ser. 2019, 300, 052021. [Google Scholar] [CrossRef]
- Li, Z.; Cao, F.; Wang, L.; Chen, Z.; Ji, X. A novel ternary MoS2/MoO3/TiO2 composite for fast photocatalytic degradation of rhodamine B under visible-light irradiation. New J. Chem. 2020, 44, 537–542. [Google Scholar] [CrossRef]
- Guo, F.; Huang, X.; Chen, Z.; Ren, H.; Li, M.; Chen, L. MoS2 nanosheets anchored on porous ZnSnO3 cubes as an efficient visible-light-driven composite photocatalyst for the degradation of tetracycline and mechanism insight. J. Hazard. Mater. 2020, 390, 122158. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Jin, C.; Li, Z.; Wang, M.; Chen, Z.; Wang, Y. Dual Z-scheme MoS2/g-C3N4/Bi24O31Cl10 ternary heterojunction photocatalysts for enhanced visible-light photodegradation of antibiotic. J. Alloys Compd. 2020, 825, 153975. [Google Scholar] [CrossRef]
- Chen, L.; Nguyen, T.B.; Lin, Y.-L.; Wu, C.-H.; Chang, J.-H.; Chen, C.-W.; Dong, C.-D. Enhanced Heterogeneous Photodegradation of Organic Pollutants by a Visible Light Harvesting CoO@meso–CN@MoS2 Nanocomposites. Catalysts 2020, 10, 722. [Google Scholar] [CrossRef]
- Li, G.; Hou, J.; Zhang, W.; Li, P.; Liu, G.; Wang, Y.; Wang, K. Graphene-bridged WO3/MoS2 Z-scheme photocatalyst for enhanced photodegradation under visible light irradiation. Mater. Chem. Phys. 2020, 246, 122827. [Google Scholar] [CrossRef]
- Patel, J.D.; Mighri, F.; Ajji, A. A facile route towards the preparation of ZnSe nanocrystals. Mater. Lett. 2014, 131, 366–369. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, H.Q.; Ma, Y.; Zhai, T.Y. ZnSe nanostructures: Synthesis, properties and applications. Prog. Mater. Sci. 2016, 83, 472–535. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, C.G.; Feng, B.; Wang, X.; Wan, B.Y. Synthesis and photocatalytic property of ZnSe flowerlike hierarchical structure. Appl. Surf. Sci. 2011, 257, 10679–10685. [Google Scholar] [CrossRef]
- Liu, H.R.; Hu, Y.C.; He, X.; Jia, H.S.; Liu, X.G.; Xu, B.S. In-situ anion exchange fabrication of porous ZnO/ZnSe heterostructural microspheres with enhanced visible light photocatalytic activity. J. Alloys Compd. 2015, 650, 633–640. [Google Scholar] [CrossRef]
- Cao, H.Q.; Xiao, Y.J.; Zhang, S.C. The synthesis and photocatalytic activity of ZnSe microspheres. Nanotechnology 2011, 22, 015604. [Google Scholar] [CrossRef]
- Zhang, L.H.; Yang, H.Q.; Xie, X.L.; Zhang, F.H.; Li, L. Preparation and photocatalytic activity of hollow ZnSe microspheres via Ostwald ripening. J. Alloys Compd. 2009, 473, 65–70. [Google Scholar] [CrossRef]
- Zhang, L.H.; Yang, H.Q.; Yu, J.; Shao, F.H.; Li, L.; Zhang, F.H.; Zhao, H. Controlled Synthesis and Photocatalytic Activity of ZnSe Nanostructured Assemblies with Different Morphologies and Crystalline Phases. J. Phys. Chem. C 2009, 113, 5434–5443. [Google Scholar] [CrossRef]
- Miao, H.; Hu, X.; Sun, Q.; Hao, Y.; Wu, H.; Zhang, D.; Bai, J.; Liu, E.; Fan, J.; Hou, X. Hydrothermal synthesis of MoS2 nanosheets films: Microstructure and formation mechanism research. Mater. Lett. 2015, 166, 121–124. [Google Scholar] [CrossRef]
- Feng, B.; Cao, J.; Han, D.L.; Liang, H.T.; Yang, S.; Li, X.Y.; Yang, J.H. ZnSe nanoparticles of different sizes: Optical and photocatalytic properties. Mater. Sci. Semicond. Process. 2014, 27, 865–872. [Google Scholar] [CrossRef]
- Nayak, S.; Swain, G.; Parida, K. Enhanced Photocatalytic Activities of RhB Degradation and H2 Evolution from in Situ Formation of the Electrostatic Heterostructure MoS2/NiFe LDH Nanocomposite through the Z-Scheme Mechanism via p–n Heterojunctions. ACS Appl. Mater. Interfaces 2019, 11, 20923–20942. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liao, Y.; Wan, X.; Tie, S.; Zhang, B.; Lan, S.; Gao, X. A high performance MoO3@MoS2 porous nanorods for adsorption and photodegradation of dye. J. Solid State Chem. 2020, 291, 121652. [Google Scholar] [CrossRef]
- Shi, L.; He, Z.; Liu, S.Q. MoS2 quantum dots embedded in g-C3N4 frameworks: A hybrid 0D–2D heterojunction as an efficient visible-light driven photocatalyst. Appl. Surf. Sci. 2018, 457, 30–40. [Google Scholar] [CrossRef]
- Wang, X.; Xiong, W.; Li, X.Y.; Zhao, Q.D.; Fan, S.Y.; Zhang, M.M.; Mu, J.C.; Chen, A.C. Fabrication of MoS2@g-C3N4 core-shell nanospheres for visible light photocatalytic degradation of toluene. J. Nanoparticle Res. 2018, 20, 243. [Google Scholar] [CrossRef]
- Lohar, G.M.; Jadhav, S.T.; Takale, M.V.; Patil, R.A.; Ma, Y.R.; Rath, M.C.; Fulari, V.J. Photoelectrochemical cell studies of Fe2+ doped ZnSe nanorods using the potentiostatic mode of electrodeposition. J. Colloid Interface Sci. 2015, 458, 136–146. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zeng, W.; Li, Y.Q. Hydrothermal synthesis and controlled growth of hierarchical 3D flower-like MoS2 nanospheres assisted with CTAB and their NO2 gas sensing properties. Appl. Surf. Sci. 2018, 455, 276–282. [Google Scholar] [CrossRef]
- Ehsan, M.F.; Qudoos, S.; Ahmad, Z.; Hamid, S.; Arfan, M.; Zia, A.; Umbreen, K.; Ashiq, M.N.; Tyagi, D. ZnTe/ZnSe heterostructures: In-situ synthesis, characterization and photocatalytic activity for Congo Red degradation. SN Appl. Sci. 2019, 1, 197. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.-H.; Li, L.; Liu, N.; Wang, D.-J.; Xue, Y.-C.; Tang, L. Molybdenum disulfide (MoS2) as a co-catalyst for photocatalytic degradation of organic contaminants: A review. Process. Saf. Environ. 2018, 118, 40–58. [Google Scholar] [CrossRef]
- Fu, Y.; Liang, W.; Guo, J.; Tang, H.; Liu, S. MoS2 quantum dots decorated g-C3N4/Ag heterostructures for enhanced visible light photocatalytic activity. Appl. Surf. Sci. 2018, 430, 234–242. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, X.; Zheng, L.; Wan, C. Fabrication of TiO2/MoS2Composite Photocatalyst and Its Photocatalytic Mechanism for Degradation of Methyl Orange under Visible Light. Can. J. Chem. Eng. 2015, 93, 1594–1602. [Google Scholar] [CrossRef]
- Kuehnel, M.F.; Creissen, C.E.; Sahm, C.D.; Wielend, D.; Schlosser, A.; Orchard, K.L.; Reisner, E. ZnSe Nanorods as Visible-Light Absorbers for Photocatalytic and Photoelectrochemical H2 Evolution in Water. Angew. Chem. 2019, 58, 5059–5063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, W.-C.; Li, X.-Y. Synthesis of MoS2/g-C3N4 as a solar light-responsive photocatalyst for organic degradation. Catal. Commun. 2014, 49, 63–67. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, M.; Liu, J.; Chuang, S.S.C.; Jana, S.C. Fabrication of Hierarchical V2O5 Nanorods on TiO2 Nanofibers and Their Enhanced Photocatalytic Activity under Visible Light. ChemCatChem 2018, 10, 3305–3318. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, N.; Yang, Y.; Wang, G.; Ng, D.H. High efficiency photocatalysis for pollutant degradation with MoS2/C3N4 heterostructures. Langmuir 2014, 30, 8965–8972. [Google Scholar] [CrossRef]
- Zheng, Z.X.; Qiao, Y.; Cai, Y.H.; He, Y.N.; Tang, Y.M.; Li, L.S. MoS2 decorated CdS hybrid heterojunction for enhanced photoelectrocatalytic performance under visible light irradiation. J. Colloid Interface Sci. 2019, 533, 561–568. [Google Scholar] [CrossRef]
- Tahir, M.B. Construction of MoS2/CND-WO3 Ternary Composite for Photocatalytic Hydrogen Evolution. J. Inorg. Organomet. Polym. Mater. 2018, 28, 2160–2168. [Google Scholar] [CrossRef]
- Chen, Y.L.; Wang, L.J.; Wang, W.Z.; Cao, M.S. Enhanced photoelectrochemical properties of ZnO/ZnSe/CdSe/Cu2-xSe core-shell nanowire arrays fabricated by ion-replacement method. Appl. Catal. B Environ. 2017, 209, 110–117. [Google Scholar] [CrossRef]
- Arya, M.; Kaur, M.; Kaur, A.; Singh, S.; Devi, P.; Kansal, S.K. Hydrothermal synthesis of rGO-Bi2WO6 heterostructure for the photocatalytic degradation of levofloxacin. Opt. Mater. 2020, 107, 110126. [Google Scholar] [CrossRef]
- Gupta, G.; Kaur, A.; Sinha, A.S.K.; Kansal, S.K. Photocatalytic degradation of levofloxacin in aqueous phase using Ag/AgBr/BiOBr microplates under visible light. Mater. Res. Bull. 2017, 88, 148–155. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sitara, E.; Ehsan, M.F.; Nasir, H.; Iram, S.; Bukhari, S.A.B. Synthesis, Characterization and Photocatalytic Activity of MoS2/ZnSe Heterostructures for the Degradation of Levofloxacin. Catalysts 2020, 10, 1380. https://doi.org/10.3390/catal10121380
Sitara E, Ehsan MF, Nasir H, Iram S, Bukhari SAB. Synthesis, Characterization and Photocatalytic Activity of MoS2/ZnSe Heterostructures for the Degradation of Levofloxacin. Catalysts. 2020; 10(12):1380. https://doi.org/10.3390/catal10121380
Chicago/Turabian StyleSitara, Effat, Muhammad Fahad Ehsan, Habib Nasir, Sadia Iram, and Syeda Aqsa Batool Bukhari. 2020. "Synthesis, Characterization and Photocatalytic Activity of MoS2/ZnSe Heterostructures for the Degradation of Levofloxacin" Catalysts 10, no. 12: 1380. https://doi.org/10.3390/catal10121380




