Abstract
Pathogenic microorganisms can spread throughout the world population, as the current COVID-19 pandemic has dramatically demonstrated. In this scenario, a protection against pathogens and other microorganisms can come from the use of photoactive materials as antimicrobial agents able to hinder, or at least limit, their spreading by means of photocatalytically assisted processes activated by light—possibly sunlight—promoting the formation of reactive oxygen species (ROS) that can kill microorganisms in different matrices such as water or different surfaces without affecting human health. In this review, we focus the attention on TiO2 nanoparticle-based antimicrobial materials, intending to provide an overview of the most promising synthetic techniques, toward possible large-scale production, critically review the capability of such materials to promote pathogen (i.e., bacteria, virus, and fungi) inactivation, and, finally, take a look at selected technological applications.
1. Introduction
Over the last decades, titanium dioxide (TiO2) has been extensively investigated for its physical-chemical properties, that, combined with its high stability, low cost, and safety for the environment and humans, have resulted in a range of environmental and energy applications.
When TiO2 is obtained at nanoscale, many relevant properties of this semiconductor are enhanced, due to the increased surface area, which results from the high surface-to-volume ratio, the excellent surface morphology, and the band edge modulation, that, overall, turn into a control on the photocatalytic behavior and performance of the nanostructured materials [1,2,3].
Upon illumination, TiO2 nanoparticles (NPs) convert incoming photons into excitons, or electron/hole pairs, which can migrate to the surface and participate in redox reactions and generate reactive oxygen species (ROS), such as hydroxyl radicals (OH·) and superoxide(O2−) [3,4].
Indeed, the photocatalytic behavior of TiO2 NPs has been widely exploited for removal of water and air contaminants and self-cleaning surfaces [1,2,3,4]. Currently, increasing concerns regarding the COVID-19 pandemic are drawing the attention of researchers and general public more and more to photocatalytic antimicrobial and antiviral treatments with the purpose of hindering virus spreading, by using light (possibly solar light) activated systems.
TiO2 NPs are among the most studied materials in the area of photocatalytic antimicrobial applications, having demonstrated a great potential for the disinfection/inactivation of harmful pathogens, including bacteria, viruses, and fungi [5], However, aside from the superior advantage of TiO2 nanostructured materials, some drawbacks have been identified, i.e., high recombination rate of the photogenerated species and limited solar light sensitivity, therefore various modifications have been developed to enhance the photocatalytic efficiency [3,4].
Upon photoactivation of TiO2 NPs, the biocidal action is a result of the modulation of charge carriers, electrons, and holes at the surface of the material, resulting in powerful and long-lasting capabilities [1], since the process does not rely on the release of metal ions, and hence the material consumption, as it happens in the case of Ag-based antimicrobial material. Moreover, TiO2 NPs-based systems have a substantial advantage due to their non-contact biocidal action. Finally, since any possible release into the environment of potentially toxic NPs—with unpredictable effects on human health—from the material or device for the final application must be prevented, the TiO2-based structures are required to be suited for immobilization onto substrate and/or incorporation in matrices. In this regard, this class of materials could be considered reasonably environmentally friendly.
The antimicrobial activity of TiO2 NPs is primarily attributed to the photocatalytic generation, under band-gap irradiation, of ROS with high oxidative potentials. However, other possible factors may be considered to explain their biocidal effect, such as free metal ions formation or synergistic effects deriving from the combination of TiO2 NPs with other materials and compounds in nanocomposite systems [1,2,5].
In the following, possible mechanisms underlying the antimicrobial behavior of the TiO2 NPs-based systems will be described, specifically highlighting the photocatalytic path, along with other possible alternative factors, that, also synergistically combined, can be responsible for microorganism inactivation.
The biocidal activity of TiO2 NPs-based materials is greatly dependent on their photocatalytic performance, which is strongly influenced by several inherent factors, including the NP morphology, size, chemistry, crystalline phase, structure, and precursor, as well as their possible modification with metals, other semiconductors, and organic compounds [5].
For this reason, here the latest tendencies on synthesis of TiO2 NPs-based materials will be illustrated, specifically addressing environmentally sustainable and ecological processes (i.e., requiring mild reaction conditions and non-toxic precursors) and scalable preparation procedures, such as sol-gel and hydrothermal methods. Nanocomposites preparation, including TiO2/metal nanocomposites, and in particular, TiO2-/Ag-based ones, and nanocomposites coupling TiO2 with other metals and metal oxides, but also with organic and even biological molecules, will be reviewed in order to explore the effect of the nanostructured material features on the overall performance. Then, the activity of TiO2 nanosized-based systems against bacteria, fungi, and viruses, in that order, will be presented, highlighting the effect of different biological factors on their overall antimicrobial performances, including cell membrane structure, metabolism, physiological state of the cells, and environmental stress. Finally, selected applications of the antimicrobial TiO2-based nanomaterials will be described, namely, environmental applications, including water treatment, anti-biofouling membranes for water treatment and disinfection of building materials, and disinfection of biomaterial and of materials for food packaging and processing. The ability to push forward the great potential of the TiO2 nanostructured materials as an antimicrobial system is strongly motivated by the need for inorganic alternatives to antibiotics, due also to the emergent multidrug resistance of some bacteria and the toxicity to the human body of some organic antimicrobial substances. Advances in preparative approaches, alongside a further elucidation and a more comprehensive understanding of the mechanisms underlying the functions of TiO2-based nanostructures, could provide additional and powerful tools to tackle the enormous incidence of bacteria and viruses and strengthen also the capacity to inactivate and destroy a wide range of microorganisms, possibly extending their biocidal action to extremely harmful strains like the SARS-CoV viruses.
2. Preparation of TiO2-Based Nanostructured Materials for Photocatalytic Inactivation of Microorganisms
2.1. Synthesis of Photocatalytic TiO2 Nanoparticles with Antimicrobial Function
For decades, nanosized TiO2 has been widely investigated because of its unique photocatalytic properties. Among the wide range of environmental applications enabled by such a material at nanoscale, the photocatalytic inactivation of harmful microorganisms is attracting increasing attention.
In the last years, many studies have described the antimicrobial activity of commercially available TiO2 NPs. However, more recently, innovative approaches aiming at the synthesis of original TiO2-based nanomaterials, specifically designed for photocatalytically assisted inactivation of bacteria and viruses, have been reported.
Such approaches vary in terms of degree of complexity and production cost, and allow access to photocatalytic TiO2-based nanomaterials with purposely selected physical-chemical properties, including controlled dimension and geometry, and thus size-dependent characteristics, engineered surface chemistry, enhanced colloidal stability even in different conditions of ionic strength, and biocompatibility, especially for application in the field of heath care and food preservation.
In the literature, a plethora of synthetic routes has been proposed for the preparation of nanosized TiO2 with photocatalytic antimicrobial properties; here we focus our attention on sol-gel and hydrothermal methods (Figure 1), as they have been regarded, so far, among the promising techniques for the large scale production of TiO2 NPs, owing to their user- and environment- friendly and cost effective procedures [6,7,8,9]. However, for the sake of brevity, herein we report only a small selection of the numerous examples reported in literature and we direct the readers to more specific papers [8,10,11].
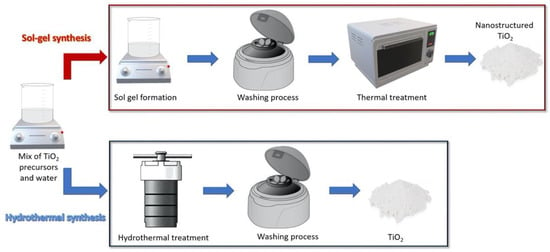
Figure 1.
Schematic representation of TiO2 NPs sol-gel method (upper panel) and hydrothermal synthetic approach (lower panel).
2.1.1. Sol-Gel Methods
The sol-gel method is based on the conversion of “sol(s)”, namely solid particles suspended in a liquid, into a network of sols defined as “gel”, containing both a liquid phase and a solid phase. Figure 1 shows a schematic representation of a general procedure typically followed for a common sol-gel synthesis. In the first step, the TiO2 precursor is dissolved in water, in an alcohol (e.g., methanol, ethanol, or isopropanol) or in a defined mixture of alcohol and water as a reaction solvent. Commonly used TiO2 precursors include (but are not limited to) titanium (IV) butoxide (Ti(OBu)4), titanium (III) chloride (TiCl3), titanium (IV) tetrachloride (TiCl4), and titanium (IV) isopropoxide (Ti[OCH(CH3)2]4, TTIP). At this stage, the formation of TiO2 takes place through the two main reactions of hydrolysis (Equation (1)) and condensation (Equations (2) and (3)). Subsequently, in most reported protocols, the obtained product is thermally treated in order to obtain TiO2 NPs with a defined size and crystalline phase [12,13,14,15].
Hydrolysis:
Ti(OR)4 + 4H2O→2Ti(OH)4 + 4 ROH
Condensation:
Ti(OH)4 + Ti(OH)4→2TiO2 + 4H2O (oxolation)
Ti(OH)4 + Ti(OR)4→2TiO2 + 4ROH (alcoxolation)
In the reactions (1) and (3), R represents organic functional group of the organometallic TiO2 precursor, such as ethyl, i-propyl, n-butyl. The dilution of the TiO2 precursor in the alcohol and/or in water before being added to the selected reaction solvent is useful to control the reaction rate of the hydrolysis process by slowing down the reaction rate as the dilution of the precursors increase. In the sol-gel method, the synthesis parameters affecting the properties and structure (crystalline phase(s)) of the resulting TiO2 NPs photocatalyst are: H2O/TiO2 precursor ratio, pH value measured during the synthesis, and experimental conditions of possible calcination step.
Vargas et al., starting from the Ti(OBu)4 as a precursor, performed a sol gel synthesis consisting of two steps: firstly, the Ti(OBu)4 was dispersed in ethanol and allowed to stir in distilled water. Then, the obtained product was dried before being thermally treated for 2 h, at increasing temperature, 350 °C, 400 °C, and 770 °C. As a result, X-ray diffraction (XRD) analysis demonstrated that a different crystalline composition was attained as a function of the thermal treatment temperature. In particular, the obtained material was found amorphous after the treatment at 350 °C, while anatase was the main crystalline phase at 400 °C, and finally the treatment at 770 °C resulted in rutile as the single crystalline phase. Scanning (SEM) and transmission (TEM) electron microscopy analysis highlighted a morphology (Figure 2) characterized by the presence of primary sub-micrometric spheroidal particles with different average sizes, namely 100 nm for the amorphous product, and 50 nm for the two crystalline samples. The thermal treatment conditions were found to also affect the specific surface area value [16] of the resulting nanomaterials, as the increase in temperature resulted in a decrease of the specific surface area [17].
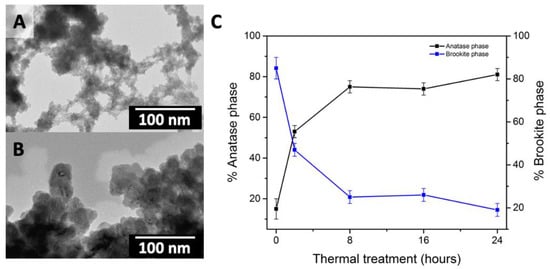
Figure 2.
Transmission electron microscopy (TEM) micrographs of synthesized TiO2 nanoparticles (NPs) before (A) and after the thermal treatment at 110 °C in an oven carried out for 16 h (B). Panel (C) percentage of anatase and brookite phase in different samples estimated by quantitative phase analysis by X-ray diffraction (XRD) pattern as a function of thermal treatment duration time at 110 °C. Reprinted with permission from ref. [16].
Dell’Edera et al. pointed out the correlation between TiO2 heat treatment and the morphological properties of the obtained nanomaterial. The synthesis was carried out by precipitation of TiOSO4 in an aqueous solution of NH4HCO3. The obtained sol was thermally treated in an oven at 110 °C for 16 h. SEM micrographs (Figure 2 panel A-B) show that small NPs were present in the untreated sample (~1 nm); after the heat treatment larger NPs were visible (23 ± 6 nm). Furthermore, Figure 2 panel C shows that the crystalline phase changed and a trend of the crystalline phase composition was observed as a function of the extent of heat treatment conditions. Initially there was a predominance of the brookite phase, then, during the thermal treatment the brookite phase progressively converted into the anatase phase. The specific surface area (SSA) depended on the thermal treatment conditions; in particular, for untreated TiO2 NPs an SSA of 503.0 m2/g was measured, while treated NPs resulted in an SSA of 336.5 m2/g. The obtained TiO2 NPs were found to be photocatalytically active, thus a potential candidate for antimicrobial applications. The dependence of the photocatalytic performance on properties such as crystalline phase composition, surface chemistry, and surface area was also highlighted [16].
In the sol-gel routes, the hydrolysis reaction, necessary for the formation of TiO2, is acid catalyzed [18,19]. It follows that pH is a critical parameter in the synthesis that has been also found to influence morphological and structural properties of the resulting nanomaterial. Indeed, Ibrahim et al. demonstrated that alkaline conditions (pH 9) promote anatase structure, while higher acidity (pH < 5) results in rutile phase [20].
On the other hand, Galkina et al. synthesized anatase and brookite nanocrystalline TiO2 using a sol-gel process by working at acid pH [21]. TTIP was dissolved in isopropylic alcohol and then added to an aqueous medium acidified by nitric acid. The obtained sol was used to modify cotton fibers, thus obtaining an antibacterial composite material. Cotton fibers were pre-treated in order to anchor the TiO2 NPs therein. In particular, a spacer, a cyclic anhydride, prepared by the reaction between 1,2,3,4-butanetetracarboxylic acid (BTCA) and NaH2PO2 in a water solution, was inserted at the fiber surface. Once pre-treated, the cotton fibers were functionalized with TiO2 NPs by adding them to the aqueous solution and heating at 70 °C for 2 h. The presence of nitric acid in the preparation of the sol was found able to drive the NP growth toward the formation of both brookite and anatase, single phase, TiO2 NPs. The obtained cotton/TiO2 composites demonstrated high bacteriostatic effects against Escherichia coli (E. coli) [21].
The solvent used to disperse the TiO2 precursor may also affect the physical-chemical characteristics of TiO2 NPs prepared by using a sol-gel method. Indeed, the morphology of the TiO2 NPs was found to be influenced by the volume ratio between alcohol and TiO2 precursor, as well as by the nature of alcohol selected as a solvent for the specific synthetic route. As an example, Bahar et al. reported that syntheses performed in ethanol lead to larger NPs (33 nm) than those obtained in butanol (26 nm) and isopropanol (30 nm) [22]. Duymaz et al. investigated the effect of the solvent composition, testing two different solvents (ethyl alcohol or water) to dissolve the TiO2 precursor. After preparing the precursor in water and alcohol solution, respectively, its oxidation was catalyzed by the addition of nitric acid to the two systems and, for both of them, the sol-gel reaction was carried out at 50 °C to obtain a homogeneous dispersion. Then, the TiO2 gel was dried for 24 h at 90 °C and finally ground to obtain fine powders, which were analyzed using a dynamic light scattering (DLS) technique. The use of ethanol was found to induce the formation of aggregates of TiO2 NPs smaller (1858 nm) than those obtained by using water (2641 nm). The report indicated that the smaller TiO2 aggregates, thanks to their size, were found to be optimal for being supported on several substrates such as silica, calcite, talc, and zinc borate. The immobilization of TiO2 on each of these substrates was performed directly in situ, by simply adding the supporting material in the reaction mixture where the oxidation reaction takes place. The obtained NPs showed antibacterial behavior against E. coli and Staphylococcus aureus (St. aureus) [23].
2.1.2. Hydrothermal Methods
Hydrothermal (HT) synthesis, along with sol-gel process, is also a very common method for preparing TiO2-based nanomaterials. In hydrothermal synthesis, that is, a solution approach, the formation of nanomaterials can take place in a wide range of temperatures, from room temperature to high temperatures (up to 400 °C [8]). Pressure can also be relevant in HT reaction, as it enables the control of morphology for the resulting nanomaterial. In a HT reaction, either low-pressure or high-pressure conditions can be applied, depending on the vapor pressure of the most abounded component of the reaction mixture under the investigated experimental conditions. Therefore, generally, a HT synthesis is carried out in an autoclave reactor, where it is essential to carefully control pressure and temperature.
The scheme of a typical HT synthetic procedure is depicted in Figure 1. In the first step, a mixture of water and TiO2 precursor is prepared; then the obtained solution is transferred in the autoclave reactor, setting specific temperature pressure conditions. At this stage, the hydrolysis and condensation reactions take place, forming a three-dimensional network, similar to what happens in a sol-gel process. HT processes can be regarded as a sort of evolution of the synthetic sol-gel processes, because they allow one to obtain NPs with a high degree of crystallinity in fewer and simpler steps relative to the procedures typically realized for the sol-gel methods.
Rasheed et al. proposed a procedure to obtain rutile phase TiO2 NPs starting from a sol obtained by mixing TiCl4, deionized water, and ethanol. The dispersion was kept under vigorous stirring for 30 min until it became colorless, then, it was introduced into a Teflon-lined stainless-steel autoclave. The autoclave was sealed and placed in an oven at 200 °C for 6 h. The XRD analysis confirmed the presence of rutile as a single crystalline phase in the resulting materials. The photocatalytic antibacterial behavior was investigated against E. coli and St. aureus [24].
Varying the synthetic parameter of the HT procedure was also possible to obtain nanomaterials with a more complex morphology, such as the flower like nanostructures, composed by rod-like TiO2 NPs reported by Korosi et al. [25]. A sol dispersion was obtained by mixing distilled water and TiCl4 previously dissolved in 2-propanol. The resulting colorless dispersion was aged for two weeks at room temperature without stirring and then left to react in autoclave, for 12 h at increasing temperature values, namely 150 °C, 200 °C, and 250 °C. The HT reaction temperature affected both the structure and the specific surface area of the obtained material. Indeed, as the temperature increased, a decrease of the specific surface area was observed, along with an increase of the rutile amount up to the 100% after treatment for 12 h at 250 °C [25]. Further, SEM micrographs (Figure 3) pointed out that the crystallite size increased with the HT treatment temperature. The obtained material was effective in the photocatalytic inactivation of Klebsiella pneumoniae (K. pneumoniae).
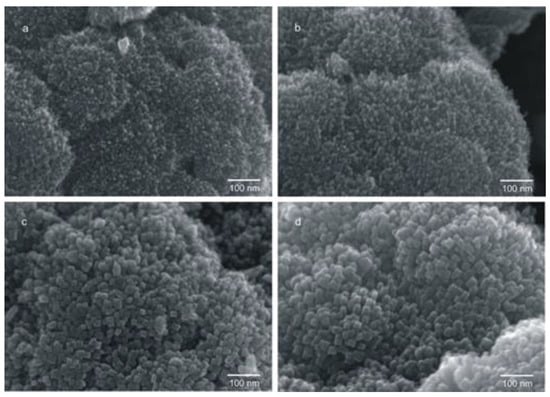
Figure 3.
High-resolution scanning electron microscopy (SEM) micrographs of flower-like rutile TiO2 (FLH-R-TiO2): (a) FLH-R-TiO2/AP (as prepared), (b) FLH-R-TiO2/150, (c) FLH-R-TiO2/200 and (d) FLH-R-TiO2/250. Reprinted from ref. [25], Copyright (2016), with permission from Elsevier.
The HT process can also be conveniently tuned to modify the morphology of pre-synthetized TiO2 NPs, as reported by Leon-Rios et al., who demonstrated the conversion of spherical TiO2 NPs into TiO2 nanowires obtained by means of a HT treatment. In detail, a water suspension of TiO2 and NaOH was introduced in a Teflon-lined autoclave reactor and heated for 6 days at a temperature of 130 °C. The resulting powder was washed with HCl, then calcined at 500 °C leading to nanowires of TiO2 in monoclinic phase, a metastable phase usually called TiO2-B. TiO2-B crystalline structure is characterized by a degree of exposure of TiO2 (010) facets higher than that found in anatase, rutile, or brookite crystalline phase. Such TiO2 (010) facets are known to be more photocatalytically active than other TiO2 facets, thus making TiO2-B a valuable candidate for photocatalytic applications [26,27]. As an example, TiO2-B demonstrated effective in the UV induced inactivation of E. coli [27].
2.2. Preparation of TiO2/Metal Nanocomposites
The increasing demand for highly efficient, visible-light-active photocatalysts can be addressed by designing and realizing hybrid nanostructured materials formed of two or more components, each characterized by peculiar size dependent properties, surface chemistry, and morphology, that are combined into one nano-object with unprecedented chemical–physical characteristics. Indeed, the presence of a metallic domain coupled to TiO2 leads to a nanomaterial particularly suited for accomplishing visible light photocatalysis, as, extending the absorption ability of TiO2 in the visible, the capacity to convert solar energy into chemical energy is increased. Several papers have demonstrated a significant enhancement in visible-light-driven photocatalytic efficiency of nanocomposites obtained upon deposition of Ag and Au NPs onto TiO2 nanostructures [3,4,28]. These composite materials are also very interesting due to the ability of metal NPs to convey bactericidal properties to the system even in the dark, thanks to the slow release of a small amount of metal ions that are known to be toxic for pathogens even at ppm level.
2.2.1. TiO2/Ag-Based Nanocomposites
One of the most investigated methods to improve the biocidal activity of TiO2 against bacteria and viruses, enhancing at the same time its photocatalytic activity in the visible range, consists of modifying TiO2 with Ag NPs thus obtaining TiO2/Ag-based nanocomposites with unique photocatalytic and antibacterial properties [29]. Remarkably, in metal/semiconductor-based nanocomposites the interaction between semiconductors and metal NPs, under UV or visible light irradiation affects the properties of the resulting nanocomposite [3]. Indeed, the role played by the metal NPs in influencing the energetics of the system, leading to an enhanced photoinduced charge separation, has been extensively investigated especially with respect to the photocatalytic reactions [30].
Several methods have been reported for the preparation of TiO2/Ag-based nanocomposites with combined antibacterial and photocatalytic properties. Sol-gel processes, that allow one to simultaneously synthesize TiO2 NPs and Ag NPs, are among the most used methods for preparing TiO2/Ag-based nanocomposites. In particular, the extensively investigated sol-gel synthetic protocols produce Ag NPs by reduction of Ag salt (typically AgNO3 or AgClO4) with a suitable reductant, such as ascorbic acid, glucose, sodium borohydride and citrate. Kedziora et al. reported a method for obtaining TiO2/Ag-based nanocomposites with a control on the amount of Ag. The synthetic procedure was based on two steps: the preparation of the Ag precursor and the sol-gel process. Firstly, a diamminosilver (I) nitrate solution was prepared by adding potassium hydroxide in a silver nitrate solution, resulting in a brown precipitate that was then dissolved in ammonium hydroxide. In the second step, first titanium n-butoxide was added drop wise in acetone, next the diamminosilver (I) and the reducing agent (glucose) were introduced in the reaction vessel obtaining a sol. Afterward, the sol was washed and dried at 80 °C and finally calcinated at 400 °C for 10 h to obtain TiO2 in anatase phase. This process made it possible to control the amount of Ag NPs nucleating on the TiO2 surface by simply repeating the process of impregnation with diamminosilver (I) nitrate solution and reduction by glucose. The TEM micrograph of TiO2/Ag nanostructures in Figure 4 points out the presence of spherical Ag NPs, appearing as high contrast round-shaped spots, in close contact with the surface of the lighter contrast TiO2 structures [31].
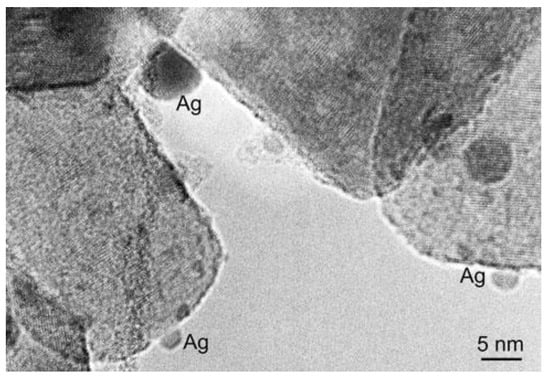
Figure 4.
TEM micrograph of TiO2/Ag nanocomposite. Reprinted from ref. [31], Copyright (2012), with permission from Springer Nature.
Another approach for the preparation of TiO2/Ag nanocomposites is based on a double step synthesis, consisting of the reduction AgNO3 in presence of TiO2 NPs previously prepared by a HT method. In this case, the obtained nanocomposite was deposited on leather for antimicrobial function in the footwear industry. The antimicrobial activity was successfully demonstrated against the bacteria Pseudomonas aeruginosa (P. aeruginosa), St. aureus, and the fungi Candida albicans (C. albicans) [32]. Moreover, other reports indicated that a post-synthesis treatment carried out at high temperature (up to 500 °C) on TiO2 NPs before growing Ag NPs resulted in a nanocomposite with a higher crystallinity of the TiO2 phase in nanocomposite prepared for inactivation of St. aureus, E. coli, and Bacillus cereus (B. cereus) [33,34,35].
Another synthetic method, widely used for the production of TiO2 nanocomposites for antibacterial applications, relies on photo-reduction of Ag salt by UV radiation onto pre-synthesized TiO2 NPs. Skorb et al. synthesized TiO2-based nanocomposites using this method. Firstly, nanostructured TiO2 was synthetized by means of a sol-gel process, and deposited by spray-coating onto different substrates such as glass microscope slides or glazed ceramic. Afterwards, the deposited material was treated at 200 °C. Subsequently, Ag NPs were deposited on the surface of TiO2 by dipping the substrate in aqueous solution of AgSO4 and irradiating with a UV lamp for 10 s. A further step allowed the preparation of TiO2/Ag/Ni nanocomposites by electrodeposition of a nickel layer onto a surface of TiO2/Ag from a Ni(CH3COO)2 solution. The antimicrobic activity of the nanocomposite was assessed against Pseudomonas fluorescens (P. fluorescens) and Lactococcus lactis (Lc. lactis) [36].
2.2.2. Coupling TiO2 with Other Metals and Metal Oxides
Current research on TiO2-based nanomaterials for antibacterial applications is also focusing on valid and cost-effective metal NPs, as alternative to Ag, to merge to TiO2, possibly characterized also by intrinsic antibacterial properties.
Kaushik et al. developed a TiO2/Al-based nanocomposite material with antibacterial activity. The synthetic method is essentially a HT process performed in the presence of an Al-based compound. Firstly, titanium (IV) isopropoxide (TTIP) and ethylene diamine were mixed in isopropyl alcohol, next aluminum isopropoxide was added, and finally the solution was transferred to Teflon-lined stainless-steel autoclave and kept at 180 °C for 18 h in oven. The obtained material was found to consist of Al doped TiO2 NPs, giving Al the twofold role of (i) extending the visible range the wavelengths suitable to activate the photocatalytic properties and (ii) significantly increasing the antibacterial activity with respect to undoped TiO2. Higher bacterial disinfection activity of the doped samples compared to that of the undoped TiO2 was observed under visible light irradiation (nearly 80%) as well as in the dark (nearly 20%) against both St. aureus and E. coli bacteria [37].
Venieri et al. studied the bactericidal activity of TiO2-based nanostructures doped with Co and Mn for inactivation of K. pneumoniae and E. coli. They synthesized by sol-gel Mn, Co, and Mn:Co doped TiO2. Each nanocomposite was synthesized starting from a sol containing both the TiO2 and the dopant precursor, afterward the pH of the sol was adjusted to pH 7, and finally, the resulting nanocomposite materials were washed with water and thermally treated at 500 °C for 3 h [38,39,40].
An original approach developed to improve both the antibacterial activity and the photocatalytic properties was based on the use of rare earth metals as dopants for TiO2 NPs. In particular, Nd is a lanthanide element that attracted a lot of attention, due to its unique optical and magnetic properties, that make it extremely useful for application in optoelectronic and magnetic devices [41]. Nithya et al., produced Nd doped TiO2 NPs by means of a sol-gel method for inactivation of E. coli and St. aureus. TTIP and Nd (III) acetate dehydrate were dissolved in isopropyl alcohol at pH 9, afterwards acetic acid was added to complete the hydrolysis reaction. Then, the obtained powder was dried at 120 °C for 2 h and annealed at 600 °C for 3 h [42].
Another effective strategy to increase the antibacterial ability of nanostructured TiO2 relies on the coupling with a suitable metal oxide semiconductor.
Siwińska-Stefańska et al. synthetized various TiO2/ZnO binary semiconductor nanocomposites, with different TiO2:ZnO ratios, by using HT route. In particular, the synthesis of the binary TiO2/ZnO semiconductor was carried out by mixing TTIP, dissolved in alcohol, with zinc acetate, dissolved in water. The obtained mixture was heated in autoclave at 160 °C. All the synthesized TiO2/ZnO binary semiconductors showed a well-defined crystalline structure and a high surface area [43]. The nanocomposite demonstrated antibacterial activity against seven different bacteria including E. coli, P. aeruginosa, St. aureus, a methicillin-resistant St. aureus, B. cereus, Bacillus licheniformis (B. licheniformis), and anaerobic Clostridium perfringens (C. perfringens).
Other hybrid nanocomposites, such as TiO2:In2O3, TiO2:g-C2N4, and TiO2:SiO2 are currently under investigation due to their promising photoactivity and antibacterial properties [36,44,45,46,47]. For instance, TiO2:In2O3 photocatalysts were prepared by sol-gel route by reacting TiCl4 and In(NO3)3 in water and then stabilized by addition of HNO3. The obtained photocatalyst was able to inactivate P. fluorescens and Lc. lactis [36]. TiO2:g-C2N4 synthesis involved the growth of TiO2 nanosheets with (001) facets exposed by a hydrothermal route based on the decomposition of TiO(Bu)4 in the presence of g-C2N4. The TiO2:g-C2N4 nanocomposite was able to inactivate E. coli [44]. Mesoporous silica nanospheres functionalized with TiO2 were prepared in a three-step procedure. The first step was conducted according to a modified Stöber sol–gel method involving the hydrolysis of tetraethyl orthosilicate (TEOS) in alkaline conditions. In a second step, a mesoporous external layer was deposited on the silica spheres by letting TEOS react in the presence of CTAB (Cetyltrimethylammonium Bromide). Finally, functionalization with TiO2 was accomplished by means of the reaction of titanium (IV) butoxide at the mesoporous silica nanosphere surface. The photocatalytic antibacterial properties of TiO2:SiO2 were investigated against E. coli [45].
Other innovative materials recently proposed for their antimicrobial properties are La-doped TiO2/calcium ferrite/diatomite and TiO2/chitosan/graphene oxide (GO@CS@TiO2) [46,47].The former was prepared by reacting ferric nitrate nonahydrate and calcium nitrate tetrahydrate in the presence of diatomite. In the next step, tetrabutyl orthotitanate and lanthanum nitrate hexahydrate were left to react in the presence of calcium ferrite/diatomite finally leading to La-doped TiO2/calcium ferrite/diatomite that was able to kill E. coli under visible light irradiation [46]. For preparing GO@CS@TiO2, in brief, GO was dispersed in ultrapure water, then CS was dissolved in acetic acid and added to GO solution obtaining nanometer film. Finally, GO@CS@TiO2 nanocomposites were prepared by adding colloidal TiO2 to the GO@CS film. GO@CS@TiO2 antibacterial properties were investigated against Aspergillus niger (A. niger) and Bacillus subtilis (B. subtilis) [47].
Moreover, many reported synthetic protocols propose the modification of commercially available TiO2 powders. For instance, TiO2 Aeroxide P25 form Evonik (20% rutile–80% anatase, specific surface area 50 m2/g) was modified with Ag NPs leading to a TiO2/Ag nanocomposite with strong antibacterial and/or antiviral characteristics [48,49,50,51].
Finally, examples of bio-hybrid nanocomposites have also been reported. For instance Kim et al. synthesized TiO2/glucose oxidase and the presence of glucose oxidase was found to increase the production of ROS under UV-A irradiation for E. coli and B. subtilis inactivation [52]. Monmaturapoj et al. synthetized TiO2/hydroxyapatite (HA) combining the antibacterial properties of TiO2 with the adsorption capability of HA to trap and photocatalytically inactivate H1N1 Influenza A Virus [53]. Li et al. reported on a modified TiO2 with tannic acid (TA), using a sol gel technique, as the presence of TA was found to enhance interfacial compatibility between TiO2 and a polyester matrix towards the fabrication of nanofiltration membrane. The TA-modified TiO2 demonstrated antibacterial properties under UV irradiation against E. coli [54].
3. Activity of TiO2-Based Nanostructured Materials against Bacteria, Fungi, and Virus
This section intends to provide an overview of the antimicrobial photocatalytic activity of the TiO2 NPs against bacteria, virus, and fungi, critically discussing the numerous parameters affecting the experimental results reported in literature. A large number of experimental variables have been found to affect the efficiency of photocatalytic TiO2-based NPs in the inactivation of microorganisms. The main parameters considered in the following are structural characteristics of the photocatalytic TiO2-based materials, morphology of bacteria such as structural, genomic, and physiological features, their metabolism, environmental conditions such as absence/presence of a light source, and environmental stress. Moreover, specific attention will be devoted to the intrinsic antibacterial activity of TiO2 and TiO2 -based nanocomposite. The antiviral and antifungal activity of TiO2 nanostructures will be also presented.
As a general scheme, the toxicity mechanism of TiO2 to pathogenic microorganisms could be summarized as follows (Figure 5): (A) cell damage and lipid peroxidation due to NPs attachments by electrostatic interaction on cell wall, (B) breaking of cytoplasmic flow due to NPs obstruction of nutrients carrier, photocatalytic degradation to (C) biological macromolecular, and (D) intracellular organelles.
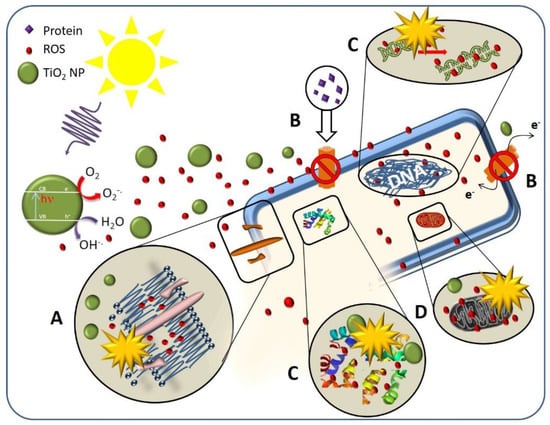
Figure 5.
Schematic diagram of TiO2 NPs toxicity mechanism on the cellular components of the pathogen microorganisms: (A) cell wall, (B) cytoplasmic flow, (C) macromolecular, (D) organelles.
Remarkably, the literature reported experimental results and their interpretation often appear debated and sometimes are in disagreement; therefore, here we aim to illustrate and critically discuss. The criteria identified to select the reported studies are the nanosize regime of the investigated TiO2 based materials (average sizes in the range 1–100 nm), the analysis of multiple experimental parameters, and the techniques to evaluate the antimicrobial activity of TiO2 NPs.
3.1. Antibacterial Activity of TiO2 Nanostructured Materials
3.1.1. Effect of TiO2 Nanostructure Characteristics
The antibacterial effect of TiO2 NPs-based nanomaterials was investigated as a function of their chemical-physical characteristics, which include NPs crystalline structure, particle size and shape, doping, and presence of co-catalysts such as metallic NPs and metal oxide NPs. Lin et al. studied the antibacterial effect of five types of TiO2 NPs, namely pure anatase NPs with average size of 10, 25, and 50 nm, 50 nm rutile NPs, and both anatase and rutile NPs with an average size of 25 nm. The photocatalytic experiments were performed under natural sunlight testing the NPs antibacterial activity against E. coli cells. The study was carried out on aqueous solutions at different pHs and ionic strengths. The smallest anatase NPs showed the highest affinity with cell surface in presence of TiO2 NPs, 50 mg/L, 3 h, and were, thus, found to induce higher oxidative cell damage. Interestingly, pH and ionic strength of the aqueous solution were also found to affect the antibacterial activity of the NPs (Figure 6) [55].
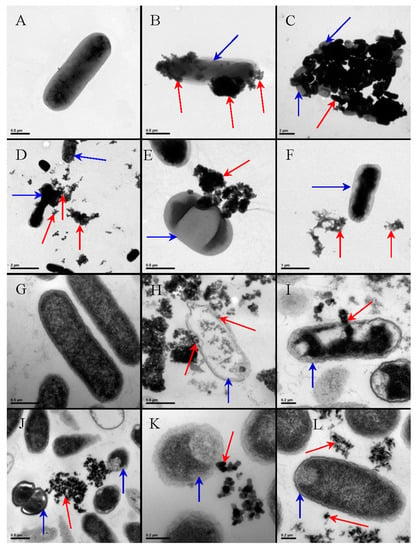
Figure 6.
TEM images showed NPs-type-dependent bacterial cell membrane localizations of TiO2 NPs and the morphological changes of the cell exposed to the NPs: TEM images of the unsliced (A–F) and sliced (G–L) Escherichia coli samples: A and G untreated; B and H upon treatment with 10 nm anatase TiO2 NPs; C and I upon treatment with 25 nm anatase TiO2 NPs; D and J upon treatment with 50 nm anatase TiO2 NPs; F and L upon treatment with 50 nm rutile TiO2 NPs; with all samples having been exposed to natural sunlight. The blue arrows highlight the cells and the red arrows point to the NP aggregates. Reprinted with permission from ref [55].
Shirai et al. compared the antibacterial activity of anatase TiO2 microparticles (5 µm) and anatase TiO2 NPs (21 nm). In particular, they studied the extent of antimicrobial effect on switching off the UV light irradiation. The antibacterial activity observed was higher for the 21 nm TiO2 NPs than the 5 µm TiO2 microparticles, even 6 h after stopping the UV exposure [56].
In the case of deposited nanostructured TiO2-based materials, the resulting antibacterial activity may also be affected by the characteristic of the nanostructured coating. Indeed, TiO2 NPs deposited on polyethylene by HIPMS (high power impulse magnetron sputtering), a pre-treatment of the polyethylene substrate with a RF-plasma (radio frequency-plasma) results in a roughness suitable for improving adhesion of bacterial cells to the substrate, that was found effective in enhancing the efficiency of the photocatalytic TiO2-assisted bacteria inactivation [57].
Sunada et al. demonstrated the bactericidal activity on E. coli and the decomposition ability on its endotoxin (detoxifying activity) of silica thin film (100 nm) modified with TiO2. Endotoxin is a toxic lipopolysaccharide (LPS) cell wall constituent of Gram-negative bacteria. The authors investigated the effect of TiO2 film photocatalysts on the concentration of endotoxin and survival ratio of E. coli, and found that while the survival ratio of the bacteria decreased either in presence and in absence of the TiO2 film (due to the germicidal effect of UV light), only in presence of TiO2 film was there also a decrease of the amount of endotoxin observed, thus highlighting the effect of the TiO2 photocatalyst on the integrity of the outer membrane of the cells [58].
In addition, modification of TiO2 nanostructures with metal NPs (Ag, Cu, Ce, Al, In, Mn, and Co), being able to improve the absorption property in the visible of TiO2 NPs-based nanocomposites, can enhance the efficiency in generation of ROS and, thus, increase the antimicrobial effect. In addition, the metal ions released from the metal NPs, once up taken by cells through their membrane, may interact with functional groups of proteins and nucleic acids, resulting in relevant damage of enzymatic activity, alteration of the cell structure, modification of the normal physiological processes, and, ultimately, in the inactivation of the microorganism [36,37,38,51,59,60,61].
Liu et al. studied antibacterial activity of a P/Ag/Ag2O/Ag3PO4/TiO2 photocatalyst against E. coli under LED lamp irradiation. The authors observed a bacterial inactivation of 100% by the composite within 20 min of photocatalytic treatment. On the contrary, under dark conditions, a reduction of E. coli was achieved (2-log cycles), attributed to the amount of metal Ag loaded on the composite. The concentration of bacteria remained almost equal in presence of TiO2 (in both the dark and light conditions). The inactivation process was also investigated by SEM analysis to evaluate cell damage. After 20 min of photocatalytic treatment, changes on the wall surface were observed, namely an increase in roughness or occurrence of holes in the cell wall. After 40 and 90 min, larger pits and holes were observed, and, subsequently the cells were found to be completely decomposed [62].
In general, metal NPs can improve the antibacterial activity both because they are able to enhance photocatalytic performance of TiO2 and release metal ions, therefore they exert an intrinsic biocidal effect interacting with cells.
3.1.2. Effect of the Cell Membrane Structure
Several types of bacteria were investigated in order to elucidate the antimicrobial activity of TiO2 NPs and TiO2 NPs-based nanocomposites. In this perspective, E. coli, as a model for Gram-negative bacteria, and St. aureus or P. aeruginosa, as models for Gram-positive bacteria, have attracted a lot of attention.
The need of investigate the behavior of both Gram-positive and Gram-negative bacteria in response to the photocatalysis-promoted antibacterial properties of TiO2 NPs arises from the intrinsic differences existing between these two classes of cells in terms of cell membrane structure, that may be responsible for a different interaction with TiO2-based NPs. In Figure 7, the difference between the cell walls of Gram-positive and -negative bacteria is shown.
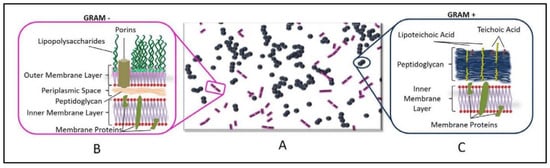
Figure 7.
(A) scheme of a Gram staining experiment with a mix of crystal violet stained cocci (Gram-positive bacteria) (blue) and safranin stained bacilli (Gram-negative) (purple) bacteria on a slide and comparison of the cell wall in Gram-negative (B) and -positive (C) bacteria.
For this reason, here, the cell membrane differences between Gram-positive and Gram-negative bacteria will be discussed first, and then relevant studies describing the TiO2 NPs antibacterial activity as a function of the characteristics of the bacterium membrane structure will be reviewed.
The cell wall of Gram-negative bacteria is characterized by the following features: (i) the presence of an outer membrane (OM) that contains phospholipids in the inner leaflet and glycolipids, mainly lipopolysaccharide (LPS) in the outer leaflet; (ii) a thin peptidoglycan cell wall, consisting of repeated units of disaccharide N-acetyl glucosamine-N-actyl muramic acid, that are cross-linked by pentapeptide side chains; and (iii) a cytoplasmic—or inner—membrane (IM), formed of a phospholipid bilayer.
The intermembrane space between the OM and the IM is characterized by a compartment defined as periplasm, that essentially contains proteins [63]. The OM, thanks to its unique composition, represents a selective barrier, that effectively protects cell from many agents, including detergents and antibiotics, still allowing penetration of specific macromolecules. Furthermore, the OM provides structural stability to the cell [64]. Gram-positive bacteria, instead, lack OM, but possess a 30–100 nm multilayer of peptidoglycans, which is thicker than that present in Gram-negative bacteria, which is a few nm thick [65].
Importantly, the cell wall of Gram-positive bacteria is porous, as the peptidoglycan multilayer can be easily crossed by teichoic acids, formed of glycerol and glucosyl or ribitol phosphate. The teichoic acids are anionic glycopolymers, that provide a high density of negative charge at the surface of the wall, thus making Gram-positive bacteria more prone than Gram-negative bacteria to interacting with positively charged NPs [65].
Several authors considered the ensemble of these features essential to explaining the observed antibacterial activity of TiO2 -based NPs being higher against Gram-positive than Gram-negative bacteria.
Fu et al. studied the effect of photocatalytic activity of TiO2 NPs at different concentrations under ambient light towards the Gram-negative E. coli and the Gram-positive Bacillus megaterium (B. megaterium). While E. coli was found to be inhibited by 5 mM TiO2 NPs, the highest tested concentration, B. megaterium was already inhibited at 1 mM [66].
Page et al. compared the antibacterial effect of TiO2 and Ag-doped TiO2 (being TiO2 in anatase phase in both cases) NPs-based coatings against E. coli (Gram-negative) and St. aureus and B. cereus (Gram-positive bacteria) under UV radiation (254 nm) for 30 min. Both coatings demonstrated photocatalytic and antimicrobial properties; however, the Ag-doped TiO2 NPs-based coating was found more effective against the Gram-positive bacteria than the bare TiO2 NPs-based one. Since the peptidoglycan multilayer forming the cell wall, along with the other mentioned components, consists of an open polymers network with peptide bridges, indeed the photogenerated hydroxyl radicals more easily interact and damage the cell wall, as they are not hindered by the surface appendages, that are, instead, present at the E. coli surface OM [34].
A similar behavior was observed by Alizadeh Sani et al. The antibacterial activity of a nanocomposite film, composed of whey protein isolate and cellulose nanofibers, 1.0% (w/w) TiO2 and 2.0% (w/v) rosemary essential oil, was evaluated and a growth inhibition effect was shown to be higher for Gram-positive bacteria, as Listeria monocytogenes (L. monocytogenes) and St. aureus, than Gram-negative bacteria, as E. coli, Salmonella enterica, and P. fluorescens [67].
Nakano et al. evaluated the microbiocidal activity of photocatalysts for various species (virus, Gram-negative and -positive bacteria). Bactericidal activity of TiO2 coated glass under UV-A irradiation for 48 h was investigated and the author confirmed that Gram-bacteria cocci (GPC) (S. aureus and Enterococcus spp.) were promptly inactivated, while Gram-negative cocci (GNC) (E. coli and P. aeruginosa) were only gradually inactivated [68].
On the contrary, other authors showed that Gram-positive bacteria are more resistant than Gram-negative bacteria. Khezerlou et al. proposed that, due to its composition, the Gram-negative bacteria OM is an attractive target for hydroxyl radicals, produced upon the TiO2 NPs photoactivation, that can react with the lipidic components of the membrane rather than crossing it. The authors inferred an antibacterial action consisting of two steps: in the first, the OM is compromised, being then involved the cytoplasmic membrane. In the second step, once the OM is breached, the radicals can disrupt cytoplasmic membrane and subsequently induce the cell death [5].
Backhaus et al. (2010) investigated the effect of TiO2 P25 on two faecal indicator strains, E. coli and Enterococcus faecalis (Ent. faecalis), in real wastewater treatment plant effluents, that is a water matrix with total organic carbon (TOC) values in the range of 8–25 mgC/L and conductivity in the range 450–750 µS/cm (effluent organic matter, EfOM). By using a UV-A bulb lamp, presenting its highest emission at 365 nm, the authors observed a different response of two types of bacteria to the photocatalytic treatment: Ent. faecalis was less sensitive than the E. coli to the photocatalytically generated TiO2 NPs. Such a result was accounted for by the occurrence of the thick peptidoglycan multilayer in Gram-positive bacteria, that might have a higher affinity with EfOM relative to the peptidoglycan layer characterizing Gram-negative bacteria (EfOM protecting Ent. faecalis by ROS) [69]. Pal et al. reached the same conclusions upon testing the antibacterial activity of TiO2 P25 NPs on different species, such as E. coli and P. fluorescens (as Gram-negative bacteria), and B. subtilis, Microbacterium spp., Microbacteriaceae, and Paenibacillus sp. (as Gram-positive bacteria). The authors used an experimental set-up, consisting of an irradiation source (UV-A, 365 nm) incident on the bacterial suspension (in contact with a filter loaded with a defined amount of TiO2 NPs) by clamping the light source on top of a suitable support, and found that photocatalytic inactivation was more effective against E. coli than against B. subtilis [70].
Other studies, conversely, observed a negligible difference between two microbial groups. Ripolles-Avila et al. tested two types of commercial TiO2 NPs, the NM105 (Degussa, Frankfurt, Germany) and the NM101 (Institute for Health and Consumer Protection at the European Commission Joint Research, Ispra, Italy), the former being based on anatase phase 7 nm NPs, while the latter is a mixture of 21 nm anatase and rutile phase (80:20 wt/wt) NPs. The experiments performed to investigate the antibacterial activity of the NM105 and the NM101 were carried out both in the dark and under UV light irradiation (315–400 nm) on Salmonella enterica var. Enteritidis, E. coli, St. aureus, and B. cereus. The results indicated that the antibacterial activity was affected by the amount of TiO2 NPs, both in the dark and under UV-light irradiation. Further, no significant difference, in terms of cell viability, was observed among the different investigated microbes [71]. Analogous results were obtained for TiO2 NP-coated implants [72] or colloidal dispersions of TiO2 NPs [73]. In summary, the authors claim that the photocatalytically assisted antibacterial activity of TiO2 NPs, defined in terms of cell inhibition, does not depend on the features of cell membrane structure. Indeed, Haider et al. used TiO2 in a self-cleaning transparent coating for windows in outdoors applications, preparing NPs by using TiCl4 as a precursor, and calcinating at different, increasing temperatures (400 °C, 600 °C, 800 °C and 1000 °C), testing E. coli and P. aeruginosa. SEM analysis showed that after 2 h of sunlight irradiation, the TiO2NPs-based coating was effective against both bacteria, as no cells survived [74]. In this case, the authors also concluded that the cell wall structure was not the main parameter involved in bacterium resistance to photocatalysis-promoted antibacterial activity of TiO2 NPs.
3.1.3. Effect of Bacterial Metabolism
The mechanisms behind cell growth inhibition and bacterial death, induced by the photocatalytic reactions promoted by TiO2 NPs, have also been analyzed considering two different cell metabolisms. Skorb et al. investigated the effect of the photocatalytic activity of several TiO2 NPs-based nanocomposites, on two bacteria strains showing a different metabolism: P. fluorescens, obligate aerobe, and Lc. lactis, facultative anaerobe. The investigated TiO2 NPs-based nanomaterials, which consisted of thin-films of TiO2, TiO2:In2O3, TiO2/Ag, or TiO2/Ag/Ni deposited on a ceramic substrate, were prepared first by spraying the oxide sols, and then performing silver photo-deposition and electroless nickel deposition in order to convey antibacterial properties. According to the results, the differences between P. fluorescens and Lc. lactis in terms of bacteria inactivation rate could be associated to the morphologies of cell envelops and to the resistance of OM to radicals generated from the photocatalytic reactions [36]. In particular, P. fluorescens uses the oxidative phosphorylation to store energy required for cell respiratory functions. Therefore, for this type of cell, the increase of ion permeability of the cytoplasmic membrane, due to the damage induced by ROS, causes the loss of the proton gradient and consequently inhibits bacteria respiratory function. Lc. lactis (facultative anaerobe), instead, stores energy thanks to other metabolic process (such as lactic acid fermentation). For this reason, the damage on the cytoplasmatic membrane detected in Lc. lactis was scarcely relevant [36].
3.1.4. Effect of Physiological State of Bacteria Cell and Environmental Stress
Rincón et al. claimed that in photocatalytic bacteria inactivation experiments, the rate of cell inactivation can be affected by the bacterial growth state (exponential or stationary phase) as well as by generation state of the culture. Indeed, E. coli cells, collected during the exponential step, were inactivated in a shorter timeframe (1 h) with respect to the same cells collected during the growth stationary phase, that, instead, required 2.5 h to be completely inactivated in presence of P25 TiO2 NPs under simulated sunlight irradiation. Moreover, E. coli cells from the third generation were found more resistant compared to those from the seventh generation.
The authors suggested that the performance of the TiO2 assisted photocatalytic inactivation was influenced by physiological state and generation of the bacteria [75]. In detail, environmental stress was thought able to induce expression of genes involved in the synthesis of a specific set of proteins, that have been found responsible for mutations in the following generations. In addition, the stationary phase response to environmental changes induces the synthesis of the proteins that convey to E. coli resistance to several types of stress (i.e., heat shock, oxidation—UV light—, hyperosmolarity, and acidity). During the stationary phase, the expression of rpoS is also induced, which encodes a regulator for expression of genes [76,77], such as those involved in the protection against oxidants (e.g., catalase) and in the reparation of oxidative damage (e.g., exonuclease III) [78,79].
Another important factor is represented by the ability of bacteria to resist to environmental stress. Upon environmental stress, the bacteria can activate several mechanisms of defence, in order to limit the damage. Indeed, it is well known that there are specific enzymatic systems able to eliminate ROS. In bacteria such as St. aureus or Staphylococcus pyogenes (St. pyogenes), anti-ROS mechanisms, often associated to pathogen virulence, were identified [80,81]. Furthermore, bacteria are able to produce a biofilm that prevents the penetration of NPs into the cell membrane, thus hampering their antibacterial functions.
Interestingly, in TiO2 NPs-assisted photocatalytic reactions, the concentration of ROS physiologically produced in presence of bacteria is another important parameter to be taken into account when considering cell damage [82]. Bonnet et al. investigated Gram-positive bacteria (St. aureus and Lactobacillus casei rhamnosus, Lb. casei rhamnosus) and Gram-negative bacteria (two different strains of E. coli) in contact with anatase TiO2 NPs under UV light irradiation. In particular, after 30 min, St. aureus was found to be more resistant than the other bacteria under the investigated experimental conditions. Such evidence was explained in terms of the detoxification ability of the catalase activity, which is typical of this bacterium, and that was exerted in presence of TiO2 NPs. Indeed, catalase is able to trigger the oxidative stress-related mechanisms against endogenous ROS (such as H2O2), metabolites naturally produced by these cells, that consequently need to remove them by activating detoxification mechanisms; the same mechanisms can thus act against TiO2 NPs-induced ROS [83]. These features, therefore, suggest that the resistance may be species-dependent.
3.1.5. Intrinsic Antibacterial Activity of TiO2
The intrinsic antibacterial activity of TiO2 NPs in the dark is an extremely controversial and debated issue, that, in fact, often hinders the use of TiO2 NPs in any kind of application that does not allow light irradiation. Erdem and other authors [84,85] confirmed that different types of TiO2 NPs were able to promote a short-term bacteria inactivation, even in the dark. In detail, in this case a possible killing mechanism is assumed to mainly involve the direct interaction between TiO2 NPs and bacterial membrane. Indeed, the OM of Gram-negative bacteria (especially in E. coli) contains porins, which are membrane proteins acting as pores (1.5–2 nm in diameter) enabling diffusion of molecules in the cytoplasmic compartment. When the TiO2 NPs and bacteria are in close contact, NPs may obstruct such pores, thus hindering the diffusion channels, preventing nutrition uptake, finally inducing cell death.
The close contact between TiO2 NPs and E. coli OM was identified by Kiwi et al. as an essential condition, responsible for the intrinsic antibacterial activity of TiO2 NPs in the dark. Such a conclusion was reached by monitoring E. coli inactivation experiments by TEM microscopy. Indeed, TEM micrographs, collected at different inactivation times, pointed out that, at suitable TiO2 NPs amounts and at a pH close to its isoelectric point, aggregates of TiO2 NPs migrate towards the OM of E. coli, where they accumulate due to electrostatic interactions, finally leading to extensive damage of the OM and therefore to the loss of E. coli cultivability [86,87,88]. Ripolles-Avila et al. investigated the antibacterial activity of TiO2 NPs both in the dark and under UV-light irradiation. The test performed against both Gram-negative and -positive bacteria demonstrated an intrinsic bactericidal activity of TiO2 NPs, since no significant differences, in terms of cell inactivation rate was observed with or without irradiation [71]. However, the presence of NPs was demonstrated to affect the bacteria growth, especially at low cell population density [89].
The antibacterial effect of P25 TiO2 NPs was also investigated by Carré et al. (2014) against E. coli, specifically considering lipid peroxidation phenomena and performing a proteomic analysis. The extent of lipid peroxidation, through the thiobarbituric acid (TBA) assay, was found to significantly increase both in the dark and under UV-A-light irradiation, after 60 min exposure to TiO2 P25 at a concentration of 0.4 g/L. The proteome of bacteria was investigated by means of electrophoresis (2-DE), that is able to identify the extent of protein damage under different experimental conditions, namely at two distinct TiO2 NPs concentration (0.1 or 0.4 g/L) with and without UV-A irradiation, and evaluated against two control tests performed without catalyst, with and without irradiation, for 30 min. In particular, in presence of TiO2 NPs, both in the dark and under UV-A irradiation, OM proteins suffered most alterations, probably due to the direct contact with TiO2 NPs [90]. On the other hand, several authors observed that TiO2 NPs in the dark did not exhibit toxic effects on bacteria [89,91].
Another possible interesting explanation for the intrinsic, non-photoinduced, antibacterial activity of TiO2, though non-nanostructured in nature, was reported by Lifen et al. who demonstrated that the germicidal activity of their sol-gel synthesized TiO2 NPs was not UV-induced, under the investigated experimental conditions, being, instead, directly related to the wettability property of TiO2 NPs [50]. The surface of TiO2 NPs was found characterized by both hydrophobic and hydrophilic areas, the latter causing a deformation of the cells they get in contact with. In fact, the cell lost its rounded shape and flattened. This phenomenon was expected to build up pressure in the cell, which resulted in a burst of the cell and release of the contents.
3.2. Virus Inactivation
Viruses are ubiquitous biological entities much smaller than bacteria (0.01–0.03 µ). However, they are not independent organisms, presenting independent metabolic activities. Indeed, they need to infect a host for their reproduction. Viruses usually involved in waterborne disease outbreak are noroviruses (NoV), hepatitis A virus (HAV), hepatitis E virus (HEV), adenovirus (AdV), astrovirus, enteroviruses (EV), and rotavirus (RV). In particular, enteric viruses have high infectious capacity, that is, a low amount of viruses is sufficient to induce an infection (i.e., <10–103 virus particles) [92]. Viruses can often be detected on surfaces, because the structure of their capsid provides them environmental stability. Surfaces can play a crucial role in the spread of microorganisms, especially in a nosocomial environment, where the presence of viruses on the surfaces of equipment, furniture, and medical devices, as well as wall surfaces, represent a potential risk for patients and operators [93]. Moreover, viruses are also widespread in public spaces, including offices, canteens, and kindergartens. Once a surface is contaminated, it immediately becomes a contamination source for individuals and, consequently, other objects. For instance, in the case of NoV, it has been observed that it is possible to transmit the virus to up to seven different surfaces by simply handling contaminated objects [94]. Finally, viruses persist on the surfaces for an extremely long timeframe, ranging from 2–3 h (for HAV and coronavirus) to 20 weeks (for vaccinia virus) [95], thus being extremely critical for infection propagation.
Recently, the photocatalysis-promoted antiviral activity of TiO2 NPs has been extensively investigated with the aim of limiting the spread of viruses, given that the antiviral behavior of TiO2 NPs is less documented than the antibacterial characteristics. In this regard, the most investigated models of viruses or phages include bacteriophage MS2 [96,97,98], t4 [99,100], Qb [101], λ [102], phi-X174 and fr [103], NoV [94], herpes simplex virus-1 (HSV-1) [104], human influenza A virus [105]; H1N1 influenza A virus [53], avian influenza virus A/h5N2 [106], and influenza virus strain A/Aichi/2/68 (H3N2) [107]. Remarkably, these models of virus/phage are interesting both from an environmental and health point of view.
When phage is used as a model, often the system involves the infection of a bacterium by a specific phage, such as the commonly investigated E. coli.
Generally, most experimental studies were carried out in aqueous solution, investigating virus exposure to TiO2 NPs, both in the dark and under different irradiation conditions, given that the virus is able to interact with the TiO2 NPs [96,97,98,99,102,108] both dispersed and immobilized on suitable substrate.
Many reports demonstrated how the environmental conditions can affect viral inhibition by photocatalytic TiO2 NPs. For example, Syngouna et al. showed that the presence of quartz sand altered the antiviral efficiency of TiO2 NPs against the bacteriophage MS2-E. coli both under sunlight irradiation and in the dark, resulting in a higher virus inactivation rate in absence of quartz sand. However, at higher virus concentration, the excessive viral density was demonstrated to inhibit the antiviral activity [97] of TiO2 NPs. Zheng et al. obtained analogous results and assumed that a high viral density saturates the reactions sites on the photocatalyst surface, thus leading to limited ROS production [108]. Syngouna et al. (2017) also tested the antiviral activity of TiO2 NPs in double distilled water (ddH2O) solution and in phosphate buffered saline solution (PBS), demonstrating that a higher MS2-E. coli inactivation could be obtained in ddH2O rather than PBS solution. This is most likely because the PBS induces MS2 aggregation, due to the ability of the proteins of MS2 to bind of phosphate ions, given that virus aggregation is known to reduce the efficiency of photocatalytic TiO2 NPs in MS2 virus inactivation [97]. Different characteristics of the aqueous medium may affect the photocatalytic antiviral activity, including temperature [102], pH [98], and presence of inorganic ions [91]. The effect of temperature on the Cu-TiO2 nanofiber-assisted removal of bacteriophage f2 under visible light irradiation was shown by Zheng et al. [108]. In particular, a relatively low temperature (15 °C) was found to negatively affect the virus removal rate, In fact, although in the first 30 min, the rate at 15 °C was the highest under experimental conditions, after 90 min it decreased significantly, resulting, instead, in the removal efficiency at 25° and 35 °C being much higher. Koizumi et al. observed that inactivation rate of the phage MS2 in presence of TiO2 P25 irradiated by black light fluorescent lamp was influenced by pH, given that it was higher at pH 6 than at pH 3.0 and 10.0 [98].
Moreover, the turbidity of water matrices, affecting the absorption of incident light [109], can reduce the photocatalytic efficiency, leading to a decrease of ROS production. Therefore, a suitable design of photocatalysis experiments needs to take into account the penetration depth of the wavelengths selected for the experiments [109]. For instance, in distilled water, 254 nm radiation loses 30% of its intensity already 40 cm below the solution surface.
Ishiguro et al. (2011) investigated the antiviral effect of TiO2-NPs (anatase) coated glass plates on bacteriophage Qβ and T4 (host E. coli), as representative models of RNA and DNA viruses, respectively. The effect of UV light intensity (0.1 and 0.25 mW/cm2 UV-A) and irradiation time (1, 2, 4, 8, and 24 h) were investigated and both bacteriophages became inactivated when in contact with the TiO2-coated glass irradiated with 0.001 mW/cm2 UV-A light. However, the inactivation of T4 was lower than that of Qβ when exposed to UV-A at an intensity lower than 0.01 mW/cm2, for 4 and 8 h. Although the photocatalysis was sufficient to inactivate both viruses, these results suggest that the ratio of inactivation varies according to the type of virus [100].
Gerrity et al. compared the disinfection potential of TiO2 P25 NPs irradiated by a low-pressure UV-lamp with respect to a UV-light source, using bacteriophage PRD1, MS2, phi-X174 and fr, which are characterized by different physical and molecular features (size, nucleic acid composition and topology, genome length, mode of infection). The authors observed that in order to reach an inactivation rate of 4-log, a different UV intensity reduction, namely 19%, 15%, and 6%, was required for PRD1, MS2, and phi-X174, respectively. Interestingly fr was found to be UV-resistant [103]. The detected differences in inactivation efficiency were associated to structural differences among the investigated viruses.
Nakano et al. tested antiviral activity of TiO2 coated glass on influenza virus (IFV) and feline calicivirus (FCV), according to the same procedure reported in Section 3.1.2. The authors found that IFV was inactivated by photocatalysts significantly faster than FCV, thus indicating that the virucidal effect of the photocatalyst may depend on the presence of a viral envelope, given that IFV is an enveloped virus, while FCV a non-enveloped virus [68].
The same authors studied the human influenza A virus as a viral model for the TiO2 NPs-assisted photocatalysis Experiments [105], carried out by tuning irradiation time and UV-A lamp intensity in the range of 0.001–1.0 mW/cm. After 8 h incubation, a reduction of approximately 3-log was observed by using low intensity UV-A-light (0.01 mW/cm). A decrease of viral inactivation was observed with the decrease of UV-A light intensity. After 8 h incubation, a reduction of approximately 3-log was observed by using low intensity UV-A-light (0.01 mW/cm2). However, even with an irradiation intensity of 0.001 mW/cm2, the TiO2-coated glass effectively inactivated the virus after 16 h of incubation, with a reduction of 4-log. At high UV-intensity (1.0 mW/cm2), a much faster viral inactivation was found (3-log after 4 h). Therefore, irradiation intensity and time affect the inactivation efficiency of the prepared TiO2-coated glass against influenza virus.
The mechanisms of virus inactivation by TiO2 NPs-assisted photocatalysis are a debated topic. Some authors suggest that ROS induce the capsid protein degradation, as demonstrated through different techniques (qPCR; sodium dodecyl sulphate–polycrylamide gel electrophoresis, SDS-page; etc) [97,100,103,110], while others (Nakano et al. (2012)) indicated, on the basis of real-time reverse-transcription PCR (RT-PCR) and SDS-page, RNA degradation only after the destruction of viral proteins involved in binding, therefore the infectability decreased [105]. Hajkova et al. demonstrated the photocatalytic effect of thin TiO2 film in presence of UV-A light on the virus HSV-1. The experimental results proved that interaction of the virus with the photocatalytic surface caused significant changes in the virus structure, for instance, inducing the loss of viral glycoprotein, gC, responsible for the first attack of the virus on the target cell, thus resulting in the virus’ inability to attack host cells [104].
The antigens, present in the capsid, were also considered to elucidate the effect of TiO2 NPs. For example, the degradation of HBsAg, hepatitis B surface antigen, typical of hepatitis B virus (HBV) was evaluated in assessing its role in the photocatalytic antiviral activity of TiO2 NPs. The degradation of the HbsAg antigen was found affected by the exposure time, amount of photocatalyst, and light-source. The inactivation of the antigen occurred after 12 h exposure. HbsAg degradation extent increased as the TiO2 concentration increased. In addition, the effect of the light source on the photodestruction level of HbsAg was also shown, following the order UV lamp > mercury lamp > natural light > weak light (i.e., in the dark while not optically filtered) [110].
Furthermore, TiO2-NPs-based nanocomposites showed promising results for photocatalytic inactivation of viruses. A palladium-modified nitrogen-doped titanium oxide (TiON/PdO) photocatalytic fiber was effectively used for the disinfection of the coliphage MS2 with its host (E. coli) by Li et al. In the dark, a significant virus adsorption was measured (95.4–96.7%), while, after 1 h irradiation with visible light (λ > 400 nm) a virus removal of 94.5–98.2% was achieved [96]. Monmaturapoj et al. showed the effect of hydroxyapatite-titania composite (HAP/TiO2) on H1N1, investigating the role played by nanocomposite amount, virus concentration, and UV-light irradiation time. An H1N1 photocatalytic inhibition efficiency dependent on the amount of photocatalyst was observed. In particular, no virus inhibition was measured for a HAP/TiO2 concentration higher than 0.5 mg/mL. The HAP/TiO2 nanocomposite was able to merge two functions in one nanomaterial: the virus adsorption on the photocatalyst surface, promoted by HAP, and ROS production, induced by UV light irradiation of TiO2 [53]. Finally, SiO2-TiO2 NPs and Ag-doped TiO2 NPs (nAg/TiO2) should be also mentioned for their high photocatalytic inactivation rates against the bacteriophage MS2 [49,111].
The current pandemic situation, related to the spreading of the SARS-CoV-2 virus, stimulated the researchers towards the understanding of its ability to persist on the surfaces [112], and towards the investigation of original and reliable solutions suitable for limiting the spread of SARS-CoV-2. Recently, Khaiboullina et al. (2020) explored the photocatalytic properties of nanosized TiO2 NPs, deposited on glass coverslips, under UV radiation, against the inactivation of HCoV-NL63, namely a human coronavirus belonging to the family of α-coronaviruses, which also includes SARS-CoV-2 [113]. The experimental results highlighted the virucidal efficacy of photoactive TiO2 NPs, as examined by quantitative RT-PCR and virus culture assays.
3.3. Fungi Inactivation
The scientific community has also started to intensively investigate antifungal properties of photocatalytic TiO2 NPs. In spite of the low number of reports on the antifungal photocatalytic activity of TiO2 nanostructured materials, increasing attention can be noticed, motivated by the ability of fungi (filamentous forms and yeasts) to produce dangerous mycotoxins in the water. Further, fungi and their spores are often present on surfaces, including foodstuff, as well as in indoor and outdoor environments. Fungi infect a host, behaving as parasites or by causing the decay of organic matter.
Fungi typically used as a model substrate to investigate antifungal propriety of TiO2 NPs are: C. albicans, Saccharomyces cerevisiae (S. cerevisiae) [114], Penicillium expansum (P. expansum) [115], Aspergillus fumigatus (A. fumigatus) [82], A. niger [114,116], Fusarium sp. [116,117,118], and Penicillium chrysogenum (P. chrysogenum) [119].
Compared to bacteria and viruses, fungi survive in much more stressful conditions (high saline/sugar concentration, high osmotic pressure and extreme pH). They have a complex structure and can show several morphologies. Indeed, molds are multicellular filaments, while yeasts are unicellular organisms. Fungi have a robust cell wall, mainly formed of chitin, with lamellar morphology, given that each layer is made up of fibrils crossing each other in different directions. Fungi (filamentous and yeast species, conidia, hyphae) showed different responses to the TiO2 NPs treatment. Rodrigues-Silva et al. tested the effect, of TiO2-assisted photocatalysis on spores of A. fumigatus, both in water and air. After 60 min UV-A-irradiation, the inactivation of spores was observed, while after 180 min the complete inhibition of the microorganism’s growth was detected. They pointed out a direct correlation between the TiO2 NPs loading and the disinfection efficiency in aqueous media, as the higher the photocatalyst loading, the greater the photoinduced inactivation of fungi [82].
Seven et al. (2004) studied the effect of TiO2 P25 (0.01 mg/mL) on different organisms (bacteria and fungi, such as filamentous and yeast species) using a sodium lamp as a light source. The TiO2 disinfection ability was tested on different bacteria, in particular C. albicans and A. niger. C. albicans were inhibited after 120 min, whereas no inhibition was observed for A. niger even if the irradiation time was extended up to 240 min [114]. Furthermore, Polo-Lopez et al. (2010) tested TiO2 P25 NPs on Fusarium sp and observed a greater resistance of the chlamydospores, followed by macroconidia and microconidia, due to their extremely complex structure, which is able to hinder the penetration of ROS [120]. The resistance to TiO2 assisted deactivation of spores belonging C. albicans and A. niger was also reported [116,121,122].
Maneerat et al. (2006) tested the photocatalytic activity of TiO2 powder and of a TiO2 NPs-based coating on a plastic film, in vitro and on fruit rot (tomato and apple) in presence of UV-A source against P. expansum. [115] The severity of fruit rot was estimated by visual appearance and scored from 0 to 4 (0 = no decay; 4 = decay covering more than 50% of the whole fruit area). No inhibition effect on fungi growth was observed for the TiO2 powder in the dark (control), while in presence of TiO2 NPs under UV-A light irradiation, the growth decreased relative to the control experiment. Moreover, the TiO2 NPs-based coating on the plastic film revealed P. expansum inhibition higher than in the control experiment (scores 1.9 vs. 3.2). Kuhn and co-workers (2003) investigated the damage induced by TiO2 NPs under UV-A light irradiation by means of SEM analysis, highlighting a grainy and partially destroyed cell surface [123].
The differences among the various species of fungi in terms of structural features have been found reflected in their response to interaction with ROS generated by photocatalysis-assisted TiO2. The main difference among spores is in the wall as unicellular microconidia without septa and larger pluricellular macroconidia with septa both have a single cell wall, while chlamydospores exhibit a double thick wall [124]. Such a difference explains the highest resistance of chlamydospores (followed by macroconidia and microconidia) to photocatalysis [112].
Furthermore, the nature of the investigation medium also contributed to inactivation of spores in presence of TiO2 and solar irradiation. Indeed inactivation rate values in distilled water were found higher than those detected in well water, most likely due to the presence of carbonates/bicarbonates [116]. Indeed, inorganic salt was reported to decrease the TiO2 photocatalytic efficiency [125,126], mainly because of the formation of an inorganic layer at the TiO2 surface.
3.4. General Considerations
In spite of the great attention attracted by the antimicrobial properties of TiO2 nanostructured materials, the experimental evidence and the interpretation of the experimental results reported in the different studies, as also seen in this review, are often debated and, somehow, controversial.
Many of the discrepancies in the results can be ascribed to the diversity of methods used to investigate the photocatalytic disinfection. In order to highlight this variety of conditions, the experimental photocatalytic and operational parameters, including details of the set-up, for bacterial, viral, and fungal disinfection tests are collected in Table 1, Table 2 and Table 3, respectively. Furthermore, as summarised in Figure 8, TiO2 NP characteristics (Figure 8A) affect the photocatalytic efficiency while the microorganism structure (Figure 8C), its metabolism (Figure 8D), and local environment adopted for its growth (Figure 8E) strongly affect the response of the microorganism to photocatalytic inactivation (Figure 8B).

Table 1.
Summary of the experimental conditions reported in the literature for TiO2-based nanomaterials photocatalyzed bacterial disinfection.

Table 2.
Summary of the experimental conditions reported in the literature for TiO2 and TiO2-based nanocomposite photocatalyzed viral disinfection.

Table 3.
Summary of the experimental conditions reported in literature for TiO2-based nanomaterials photocatalyzed fungal disinfection.
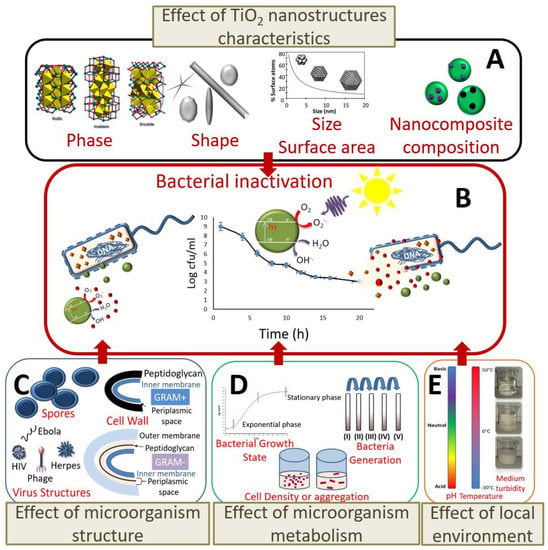
Figure 8.
Effect of factors influencing the photocatalytic action of TiO2 NPs and TiO2-based nanocomposites on bacterial and viral inactivation: (A) crystalline phase, NP size, and surface area and composition of the nanocomposite affect the photocatalytic efficiency; (C) microorganism structure, (D) its metabolism, and (E) local environment adopted for its growth strongly affect the response of the microorganism to photocatalytic inactivation (B).
Such considerations are intended to point out the relevance of a suitable experimental design, and the need to define reliable and reproducible analytical approaches to studying antimicrobial activity, which could, possibly, also be standardized in order to provide comparable data. In Figure 9, common methods for testing the microorganism inactivation ability of a specific system are outlined.
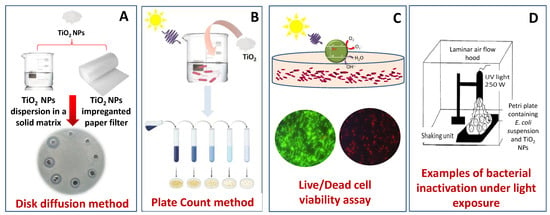
Figure 9.
Summary of the reviewed methods to study the antimicrobial activity: (A) disk diffusion method, testing TiO2 NPs dispersion directly in a solid matrix or impregnated paper filter; (B) plate count of cell suspension in contact with catalyst; (C) live/dead cell viability assay, where the two micrographs show healthy (green) and dead (red) bacteria; (D) examples of experimental set-up by Reddy et al., 2007. Reprinted from ref [82,127], Copyright (2020 and 2016, respectively), with permission from Elsevier.
Antibacterial, antiviral, and antifungal activity on surfaces (fine ceramics) can be evaluated by means of ISO, 27447:2019 (Organization for Standardization) or JIS, R 1702:2012 (Japanese Industrial Standards Committee), ISO 18061:2014 or JIS R1706:2013, and ISO 13125:2013 or JIS R1705:2016 methods, respectively. However, such international standard methods are, in most of the reported cases, not used, or are applied with modification, thus resulting in a large variability in set-up and experimental conditions, that prevent a clear comparison of the performances of the different systems [128,129,130,131,132,133].
Commonly, antibacterial activity is investigated using the disk diffusion method. According to this approach, NPs are incorporated in a solid matrix or impregnate a paper filter placed on an agar plate (Kirby Bauer agar plating technique). At this stage, bacteria are allowed to grow on the agar plate and the evaluation of the diameter of the inhibitory zones allows to determine the efficiency of bacterial growth inhibition [25,33,134]. In the disk diffusion method, a high number of experimental parameters (different dimensions of spot diameter; cell density used; amount of catalyst) is present, that can, in principle, result in a misled interpretation of the results, thus leading to contradictory conclusions as a function of the different parameters. Although the disk diffusion method is simple and widely used for testing the antibacterial efficiency, it shows some limitations, such as the limited diffusion of the material to be tested in the agar matrix, a poorly resolved concentration gradient around the disks, and the absence of a suitable light source that makes this tool not ideal for the photocatalytic experiments, which require an irradiation source. Therefore, to specifically study the TiO2-based NPs-assisted photocatalytic inactivation of microorganisms, dedicated experimental set-ups have been proposed, including a proper light source with specifications suitable for the investigation to be carried out [37,61,127,135].
Another issue is caused by the need to evaluate viability or condition of bacteria. For this purpose, a viable count method or the live/dead cell viability assay can be used. The former test allows to simultaneously control the viability of bacteria (live and viable, but not culturable cells), while the latter method allows the simultaneous determination of live and dead cells using two fluorescence dyes: ethidium homodimer III (EthD-III) and calcein acetoxymethyl (calcein AM).
Therefore, the method used to investigate the antimicrobial activity could strongly affect the reliability and interpretation of the experimental results.
Another important issue represented by the need for a standardization of test method is the selection of the microorganism to be used as a target for photocatalytic antimicrobial experiments, especially for a hypothetical future application of the material studied (water-, air-, and food-borne). Beyond the structural properties (i.e., Gram-positive vs. Gram-negative bacteria), the final applications field of the NPs studied should also be considered. Therefore, it is also important to consider the correct indicator microorganism. It follows that the target microorganism can also be selected as a function of the specific application the investigated TiO2 NPs-based nanocomposite is designed for. In other words, E. coli, Shigella flexneri (S. flexneri), L. monocytogenes, and Vibrio parahaemolyticus (V. parahaemolyticus) are indicators of contaminated waters. They can therefore be selected as target microorganisms for TiO2 NPs-based nanocomposites specifically designed for water remediation, while indicators of nosocomial infections such as Streptococcus pyogenes (Str. pyogenes), Acinetobacter baumannii (Acin. baumanii), St. aureus, and P. aeruginosa can be used as target microorganisms to investigate TiO2 NPs-based nanocomposites designed to be used in healthcare facilities.
Taking into account the above reported considerations, it is apparent that the lack of standardized techniques suitable for evaluating the photocatalytic antimicrobial activity of TiO2 NPs underlining the different actions on the microbial inhibition is at present a strong limitation in unequivocally assessing the photocatalytic antimicrobial properties of TiO2.
Furthermore, for fungi, the proposed methods to test the antifungal activity may vary significantly in the different reports. Some authors investigate antifungal activity in a fungal suspension containing the TiO2-based powder irradiating with a light source that can be very different in term of light flux and emission wavelength [82,114,116,117,123]. The photocatalytic activity is evaluated by examining the growth of the investigated fungus by plate count relative to an untreated control sample. As an alternative, the photocatalysts can be applied directly to fruit or vegetable surfaces (e.g., apple, tomato, or lemon) [115,117] and the photocatalytic activity can be evaluated by simply measuring the diameter of the fungal colonies grown with relative to an untreated control sample.
In conclusion, photocatalytically active TiO2 NPs show great potential for antiviral and antifungal related applications. However, while antiviral activity is well documented, the photocatalysis-promoted antifungal activity of TiO2 NPs, still deserves more investigation, considering, in particular, the role played by the structural features of the microorganisms in terms of thickness of spores and cell wall.
4. Technological Applications of Antimicrobial TiO2-Based Nanostructured Materials
In this section, selected applications of the antimicrobial TiO2-based materials will be described focusing the attention on environmental applications, including water treatment, anti-biofouling membranes for water treatment, disinfection of building materials, and disinfection of (i) biomaterial and (ii) food packaging and processing materials.
4.1. Environmental Applications
4.1.1. TiO2-Based Nanostructured Materials for Water Disinfection
TiO2 based nanocomposites are well known and extensively used materials in water treatment technologies [3,4,136]. Indeed they are able, due to their photocatalytic activity, to degrade organic pollutants such as dyes, [137] pesticides [138], pharmaceuticals [3,139], and personal care products [140], that pose a great danger for human and aquatic life. Moreover, their antimicrobial properties are effective against waterborne pathogens such as bacteria, viruses, and fungi that originate from the urban microbiome and tend to be released and accumulated in sewage and urban runoff. The mechanism is summarized in Figure 10 and will be explained in the following. Waterborne diseases derive often from bacteria such as E. coli, Legionella pneumophila, Mycobacterium avium, S. flexneri, L. monocytogenes, V. parahaemolyticus and, to a lesser extent, from viruses, that are typically present at lower concentration. Every year, waterborne infections cause nearly 200 million deaths worldwide, mainly localized in low income countries [129]. One of the most critical classes of bacteria in wastewater treatment plants (WWTPs) is antibiotic resistant bacteria (ARB), which derive from the extensive use and abuse of antibiotics. WWTPs offer an ideal environment for ARB proliferation and, under these conditions, the antibiotic resistance genes (ARG) that induce the resistance in the bacterial population, can spread easily and quickly. Therefore, as the use of common antibiotics becomes ineffective, it is fundamental to develop alternative strategies to treat microbial infections and to promptly take action against the spreading of ARB in water as well as in the air [141]. The present section will focus on TiO2-based nanomaterials as an excellent alternative to conventional methods for water disinfection. In addition, nanomaterials applied to counteract the biofouling will be reviewed, as it is another great problem affecting water treatment plants as well as materials in contact with water, including distribution pipes and filtration membranes [142].
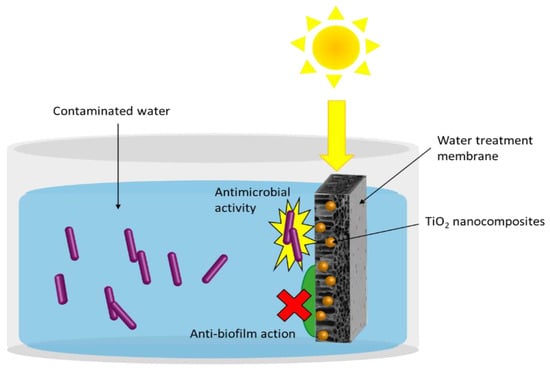
Figure 10.
General scheme of TiO2 NPs-based nanocomposite application for water disinfection, that highlights the ability to prevent biofilm formation at the surface of the substrate, such as a membrane, and the antibacterial activity against water pathogens driven by photocatalysis.
TiO2 NPs-based nanocomposites for water disinfection are often investigated as colloidal suspensions, since, under these conditions, the whole NPs surface is available, exposing all the active catalytic sites to the aqueous environment dispersing the pathogens. However, while suspended NPs are typically found to display a higher photocatalytic activity in comparison with NPs immobilized on a substrate [143], the dispersed NPs present technological problems, as they need to be recovered to prevent them from becoming a source of pollution in the environment.
Biancullo et al. investigated the effect of TiO2 NPs on the inactivation of ARBs and antibiotics in urban wastewater and showed that the photocatalytic treatment inactivated E. coli and Enterococci sp. However, bacteria regrowth experiments demonstrated that the bacteria population over time was not inhibited and that the ratio between resistant and non-resistant cells, detected before and after the TiO2-assisted photocatalysis treatment, stayed unchanged [144]. Venieri et al. proposed metal-doped TiO2 (Mn-, Co-, and binary Mn/Co-doping) nanomaterial against K. pneumoniae in real wastewater. After 90 min of exposure to artificial sunlight, a decrease in bacterial population, in the range from 4 to 6 logs cycles, was measured in each investigated case and the co-doped material (0.04% wt) was found to be effective like the single doped (0.1% wt) samples. Nevertheless, the experiment performed under natural sunlight irradiation showed a lower photocatalytic efficiency, as a 2-log bacteria population reduction was detected in presence of the Mn/Co-TiO2 nanocomposite. Moreover, the resistance of the bacteria surviving cells to some antibiotics was found to be decreased even though ARGs were still detectable, and thus found to be potentially able to develop antibiotic resistance [39]. Rizzo et al. reported the antibacterial activity of N-doped TiO2 NPs against the antibiotic resistant E. coli from urban wastewater. They found a higher activity of N-doped TiO2 NPs compared to the commercial benchmark TiO2 P25 and identified 0.2 g/L NPs concentration suited to obtain, under simulated sunlight irradiation, the highest inactivation rate (8.5 × 105 CFU 100 mL−1min−1) after 10 min of irradiation and total inactivation after 60 min. No significant change in E. coli resistance to selected antibiotics (ciprofloxacin, cefuroxime, tetracycline, and vancomycin) was observed after treatment [145]. The same authors investigated the effect of TiO2 P25 on the same bacteria as a function of irradiation type, testing four different light sources: a wide spectrum 250 W lamp, the same lamp equipped with a filter to simulate solar radiation, a 125 W black light fluorescent lamp, and real solar light. A higher efficiency was found when artificial solar light was used to irradiate for 60 min 0.05 g/L of TiO2 suspension with respect to solar light. Differences in antibiotic resistance were detected as a function of the irradiation source. On the other hand, the unfiltered 250 W lamp was able to inactivate E. coli without any catalysts, whereas the 125W black light fluorescent lamp catalyzed a 70% inactivation of E. coli in the presence of 0.1 g/L of TiO2 [146].
Ghosh et al. described an antibacterial activity, under UV-vis irradiation of a Ag-TiO2 nanocomposite, obtained upon ball-milling of Ag and P25, against E. coli and St. aureus higher than that of the bare TiO2 NPs. A similarly high photocatalytic efficiency was observed for the degradation of organic molecules. The nanocomposite was found not to be cytotoxic below a 50 μg/mL concentration [49]. Liu et al. proposed a vertical face-to-face heterojunction obtained by assembling horizontally TiO2 nanosheets, exposing 001 facet, with graphitic carbon nitride (g-C3N4) sheets, obtaining a Z-scheme electron transfer that enhances the charge separation and therefore the photocatalytic and antibacterial activity. In particular, a 96.8% reduction of E. coli was achieved after 30 min of simulated solar light irradiation, against a 50% reduction obtained with unmodified TiO2. In the dark, the nanocomposite promoted only a 5% reduction of the E. coli population after 30 min, thus confirming that the bacteria inactivation occurred mainly due to photocatalytic ROS production [45]. Liga et al. evaluated the biocide activity, both at dark and under UV-A irradiation, of Ag-TiO2 obtained by photoreduction of AgNO3, against bacteriophage MS2, a model virus for common waterborne pathogens, characterized by a high resistance to common water disinfection methods. The inactivation activity of this system under UV was up to five times higher than that observed for commercial TiO2 P25 and was found to enhance with the increase of the Ag amount [49].
Kim et al. reported on a nanocomposite based on TiO2 NPs coupled with glucose oxidase (GOx), an enzyme acting as organic biocatalyst in presence of glucose, purposely selected as a high concentration of glucose favours the proliferation of heterotrophic bacteria, thus providing a (photo)catalytic system able to decrease the amount of available glucose and, concomitantly, to increase the production of reactive species, responsible for bacteria inactivation. Under UV irradiation with a 4W UV lamp (λmax = 352 nm), inactivation of E. coli assisted by TiO2-GOx was higher than that assisted by bare TiO2, and such enhancement was much more relevant in the presence of glucose [52].
4.1.2. Immobilization of Nanocomposites on Membranes or Recoverable Supports
The immobilization of photocatalytic TiO2 NPs and their nanocomposites onto a suitable substrate is essential to accomplish technologically feasible applications. As mentioned above, a robust and durable immobilization of NPs onto appropriate support is fundamental, not only for enabling the nanosized photocatalyst recovery, but also to prevent the accidental release of NPs in the environment, that, becoming themselves a possible contaminant, may become harmful [4]. Here, some of the most original proposed solutions for the deposition and immobilization of TiO2 NPs-based nanomaterials specifically designed for photocatalytic microorganism inactivation are summarized.
Li et al. developed a foam based on photocatalytic TiO2 NPs; a promptly separable system, and, as it floats at water surface, it is able to efficiently exploit sunlight for photocatalytic generation of ROS [147]. Another ingenious solution is based on nanocomposites also incorporating a magnetic domain, able to convey to the system magnetic properties effective in enabling their simple recovery at the end of the treatment by applying a magnetic field. Wu et al. synthetized a ternary composite based on lanthanum-doped TiO2/calcium ferrite/diatomite with magnetic properties, and enhanced photocatalytic antibacterial activity against E. coli [46]. Another interesting alternative to achieve recoverable photoactive materials is given by nanocomposites formed of NPs coupled with micrometer structures, thus combining the advantages of the nanoscale properties with the possibility of recovering the composite by using filtration methods. Indeed, Xiao et al. reported an original multifunctional system, based on chitosan (CS) beads decorated with TiO2 nanostructures modified with Ag NPs, able to effectively merge the advantage of a recoverable and reusable material and an enhanced antibacterial activity, synergistically accomplished by each of the three components of the nanocomposite, as effectively demonstrated against E. coli upon UV-A irradiation [50].
Negishi et al. used a similar approach with commercial TiO2 coated silica gel beads (4 mm diameter). The coated beads were applied in a solar water purification system for disinfection of tap water from a village in Thailand. A reduction of coliform and general bacteria was observed during three days, together with a degradation, after long time exposure, of the silica component of the bead, resulting in loss of the TiO2 layer. [148]
However, the most extensively explored immobilization approach relies on the incorporation of the photocatalyst in polymeric materials [149]. In particular, polymeric membranes have been thoroughly applied in water treatment technologies as microfiltration, ultrafiltration, nanofiltration, membrane bioreactors, and membrane with biocide activity for water disinfection, through the integration of TiO2 nanostructured material [150]. Haghighat et al. designed a nanocomposite Ag/TiO2/polyvinyl chloride (PVC) membrane for ultrafiltration (UF) by incorporating well dispersed Ag/TiO2 NPs in the host polymer. Such a nanocomposite-based membrane displayed bacterial inactivation ability against P. aeruginosa, E. coli, and St. aureus, together with antifouling properties and degradation activity against an organic dye [51]. Li et al. reported a nanofiltration (NF) membrane obtained by coating a polyethersulfone (PES) substrate with a film of nanocomposite consisting of TiO2 NPs (functionalized with tannic acid) embedded in polyester. In particular, the membrane prepared with 0.020 wt% TiO2 tannic acid (TA) solution showed an E. coli population reduction of 77.2% under dark conditions and a 99.2% E. coli population reduction after UV irradiation at 365 nm [54].
Photocatalytic membrane reactors (PMRs) combine the membrane technology with the advantages of photocatalysis assisted reactions. Two main configurations are reported in the literature: PMRs with the photocatalyst immobilized on the membrane or incorporated therein, and PMRs based on the photocatalyst dispersed in a colloidal suspension. In both cases the photocatalyst is confined in the reaction environment. However, the former configuration allows an effective recovery and reuse of the catalyst, thanks to its stable immobilization, while the recovery is more complicated in the latter case, although the whole NPs surface can be more effectively exploited for adsorption and catalysis [151]. Among the possible photocatalysts for PMR fabrication, TiO2 NPs and TiO2 NPs-based nanocomposites have been the most investigated [152]. Cheng et al. fabricated a PMR based on the combination of a polyvinylidene fluoride (PVDF) membrane and a suspension of TiO2 P25 in tap water and studied the biocide effect for virus removal, using phage f2 as a model. The effect of the amount of humic acid (HA) in the water dispersion on the efficiency of the system was also investigated. Moreover, HA was found to compete with phage f2 at the adsorption sites on TiO2 NPs surface, and the UV light absorbed by HA overall led to a reduction of biocide activity at high HA concentration [153]. A PMR based on a microfiltration hollow polyethylene (PE) fiber membrane coupled with a suspension of TiO2 P25 was investigated for the reduction in bacterial population of secondary effluent water sample, that was assessed, down to 2 log, when a 1g/L TiO2 NPs suspension was used [154]. Another typical and efficient approach for nanocatalyst immobilization, able to preserve their activity, relies on their immobilization onto fibers that, possessing a high surface, allow optimal interaction between the target substrate dispersed in water and the photocatalyst. Amarjargal et al. functionalized the surface of electrospun polyurethane (PU) fibers with Ag-TiO2 NPs by simply dipping the fiber in hot colloidal photocatalyst dispersion for 4 h and 12 h. After 3 h UV irradiation (320–500nm), a 6-log cycles reduction of E. coli population was found for the sample obtained after 4 h immersion and a 5-log cycles reduction for that immersed for 12 h. Interestingly, only 2-log cycles reduction was detected after exposure to UV, in absence of Ag-TiO2 and no reduction was observed upon exposure of the nanocomposite to ambient light [35]. The antibacterial activity of electrospun Ag-TiO2 nanofibers was tested against E. coli. The study demonstrated that, under the investigated experimental conditions: (i) no biocidal activity was obtained upon exposure to light (solar simulator) of the bare fiber, nor in the dark for TiO2/Ag fibers and P25-coated fibers, (ii) fibers functionalized with the synthesized TiO2 performed better, in terms of photocatalytic antibacterial activity, than fibers coated with TiO2 P25, and (iii) the antibacterial activity in presence of Ag NPs was found higher in the dark than under light irradiation [155]. A TiO2/Polyamide 6 (PA-6) electrospun fiber was tested by Daels et al. for the removal of St. aureus and HA from a secondary effluent sample. HA reduction of 83% after 2 h irradiation with simulated solar light (300 W Osram Ultra-Vitalux lamp) with an intensity of about 5 mW/cm2 and 99.99% bacteria reduction after 6h UV irradiation was detected. The same fibers, tested in a filtration process (flow rate 11 m3/(m2h)) in a photocatalytic experiment performed under UV irradiation achieved 37% HA reduction and 76% St. aureus inactivation [18]. Zheng et al. electrospun Cu-TiO2 nanofibers and tested the biocide activity under visible light against f2 phage and the system formed by f2 phage and E. coli as its host, and reported a photocatalytic inactivation of E. coli higher than that of f2 phage. Remarkably, the E. coli inactivation did not seem affected by the presence of f2 phage, while the inactivation of the phage f2 in the bacteria host system was lower than that found in absence of E. coli. Such a result was explained by considering that E. coli can behave as a ROS scavenger, probably due to its membrane structure, as discussed above, thus reducing amount of ROS effective for f2 inactivation, thus somehow preventing its inactivation [108].
Ishikawa et al. proposed an interesting approach to obtain ceramic fibers characterized by an outer TiO2 NPs enriched layer. Namely polycarbosilane, -SiH(CH3)-CH2-)n, was mixed with Ti(OC4H9)4 (50 wt%) in order to obtain a fiber by melt spinning, that was then exposed to air at 70 °C for 100 h. The resulting fiber was composed of amorphous SiO2 and nanometric TiO2 in anatase phase that, for a bleed-out process, migrated to the surface of the fiber. The functionalized fiber demonstrated an ability to completely remove coliform from wastewater after 3 h UV irradiation (352 nm, 2 mW/cm2) [156].
4.1.3. Anti-Biofouling Membranes for Water Treatment
The biofouling process is defined as the accumulation of microorganisms on a surface, and is realized in four steps: (i) organic matters are adsorbed on the surface, (ii) microorganisms adsorb and adhere to the surface, and (iii) start to grow and reproduce on the surface, and finally (iv) extracellular polymers are secreted and mutual adhesion among the clonal cells takes place [157]. These processes ultimately result in the formation of a biofilm, namely an assembly of microbial cells, irreversibly bound to a surface and embedded in a matrix of polysaccharide material [158]. When biofouling takes place at the surface of a membrane, its flux is limited, thus interfering with rejection and reducing the lifetime of the membrane itself, with the consequent drawbacks. In order to prevent biofilm formation, two main strategies can be employed. The first relies on the ability to enhance the hydrophilicity of the membrane, which reduces the affinity of the organic matter, and enhances its affinity with water, that can, thus, form a thin layer on the membrane surface, finally hampering the microorganisms’ adhesion. The second route is based on the incorporation of antibacterial agents into the membrane, such as TiO2-based nanomaterials [51]. TiO2-based nanocomposites are greatly suggested for this application since they display antibacterial properties and, concomitantly, convey, as a function of their chemical nature, hydrophilicity to the host membrane [159]. The combination of the intrinsic antibacterial properties with the photocatalytic properties of TiO2 NPs-based nanocomposites offers multiple advantages for hindering the biofouling. As an example, Kim et al. deposited TiO2 NPs on a polyamide thin film composite (TFC) membrane and tested their anti-biofouling properties, along with the antibacterial activity against E. coli. The water flux was measured for three days, for pristine and TiO2 NPs-modified membrane, and with and without UV-light irradiation. After pipetting E. coli dispersion onto the membranes and incubating at 37 °C, a fast and significant reduction of the flux, a clear indication of the occurrence of a higher fouling, was observed for the membranes not exposed to UV, while a slow and low reduction of the flux was found for TiO2-modified membranes, especially upon UV irradiation, finally demonstrating how the anti-biofouling activity is tightly connected to antibacterial behavior of UV-active TiO2 photocatalysts [19].
4.2. TiO2 NPs-Based Nanocomposite against Biofouling on Building Materials
In the construction field, nanostructured TiO2 is commonly employed to degrade organic pollutants in the air or to protect the building materials from soot, due to its photocatalytic and hydrophobic properties. The biocide activity of TiO2 can also be exploited to reduce the formation of biofilms formed on the surface of buildings [160]. Indeed, biofouling causes, not only aesthetic and structural degradation of construction materials and surfaces, but also bacteria and fungi proliferation that may pose a great health concern [149]. The application of photocatalytic TiO2 NPs as a biocidal agent has considerable relevance, especially for locations strongly sensitive to biological safety, such as medical facilities and food industries, where usually ceramic tiles are used to coat walls and floor. The photocatalytic activity of TiO2 nanomaterials was found to successfully reduce the bacterial proliferation and the fungi population (and, consequently, mycotoxins production) indoor when applied to surfaces, tiles, furniture etc. [161]. Dyshlyuk et al. tested suspensions of TiO2, ZnO, and SiO2 against microorganisms known for damaging construction materials, namely a bacterium (B. subtilis) and several common fungi (A. niger, Aspergillus terreus, Aureobasidium pullulans var. pullulans, Cladosporium cladosporioides, Penicillium ochrochloron, Trichoderma viride, and Paecilomyces variotii). They found TiO2 and SiO2 less active under sunlight than ZnO [162]. Sikora et al. fabricated core-shell nanocomposites of mesoporous silica (mSiO2, core) and TiO2 (shell) to be applied on cement mortars. The merging of the two nanomaterials in one nanocomposite was found to play the two roles of introducing in the building material a filler, mSiO2, able to improve its mechanical properties, and conveying, with TiO2, anti-biofouling and self-cleaning activity upon UV exposure. The mSiO2/TiO2 composite antibacterial activity was tested against E. coli and, after 2 h, a 67% and a 42% reduction were measured after exposure to UV light and at dark, respectively [46].
4.3. Photocatalytic TiO2 NPs-Based Nanocomposites for Biomaterials Disinfection
Antibacterial NPs find a wide range of applications in the field of biomaterials. Materials designed for wound dressing or for tissue engineering-related applications need to comply with several requirements as summarized in Figure 11, as they need to be biocompatible, non-allergenic, easily removable, and degradable after implantation.
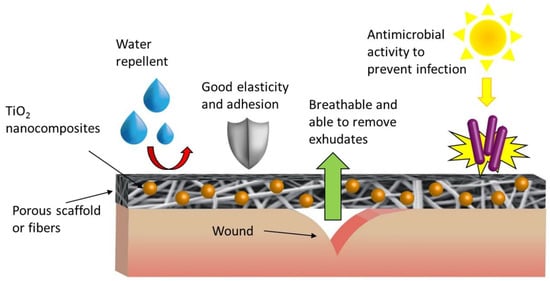
Figure 11.
General scheme of TiO2 NPs-based nanocomposite application for biomaterial-related applications highlighting the ability to repel water, to adhere to the skin following its movements, to remove exudates, to be permeable to air, and to prevent infections due to the antimicrobial activity driven by photocatalysis.
Antibacterial behavior is strongly desirable in this kind of material to prevent infections during wound recovery [163,164]. A great deal of work has been carried out in the development of biocompatible TiO2-NPs-based nanocomposites, with intrinsic antimicrobial activity, specifically designed for tissue engineering-related applications to be used in healthcare facilities [33,165,166,167,168,169].
In this scenario, several research groups have explored the advantages of TiO2-based nanocomposites considering both their biocompatibility and the intrinsic antibacterial properties, focusing also on their excellent photocatalytic properties that can be extremely advantageous in specific biomedical applications. Monmaturapoj et al. fabricated a nanocomposite made of TiO2 and HAP NPs for face masks serving as filtration devices. HAP is a type of calcium phosphate-based ceramic material widely used in the biomaterial field, especially in orthopaedic implants, being biocompatible and a very good substitute for bone. After assessing the antibacterial activity of the nanocomposite, [170] strong antiviral activity against H1N1 influenza A virus was observed under UV-light irradiation, with a synergic effect of HA and TiO2 NPs that revealed optimal for HA/TiO2 (50:50) [53]. Li et al. coated a face mask for medical purposes with NPs formed of a mixture of silver nitrate and TiO2 NPs in order to work as photocatalyst, reduce the bacteria population and to inhibit their growth on the surface, due to the presence of the exhaled moisture from breathing. A 100% reduction of bacteria on the surface of the coated mask 24 h after E. coli and St. aureus inoculation was found against a 25% and 50% increase measured on the uncoated masks for the two bacteria, respectively [171].
4.4. TiO2 NPs-Based Nanocomposites Designed for Disinfection of Food Packaging and Processing Materials
Food packaging materials containing TiO2 NPs-based nanocomposites have been thoroughly investigated materials for food contact-related applications and a steadily increasing number of technologies has been in development in the last years. This is in order to accomplish multiple purposes including improved mechanical, thermal, optical and antimicrobial properties, control of gas and moisture permeability, UV shielding, nutraceuticals release, and installing sensors for pathogens and harmful substances (Figure 12) [172,173,174]. Indeed, surfaces of food-processing plant components need to comply with the same requirements, as they are often in contact with food and the presence of microorganisms therein can easily lead to food contamination, causing transmission of diseases [153]. Indeed, the presence of antimicrobial substances in food packaging materials may inhibit growth of harmful microorganisms and pathogens on food, improving its safety and shelf life. NPs and nanocomposites can be integrated in food packaging materials as growth inhibitors, antimicrobial agents, or carriers [175], and are usually applied to the packaging of meat, fish, poultry, bread, cheese, fruits, and vegetables [176]. TiO2 NPs, aside from antibacterial properties, also possess unique characteristics that may prove particularly useful for such applications. For example, TiO2 shields UV light, a property that is generally exploited in the formulation of sunscreen lotions, and therefore may be an effective additive in packaging to preserve food from irradiation, and thus limit its deterioration [177,178]. Moreover, when TiO2 NPs are contained in a packaging material, upon UV exposure they can photocatalytically degrade the ethylene molecules produced during the ripening process, which are responsible for food degradation [179].
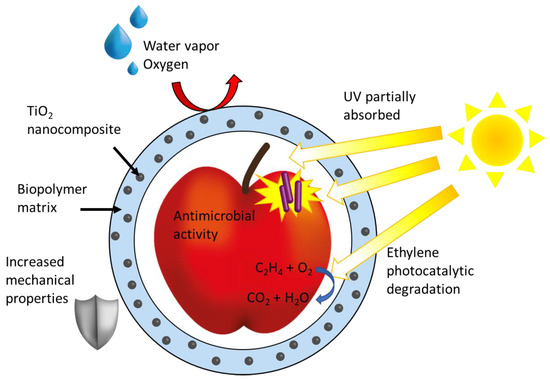
Figure 12.
General scheme of TiO2 NPs-based nanocomposite application for food packaging highlighting the advantages of TiO2 in the coating as improved mechanical properties, water and oxygen repellence, UV shielding, and ethylene scavenger and antimicrobial activity.
In the last years, the choice of a polymer suitable and environmentally sustainable for food packaging has oriented towards biopolymers, which can be easily degraded and recycled, for a more ecological fingerprint in a field that has been, so far, heavily relying on petroleum-based polymers. Such biopolymers include poly hydroxybutyrates (PHB), polylactic acid (PLA), poly caprolactone (PCL), polyvinyl alcohol (PVA), poly butylene succinate, lipids (wax and free fatty acids), proteins (casein, whey, and gluten), polysaccharides (starch and cellulose derivatives, alginates, and chitosan) and their possible blends [180]. In particular, chitosan, a polysaccharide derived from chitin, is especially suited for food packaging, considering, as reported above, its biocompatibility, biodegradability, and antibacterial and antifungal behavior, along with good film forming properties [177,181].
Zhang et al. developed a coating for food packaging based on TiO2 NPs embedded in a chitosan (CS) matrix. The presence of TiO2 NPs in a chitosan film served to improve, upon exposure to visible light, its mechanical properties, wettability and antibacterial activity against typical food pathogens (E. coli, St. aureus, C. albicans, and A. niger) with respect to film formed by bare chitosan. The effect of the direct application of the CS/TiO2 coating on red grapes was also tested and was shown to preserve the fruit better than conventional plastic coating [181]. Li et al. reported a composite film where Ag-TiO2 nanocomposites where embedded in a mixture of fish gelatin (FG) and CS, given that FG is a protein with good film forming, and antioxidant and water locking properties. The addition of CS imparted to FG enhanced mechanical properties while the integration of the Ag-TiO2 nanocomposite, to a different extent, conveyed antibacterial activity. Antibacterial activity (against E. coli, St. aureus, and Botrytis cinereal) and UV-blocking function were found to increase as a function of Ag-TiO2 concentration. However, its very high content led to a deterioration of the mechanical properties of the composite, that were observed, which instead, improved at low Ag-TiO2 concentration [182]. Xu et al. realized a coating based on three different antimicrobial agents: graphene oxide (GO), chitosan and TiO2 NPs, in increasing amounts. The sample with intermediate TiO2 NPs concentration (GO:CS:TiO2 ratio of 1:20:4) showed a good antimicrobial activity against B. subtilis and A. niger, and proved able to destroy bacterial cell membrane and to avoid the formation of a biofilm. Such a coating, when applied to fruit and vegetables, such as strawberries and mango, was found suitable to delay weight loss, which is caused by deterioration, to reduce polyphenol oxidase (PPO)—the enzyme responsible for food browning—activity, and to increase superoxide dismutase (SOD) activity, which is usually high in low stress conditions for the fruit. [47]
Besides chitosan-based blends, many other polymers have been used recently to develop antimicrobial composite coatings containing TiO2 NPs for food packaging. Xie et al., for example, embedded TiO2 NPs into three different biodegradable polymers: cellulose acetate (CA), polycaprolactone (PCL), and PLA. A higher compatibility of TiO2 with CA and PLA was observed, along with improved film forming properties, resulting in uniform films. Moreover, the CA/TiO2-based films showed a high transparency, and good photocatalytic activity, as demonstrated by degradation of methylene blue upon irradiation with a UV-A light. The best antibacterial activity under the investigated experimental conditions, when compared to the other systems, was found against E. coli upon 2 h UV-A light irradiation with light intensity of 1.30 ± 0.15 mW/cm2, and was found to increase at higher TiO2 concentration, achieving a 1.69 log CFU/mL reduction. Very low reduction was, instead, observed, even at the highest TiO2 concentration, without irradiation [183]. Xie et al. thoroughly investigated the behavior of the best performing CA/TiO2 coatings when the bacteria were inoculated under the coating itself and—upon UV-A exposure—a higher TiO2 concentration was found to act as a screen, decreasing the intensity of the light reaching the inoculum and resulting in reduced antibacterial activity [184].
He et al. realized a uniform coating using fish skin gelatin and TiO2 in different ratios (30:1, 20:1, 10:1). The film showed antibacterial activity increased when TiO2 was embedded, reaching values of 54.38% and 44.89% for E. coli and St. aureus, respectively, for the sample with the highest TiO2 content (10:1 ratio) after 2 h UV irradiation. Moreover, the presence of TiO2 enhanced tensile strength and elongation at break and reduced water vapor permeability of the coating. The coating, transparent in the visible region, was found to act as a barrier against UV-C light [175]. Teymoupour et al. reported a composite coating based on a soluble soybean polysaccharide (SSPS) as biopolymer. The SSPS/TiO2 coating, obtained at increasing TiO2 concentration—0%, 1%, 3%, and 5% (w/w)—resulted in an increased antibacterial activity against both E. coli and St. aureus, being particularly effective against St. aureus. Properties such as water vapor permeability, oxygen permeability, and moisture content decreased upon the addition of TiO2, while the mechanical properties improved [185].
All the application of any kind of NPs in materials that come into contact with food must cope with NP migration and their release, as it may become a severe environmental hazard [177,178]. Although in the last decade many strategies have been proposed to improve quality standard for food-contact materials and to reduce its impact on the environment, the regulations concerning nanocomposite based coatings for food safety remain behind, thus further delaying their development up to market [179].
5. Conclusions and Perspectives
Pathogenic microorganisms have demonstrated the ability to spread easily throughout the world, thus potentially very severely threatening human health, as the current COVID-19 pandemic has shown in the last year. Therefore, inactivation of pathogenic microorganisms in water, on surfaces, and on food strongly demands the attention of the scientific community. Photocatalytic TiO2-based nanomaterials have demonstrated their potential to tackle pathogens through multiple paths, in particular by exploiting light (even sun or ambient light, in some cases) to photocatalytically generate ROSs able to kill them or inhibit their growth without using chemical products, that may, instead, be even more harmful for human health and the environment. However, to realistically make the great promise of these photocatalytic nanomaterials viable, it is necessary to easily access large amounts of TiO2-based nanomaterials with controlled size, crystalline phase, and surface chemistry. Such a consideration has been, therefore, briefly summarized in the first part of the review, reporting examples of promising synthetic approaches, specifically selected for their potential scalability towards TiO2-based nanomaterial production, thus ultimately showing the great contribution of modern materials science in providing an extremely rich toolbox for manufacturing nanomaterials with designed photocatalytic properties.
In the second part of the review, we critically analyzed selected approaches within the plethora of reported ones to investigate the antimicrobial properties of TiO2-based nanomaterials. While all the described examples have shown the capability of TiO2-based nanomaterials in inactivating bacteria, fungi, and viruses, the great variability of the experimental parameters (catalyst loading, light source and intensity, target microorganism, environmental conditions of microorganism growth, method of detection of antimicrobial efficacy) used in the antimicrobial tests does not allow an objective comparison of the reported results nor to trace a profile of antimicrobial activity efficiency across the different proposed and investigated TiO2-based nanostructured materials. In addition, the selection of the target microorganism needs to be carefully designed as a function of the intended application. Moreover, a more fundamental issue that makes it very complex to correctly interpret the outcome of experiments is given by the multiple mechanisms of TiO2 NPs antimicrobial activity concomitantly occurring, that, as reported, could involve an intrinsic antimicrobial effect of TiO2-based nanomaterials in the dark. It follows a strong need for standardized protocols and methods specifically suited to quantitatively evaluate the photocatalytic antimicrobial activity of TiO2 NPs, and thus compare the performance of the materials. In this way, it would be possible to highlight the role of the different inhibition mechanisms of the investigated materials, under the specific conditions.
Finally, established and standardized characterization tools to elucidate photocatalytic antimicrobial activities would also fully empower a sound investigation of a systematic structure-function relationship that could be applied not only to TiO2-based nanomaterials but also to whole classes of photocatalytic nanomaterials.
Nonetheless, the results reported so far are promising, as can be inferred by the maturity level of the technological applications that, in a few cases, have been already obtained, as discussed in the third part of the review. Indeed, photocatalytic TiO2-based nanomaterials have been recently successfully exploited in the disinfection of urban wastewater, in the production of photocatalytic anti-fouling membrane for ultra- and nanofiltration, to prevent building materials from bio-fouling, for biomaterials disinfection in wound dressing application and mask filters, and for the disinfection of food packaging and processing materials.
In conclusion, photocatalytic TiO2-based nanomaterials hold great promise in the fight against a wide range of pathogenic microorganisms that can be effectively inactivated and destroyed in different matrices, including water, air, surfaces, and food, possibly also providing additional weapons against extremely harmful and emerging strains like the SARS-CoV viruses.
Author Contributions
I.D.P., R.C. and M.L.C. conceived and drafted the work. I.D.P., F.P., C.L.P. and M.D. designed the article, and acquired, analyzed, and interpreted the literature reports. A.A. critically revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by the Apulia Region Funded Project FONTANAPULIA (WOBV6K5) Italy and the European H2020 funded Project InnovaConcrete (G.A. n. 760858), PON Energy for TARANTO (ARS01_00637). PON Ricerca e Innovazione 2014–2020 (DOT1302393).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Laxma Reddy, P.V.; Kavitha, B.; Kumar Reddy, P.A.; Kim, K.-H. TiO2-based photocatalytic disinfection of microbes in aqueous media: A review. Environ. Res. 2017, 154, 296–303. [Google Scholar] [CrossRef]
- Li, Q.; Mahendra, S.; Lyon, D.Y.; Brunet, L.; Liga, M.V.; Li, D.; Alvarez, P.J.J. Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Res. 2008, 42, 4591–4602. [Google Scholar] [CrossRef]
- Petronella, F.; Truppi, A.; Sibillano, T.; Giannini, C.; Striccoli, M.; Comparelli, R.; Curri, M.L. Multifunctional TiO2/FexOy/Ag based nanocrystalline heterostructures for photocatalytic degradation of a recalcitrant pollutant. Catal. Today 2017, 284, 100–106. [Google Scholar] [CrossRef]
- Petronella, F.; Truppi, A.; Ingrosso, C.; Placido, T.; Striccoli, M.; Curri, M.L.; Agostiano, A.; Comparelli, R. Nanocomposite materials for photocatalytic degradation of pollutants. Catal. Today 2017, 281, 85–100. [Google Scholar] [CrossRef]
- Khezerlou, A.; Alizadeh-Sani, M.; Azizi-Lalabadi, M.; Ehsani, A. Nanoparticles and their antimicrobial properties against pathogens including bacteria, fungi, parasites and viruses. Microb. Pathog. 2018, 123, 505–526. [Google Scholar] [CrossRef]
- Lusvardi, G.; Barani, C.; Giubertoni, F.; Paganelli, G. Synthesis and Characterization of TiO2 Nanoparticles for the Reduction of Water Pollutants. Materials 2017, 10, 1208. [Google Scholar] [CrossRef] [PubMed]
- Qarni, F.; Alomair, N.; Mohamed, H. Environment-Friendly Nanoporous Titanium Dioxide with Enhanced Photocatalytic Activity. Catalysts 2019, 9, 799. [Google Scholar] [CrossRef]
- Petronella, F.; Truppi, A.; Dell’Edera, M.; Agostiano, A.; Curri, M.L.; Comparelli, R. Scalable Synthesis of Mesoporous TiO2 for Environmental Photocatalytic Applications. Materials 2019, 12, 1853. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.H.; Jun, Y.; Li, H.G.; Du, S.G. Using a Sol-Gel Method to Prepare the TiO2/CNTs Nanocomposite. Appl. Mech. Mater. 2014, 529, 108–111. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, L.; Cheng, B.; Yu, J. Dual Cocatalysts in TiO2 Photocatalysis. Adv. Mater. 2019, 31, 1807660. [Google Scholar] [CrossRef]
- Kang, X.; Liu, S.; Dai, Z.; He, Y.; Song, X.; Tan, Z. Titanium Dioxide: From Engineering to Applications. Catalysts 2019, 9, 191. [Google Scholar] [CrossRef]
- Cassaignon, S.; Koelsch, M.; Jolivet, J.-P. From TiCl3 to TiO2 nanoparticles (anatase, brookite and rutile): Thermohydrolysis and oxidation in aqueous medium. J. Phys. Chem. Solids 2007, 68, 695–700. [Google Scholar] [CrossRef]
- Lee, D.S.; Liu, T.K. Preparation of TiO2 Sol Using TiCl4 as a Precursor. J. Sol-Gel Sci. Technol. 2002, 25, 121–136. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, C.; Cao, L. The synthesis of nanosized TiO2 powder using a sol-gel method with TiCl4 as a precursor. J. Mater. Sci. 2000, 35, 4049–4054. [Google Scholar]
- Ullattil, S.; Periyat, P. Sol-Gel Synthesis of Titanium Dioxide. In Sol-Gel Materials for Energy, Environment and Electronic Applications. Advances in Sol-Gel Derived Materials and Technologies; Springer: Cham, Switzerland, 2017; pp. 271–283. [Google Scholar]
- Dell’Edera, M.; Petronella, F.; Truppi, A.; Liotta, L.F.; Gallì, N.; Sibillano, T.; Giannini, C.; Brescia, R.; Milano, F.; Striccoli, M.; et al. Low Temperature Synthesis of Photocatalytic Mesoporous TiO2 Nanomaterials. Catalysts 2020, 10, 893. [Google Scholar] [CrossRef]
- Vargas, M.A.; Rodríguez-Páez, J.E. Facile Synthesis of TiO2 Nanoparticles of Different Crystalline Phases and Evaluation of Their Antibacterial Effect Under Dark Conditions Against E. coli. J. Clust. Sci. 2019, 30, 379–391. [Google Scholar] [CrossRef]
- Daels, N.; Radoicic, M.; Radetic, M.; De Clerck, K.; Van Hulle, S.W.H. Electrospun nanofibre membranes functionalised with TiO2 nanoparticles: Evaluation of humic acid and bacterial removal from polluted water. Sep. Purif. Technol. 2015, 149, 488–494. [Google Scholar] [CrossRef]
- Kim, S.H.; Kwak, S.Y.; Sohn, B.H.; Park, T.H. Design of TiO2 nanoparticle self-assembled aromatic polyamide thin-film-composite (TFC) membrane as an approach to solve biofouling problem. J. Membr. Sci. 2003, 211, 157–165. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Sreekantan, S. Effect of pH on TiO2 Nanoparticles via Sol-Gel Method. Adv. Mater. Res. 2011, 173, 184–189. [Google Scholar] [CrossRef]
- Galkina, O.L.; Sycheva, A.; Blagodatskiy, A.; Kaptay, G.; Katanaev, V.L.; Seisenbaeva, G.A.; Kessler, V.G.; Agafonov, A.V. The sol–gel synthesis of cotton/TiO2 composites and their antibacterial properties. Surf. Coat. Technol. 2014, 253, 171–179. [Google Scholar] [CrossRef]
- Bahar, M.; Mozaffari, M.; Esmaeili, S. Effect of different alcohols, gelatinizing times, calcination and microwave on characteristics of TiO2 nanoparticles synthesized by sol–gel method. J. Theoretic. Appl. Phys. 2017, 11, 79–86. [Google Scholar] [CrossRef]
- Duymaz, B.; Yigit, Z.V.; Şeker, M.G.; Dündar, F. Antibacterial Properties of Sol-Gel Derived TiO2 Nanoparticles. Acta Phys. Pol. A 2016, 129, 872–874. [Google Scholar] [CrossRef]
- Rasheed, R.; Algawi, S.; Rhoomi, Z. Synthesis and Antibacterial Activity of Rutile-TiO2 Nano Powder Prepared by Hydrothermal Process. J. Univ. Babylon Pure Appl. Sci. 2017, 25, 1744–1754. [Google Scholar]
- Kőrösi, L.; Prato, M.; Scarpellini, A.; Kovács, J.; Dömötör, D.; Kovács, T.; Papp, S. H2O2-assisted photocatalysis on flower-like rutile TiO2 nanostructures: Rapid dye degradation and inactivation of bacteria. Appl. Surf. Sci. 2016, 365, 171–179. [Google Scholar] [CrossRef]
- Zárate, R.A.; Fuentes, S.; Wiff, J.P.; Fuenzalida, V.M.; Cabrera, A.L. Chemical composition and phase identification of sodium titanate nanostructures grown from titania by hydrothermal processing. J. Phys. Chem. Solids 2007, 68, 628–637. [Google Scholar] [CrossRef]
- León-Ríos, S.; Espinoza González, R.; Fuentes, S.; Chávez Ángel, E.; Echeverría, A.; Serrano, A.E.; Demergasso, C.S.; Zárate, R.A. One-Dimensional TiO2-B Crystals Synthesised by Hydrothermal Process and Their Antibacterial Behaviour on Escherichia coli. J. Nanomater. 2016, 2016, 7213672. [Google Scholar] [CrossRef]
- Ben-Shahar, Y.; Banin, U. Hybrid Semiconductor–Metal Nanorods as Photocatalysts. Top. Curr. Chem. 2016, 374, 54. [Google Scholar] [CrossRef]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Banin, U.; Ben-Shahar, Y.; Vinokurov, K. Hybrid Semiconductor–Metal Nanoparticles: From Architecture to Function. Chem. Mater. 2014, 26, 97–110. [Google Scholar] [CrossRef]
- Kedziora, A.; Strek, W.; Kepinski, L.; Bugla-Ploskonska, G.; Doroszkiewicz, W. Synthesis and antibacterial activity of novel titanium dioxide doped with silver. J. Sol-Gel Sci. Technol. 2012, 62, 79–86. [Google Scholar] [CrossRef]
- Carvalho, I.; Ferdov, S.; Mansilla, C.; Marques, S.M.; Cerqueira, M.A.; Pastrana, L.M.; Henriques, M.; Gaidau, C.; Ferreira, P.; Carvalho, S. Development of antimicrobial leather modified with Ag–TiO2 nanoparticles for footwear industry. Sci. Technol. Mater. 2018, 30, 60–68. [Google Scholar] [CrossRef]
- Ahmed, A.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Hussain, A.; Pervaiz, E.; Janjua, H.A.; Hussain, Z. In-vitro and in-vivo study of superabsorbent PVA/Starch/g-C3N4/Ag@TiO2 NPs hydrogel membranes for wound dressing. Eur. Polym. J. 2020, 130, 109650. [Google Scholar] [CrossRef]
- Page, K.; Palgrave, R.G.; Parkin, I.P.; Wilson, M.; Savin, S.L.P.; Chadwick, A.V. Titania and silver–titania composite films on glass—potent antimicrobial coatings. J. Mater. Chem. 2007, 17, 95–104. [Google Scholar] [CrossRef]
- Amarjargal, A.; Tijing, L.D.; Ruelo, M.T.G.; Lee, D.H.; Kim, C.S. Facile synthesis and immobilization of Ag-TiO2 nanoparticles on electrospun PU nanofibers by polyol technique and simple immersion. Mater. Chem. Phys. 2012, 135, 277–281. [Google Scholar] [CrossRef]
- Skorb, E.V.; Antonouskaya, L.I.; Belyasova, N.A.; Shchukin, D.G.; Möhwald, H.; Sviridov, D.V. Antibacterial activity of thin-film photocatalysts based on metal-modified TiO2 and TiO2:In2O3 nanocomposite. Appl. Catal. B 2008, 84, 94–99. [Google Scholar] [CrossRef]
- Kaushik, R.; Samal, P.K.; Halder, A. Degradation of Fluoroquinolone-Based Pollutants and Bacterial Inactivation by Visible-Light-Active Aluminum-Doped TiO2 Nanoflakes. ACS Appl. Nano Mater. 2019, 2, 7898–7909. [Google Scholar] [CrossRef]
- Venieri, D.; Gounaki, I.; Bikouvaraki, M.; Binas, V.; Zachopoulos, A.; Kiriakidis, G.; Mantzavinos, D. Solar photocatalysis as disinfection technique: Inactivation of Klebsiella pneumoniae in sewage and investigation of changes in antibiotic resistance profile. J. Environ. Manag. 2017, 195, 140–147. [Google Scholar] [CrossRef]
- Binas, V.D.; Sambani, K.; Maggos, T.; Katsanaki, A.; Kiriakidis, G. Synthesis and photocatalytic activity of Mn-doped TiO2 nanostructured powders under UV and visible light. Appl. Catal. B 2012, 113–114, 79–86. [Google Scholar] [CrossRef]
- Venieri, D.; Fraggedaki, A.; Kostadima, M.; Chatzisymeon, E.; Binas, V.; Zachopoulos, A.; Kiriakidis, G.; Mantzavinos, D. Solar light and metal-doped TiO2 to eliminate water-transmitted bacterial pathogens: Photocatalyst characterization and disinfection performance. Appl. Catal. B 2014, 154–155, 93–101. [Google Scholar] [CrossRef]
- Hassan, M.S.; Amna, T.; Yang, O.B.; Kim, H.-C.; Khil, M.-S. TiO2 nanofibers doped with rare earth elements and their photocatalytic activity. Ceram. Int. 2012, 38, 5925–5930. [Google Scholar] [CrossRef]
- Nithya, N.; Bhoopathi, G.; Magesh, G.; Kumar, C.D.N. Neodymium doped TiO2 nanoparticles by sol-gel method for antibacterial and photocatalytic activity. Mater. Sci. Semicond. Process 2018, 83, 70–82. [Google Scholar] [CrossRef]
- Siwińska-Stefańska, K.; Kubiak, A.; Piasecki, A.; Dobrowolska, A.; Czaczyk, K.; Motylenko, M.; Rafaja, D.; Ehrlich, H.; Jesionowski, T. Hydrothermal synthesis of multifunctional TiO2-ZnO oxide systems with desired antibacterial and photocatalytic properties. Appl. Surf. Sci. 2019, 463, 791–801. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, X.K.; Hu, X.Y.; Hu, J.; Wang, Z.Y.; Yin, Y.C.; Sun, C.H.; Zhang, X.W. Two-dimensional g-C3N4/TiO2 nanocomposites as vertical Z-scheme heterojunction for improved photocatalytic water disinfection. Catal. Today 2019, 335, 243–251. [Google Scholar] [CrossRef]
- Sikora, P.; Cendrowski, K.; Markowska-Szczupak, A.; Horszczaruk, E.; Mijowska, E. The effects of silica/titania nanocomposite on the mechanical and bactericidal properties of cement mortars. Constr. Build. Mater. 2017, 150, 738–746. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, Z.H. The Fabrication of Magnetically Recyclable La-Doped TiO2/Calcium Ferrite/Diatomite Composite for Visible-Light-Driven Degradation of Antibiotic and Disinfection of Bacteria. Environ. Eng. Sci. 2020, 37, 109–119. [Google Scholar] [CrossRef]
- Xu, W.R.; Xie, W.J.; Huang, X.Q.; Chen, X.; Huang, N.; Wang, X.; Liu, J. The graphene oxide and chitosan biopolymer loads TiO2 for antibacterial and preservative research. Food Chem. 2017, 221, 267–277. [Google Scholar] [CrossRef]
- Ghosh, M.; Mondal, M.; Mandal, S.; Roy, A.; Chakrabarty, S.; Chakrabarti, G.; Pradhan, S.K. Enhanced photocatalytic and antibacterial activities of mechanosynthesized TiO2-Ag nanocomposite in wastewater treatment. J. Mol. Struct. 2020, 1211, 11. [Google Scholar] [CrossRef]
- Liga, M.V.; Bryant, E.L.; Colvin, V.L.; Li, Q.L. Virus inactivation by silver doped titanium dioxide nanoparticles for drinking water treatment. Water Res. 2011, 45, 535–544. [Google Scholar] [CrossRef]
- Xiao, G.; Zhang, X.; Zhang, W.Y.; Zhang, S.; Su, H.J.; Tan, T.W. Visible-light-mediated synergistic photocatalytic antimicrobial effects and mechanism of Ag-nanoparticles@chitosan-TiO2 organic-inorganic composites for water disinfection. Appl. Catal. B-Environ. 2015, 170, 255–262. [Google Scholar] [CrossRef]
- Haghighat, N.; Vatanpour, V.; Sheydaei, M.; Nikjavan, Z. Preparation of a novel polyvinyl chloride (PVC) ultrafiltration membrane modified with Ag/TiO2 nanoparticle with enhanced hydrophilicity and antibacterial activities. Sep. Purif. Technol. 2020, 237, 116374. [Google Scholar] [CrossRef]
- Kim, B.C.; Jeong, E.; Kim, E.; Hong, S.W. Bio-organic–inorganic hybrid photocatalyst, TiO2 and glucose oxidase composite for enhancing antibacterial performance in aqueous environments. Appl. Catal., B 2019, 242, 194–201. [Google Scholar] [CrossRef]
- Monmaturapoj, N.; Sri-On, A.; Klinsukhon, W.; Boonnak, K.; Prahsarn, C. Antiviral activity of multifunctional composite based on TiO2-modified hydroxyapatite. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xiao, Y.R.; Guo, D.X.; Shen, L.G.; Li, R.J.; Jiao, Y.; Xu, Y.C.; Lin, H.J. In-situ coating TiO2 surface by plant-inspired tannic acid for fabrication of thin film nanocomposite nanofiltration membranes toward enhanced separation and antibacterial performance. J. Colloid Interface Sci. 2020, 572, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Li, J.; Ma, S.; Liu, G.; Yang, K.; Tong, M.; Lin, D. Toxicity of TiO2 Nanoparticles to Escherichia coli: Effects of Particle Size, Crystal Phase and Water Chemistry. PLoS ONE 2014, 9, e110247. [Google Scholar] [CrossRef]
- Shirai, R.; Miura, T.; Yoshida, A.; Yoshino, F.; Ito, T.; Yoshinari, M.; Yajima, Y. Antimicrobial effect of titanium dioxide after ultraviolet irradiation against periodontal pathogen. Dent. Mater. J. 2016, 35, 511–516. [Google Scholar] [CrossRef]
- Rtimi, S.; Giannakis, S.; Bensimon, M.; Pulgarin, C.; Sanjines, R.; Kiwi, J. Supported TiO2 films deposited at different energies: Implications of the surface compactness on the catalytic kinetics. Appl. Catal. B 2016, 191, 42–52. [Google Scholar] [CrossRef]
- Sunada, K.; Kikuchi, Y.; Hashimoto, K.; Fujishima, A. Bactericidal and Detoxification Effects of TiO2 Thin Film Photocatalysts. Environ. Sci. Technol. 1998, 32, 726–728. [Google Scholar] [CrossRef]
- Wang, W.; Li, G.; Xia, D.; An, T.; Zhao, H.; Wong, P.K. Photocatalytic nanomaterials for solar-driven bacterial inactivation: Recent progress and challenges. Environ. Sci. Nano 2017, 4, 782–799. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, Q.; Yang, H.; Shi, D.; Qian, J. Photocatalytic antibacterial properties of copper doped TiO2 prepared by high-energy ball milling. Ceram. Int. 2020, 46, 16716–16724. [Google Scholar] [CrossRef]
- Ren, Y.; Han, Y.; Li, Z.; Liu, X.; Zhu, S.; Liang, Y.; Yeung, K.W.K.; Wu, S. Ce and Er Co-doped TiO2 for rapid bacteria- killing using visible light. Bioact. Mater. 2020, 5, 201–209. [Google Scholar] [CrossRef]
- Liu, N.; Zhu, Q.; Zhang, N.; Zhang, C.; Kawazoe, N.; Chen, G.; Negishi, N.; Yang, Y. Superior disinfection effect of Escherichia coli by hydrothermal synthesized TiO2-based composite photocatalyst under LED irradiation: Influence of environmental factors and disinfection mechanism. Environ. Pollut. 2019, 247, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 2003, 67, 593–656. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Vary, P.S.; Lin, C.-T. Anatase TiO2 Nanocomposites for Antimicrobial Coatings. J. Phys. Chem. B 2005, 109, 8889–8898. [Google Scholar] [CrossRef]
- Alizadeh Sani, M.; Ehsani, A.; Hashemi, M. Whey protein isolate/cellulose nanofibre/TiO2 nanoparticle/rosemary essential oil nanocomposite film: Its effect on microbial and sensory quality of lamb meat and growth of common foodborne pathogenic bacteria during refrigeration. Int. J. Food. Microbiol. 2017, 251, 8–14. [Google Scholar] [CrossRef]
- Nakano, R.; Hara, M.; Ishiguro, H.; Yao, Y.; Ochiai, T.; Nakata, K.; Murakami, T.; Kajioka, J.; Sunada, K.; Hashimoto, K.; et al. Broad Spectrum Microbicidal Activity of Photocatalysis by TiO2. Catalysts 2013, 3, 310–323. [Google Scholar] [CrossRef]
- Backhaus, K.; Marugan, J.; van Grieken, R.; Sordo, C. Photocatalytic inactivation of E. faecalis in secondary wastewater plant effluents. Water. Sci. Technol. 2010, 61, 2355–2361. [Google Scholar] [CrossRef]
- Pal, A.; Pehkonen, S.O.; Yu, L.E.; Ray, M.B. Photocatalytic inactivation of Gram-positive and Gram-negative bacteria using fluorescent light. J. Photochem. Photobiol. A 2007, 186, 335–341. [Google Scholar] [CrossRef]
- Ripolles-Avila, C.; Martinez-Garcia, M.; Hascoët, A.-S.; Rodríguez-Jerez, J.J. Bactericidal efficacy of UV activated TiO2 nanoparticles against Gram-positive and Gram-negative bacteria on suspension. J. Food 2019, 17, 408–418. [Google Scholar] [CrossRef]
- Tsuang, Y.H.; Sun, J.S.; Huang, Y.C.; Lu, C.H.; Chang, W.H.; Wang, C.C. Studies of photokilling of bacteria using titanium dioxide nanoparticles. Artif. Organs. 2008, 32, 167–174. [Google Scholar] [CrossRef] [PubMed]
- van Grieken, R.; Marugán, J.; Pablos, C.; Furones, L.; López, A. Comparison between the photocatalytic inactivation of Gram-positive E. faecalis and Gram-negative E. coli faecal contamination indicator microorganisms. Appl. Catal. B 2010, 100, 212–220. [Google Scholar] [CrossRef]
- Haider, A.J.; AL-Anbar, R.H.; Kadhim, G.R.; Salame, C.T. Exploring potential Environmental applications of TiO2 Nanoparticles. Energy Proc. 2017, 119, 332–345. [Google Scholar] [CrossRef]
- Rincón, A.-G.; Pulgarin, C. Bactericidal action of illuminated TiO2 on pure Escherichia coli and natural bacterial consortia: Post-irradiation events in the dark and assessment of the effective disinfection time. Appl. Catal. B 2004, 49, 99–112. [Google Scholar] [CrossRef]
- Child, M.; Strike, P.; Pickup, R.; Edwards, C. Salmonella typhimurium displays cyclical patterns of sensitivity to UV-C killing during prolonged incubation in the stationary phase of growth. FEMS Microbiol. Lett. 2002, 213, 81–85. [Google Scholar] [CrossRef]
- Munro, P.M.; Flatau, G.N.; Clément, R.L.; Gauthier, M.J. Influence of the RpoS (KatF) sigma factor on maintenance of viability and culturability of Escherichia coli and Salmonella typhimurium in seawater. Appl. Environ. Microbiol. 1995, 61, 1853. [Google Scholar] [CrossRef]
- Eisenstark, A.; Calcutt, M.J.; Becker-Hapak, M.; Ivanova, A. Role of escherichia coli rpos and associated genes in defense against oxidative damage. Free Radic. Biol. Med. 1996, 21, 975–993. [Google Scholar] [CrossRef]
- Prieto-Calvo, M.A.; López, M.; Prieto, M.; Alvarez-Ordóñez, A. Variability in resistance to Cold Atmospheric Plasma (CAP) and Ultraviolet light (UV) and multiple stress resistance analysis of pathogenic verocytotoxigenic Escherichia coli (VTEC). Food Res. Int. 2016, 79, 88–94. [Google Scholar] [CrossRef]
- Janulczyk, R.; Ricci, S.; Bjorck, L. MtsABC is important for manganese and iron transport, oxidative stress resistance, and virulence of Streptococcus pyogenes. Infect. Immun. 2003, 71, 2656–2664. [Google Scholar] [CrossRef]
- Mandel, G.L. Catalase, superoxide dismutase, and virulence of Staphylococcus aureus. In vitro and in vivo studies with emphasis on staphylococcal--leukocyte interaction. J. Clin. Investig. 1975, 55, 561–566. [Google Scholar] [CrossRef]
- Rodriguez-Gonzalez, V.; Obregon, S.; Patron-Soberano, O.A.; Terashima, C.; Fujishima, A. An approach to the photocatalytic mechanism in the TiO2-nanomaterials microorganism interface for the control of infectious processes. Appl. Catal. B 2020, 270, 118853. [Google Scholar] [CrossRef]
- Bonnet, M.; Massard, C.; Veisseire, P.; Camares, O.; Awitor, K.O. Environmental Toxicity and Antimicrobial Efficiency of Titanium Dioxide Nanoparticles in Suspension. J. Biomater. Nanobiotechnol. 2015, 06, 213–224. [Google Scholar] [CrossRef]
- Pagnout, C.; Jomini, S.; Dadhwal, M.; Caillet, C.; Thomas, F.; Bauda, P. Role of electrostatic interactions in the toxicity of titanium dioxide nanoparticles toward Escherichia coli. Colloids Surf. B Biointerfaces 2012, 92, 315–321. [Google Scholar] [CrossRef]
- Adams, L.K.; Lyon, D.Y.; Alvarez, P.J. Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res 2006, 40, 3527–3532. [Google Scholar] [CrossRef]
- Nesic, J.; Rtimi, S.; Laub, D.; Roglic, G.M.; Pulgarin, C.; Kiwi, J. New evidence for TiO2 uniform surfaces leading to complete bacterial reduction in the dark: Critical issues. Colloids Surf. B Biointerfaces 2014, 123, 593–599. [Google Scholar] [CrossRef]
- Kiwi, J.; Rtimi, S.; Sanjines, R.; Pulgarin, C. TiO2 and TiO2-Doped Films Able to Kill Bacteria by Contact: New Evidence for the Dynamics of Bacterial Inactivation in the Dark and under Light Irradiation. Int. J. Photoenergy 2014, 2014, 785037. [Google Scholar] [CrossRef]
- Rtimi, S.; Nesic, J.; Pulgarin, C.; Sanjines, R.; Bensimon, M.; Kiwi, J. Effect of surface pretreatment of TiO2 films on interfacial processes leading to bacterial inactivation in the dark and under light irradiation. Interface Focus 2015, 5, 20140046. [Google Scholar] [CrossRef]
- Erdem, A.; Metzler, D.; Cha, D.; Huang, C.P. Inhibition of bacteria by photocatalytic nano-TiO2 particles in the absence of light. Int. J. Environ. Sci. Technol. 2014, 12, 2987–2996. [Google Scholar] [CrossRef][Green Version]
- Carré, G.; Hamon, E.; Ennahar, S.; Estner, M.; Lett, M.C.; Horvatovich, P.; Gies, J.P.; Keller, V.; Keller, N.; Andre, P. TiO2 photocatalysis damages lipids and proteins in Escherichia coli. Appl. Environ. Microbiol. 2014, 80, 2573–2581. [Google Scholar] [CrossRef]
- Sułek, A.; Pucelik, B.; Kuncewicz, J.; Dubin, G.; Dąbrowski, J.M. Sensitization of TiO2 by halogenated porphyrin derivatives for visible light biomedical and environmental photocatalysis. Catal. Today 2019, 335, 538–549. [Google Scholar] [CrossRef]
- Gibson, K.E. Viral pathogens in water: Occurrence, public health impact, and available control strategies. Curr. Opin. Virol. 2014, 4, 50–57. [Google Scholar] [CrossRef]
- Otter, J.A.; Yezli, S.; Salkeld, J.A.; French, G.L. Evidence that contaminated surfaces contribute to the transmission of hospital pathogens and an overview of strategies to address contaminated surfaces in hospital settings. Am. J. Infect. Control 2013, 41, S6–S11. [Google Scholar] [CrossRef]
- Barker, J.; Vipond, I.B.; Bloomfield, S.F. Effects of cleaning and disinfection in reducing the spread of Norovirus contamination via environmental surfaces. J. Hosp. Infect. 2004, 58, 42–49. [Google Scholar] [CrossRef]
- Kramer, A.; Schwebke, I.; Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006, 6, 130. [Google Scholar] [CrossRef]
- Li, Q.; Page, M.; Marinas, B.J.; Ahang, F.K. Treatment of Coliphage MS2 with Palladium-Modified Nitrogen-Doped Titanium Oxide Photocatalyst Illuminated by Visible Light. Environ. Sci. Technol. 2008, 42, 6148–6153. [Google Scholar] [CrossRef]
- Syngouna, V.I.; Chrysikopoulos, C.V. Inactivation of MS2 bacteriophage by titanium dioxide nanoparticles in the presence of quartz sand with and without ambient light. J. Colloid Interface Sci. 2017, 497, 117–125. [Google Scholar] [CrossRef]
- Koizumi, Y.; Taya, M. Kinetic evaluation of biocidal activity of titanium dioxide against phage MS2 considering interaction between the phage and photocatalyst particles. Biochem. Eng. J. 2002, 12, 107–116. [Google Scholar] [CrossRef]
- Ditta, I.B.; Steele, A.; Liptrot, C.; Tobin, J.; Tyler, H.; Yates, H.M.; Sheel, D.W.; Foster, H.A. Photocatalytic antimicrobial activity of thin surface films of TiO2, CuO and TiO2/CuO dual layers on Escherichia coli and bacteriophage T4. Appl. Microbiol. Biotechnol. 2008, 79, 127–133. [Google Scholar] [CrossRef]
- Ishiguro, H.; Nakano, R.; Yao, Y.; Kajioka, J.; Fujishima, A.; Sunada, K.; Minoshima, M.; Hashimoto, K.; Kubota, Y. Photocatalytic inactivation of bacteriophages by TiO2-coated glass plates under low-intensity, long-wavelength UV irradiation. Photochem. Photobiol. Sci. 2011, 10, 1825–1829. [Google Scholar] [CrossRef]
- Ishiguro, H.; Yao, Y.; Nakano, R.; Hara, M.; Sunada, K.; Hashimoto, K.; Kajioka, J.; Fujishima, A.; Kubota, Y. Photocatalytic activity of Cu2+/TiO2-coated cordierite foam inactivates bacteriophages and Legionella pneumophila. Appl. Catal. B 2013, 129, 56–61. [Google Scholar] [CrossRef]
- Soylemez, E.; de Boer, M.P.; Sae-Ueng, U.; Evilevitch, A.; Stewart, T.A.; Nyman, M. Photocatalytic Degradation of Bacteriophages Evidenced by Atomic Force Microscopy. PLoS ONE 2013, 8, e53601. [Google Scholar] [CrossRef]
- Gerrity, D.; Ryu, H.; Crittenden, J.; Abbaszadegan, M. Photocatalytic inactivation of viruses using titanium dioxide nanoparticles and low-pressure UV light. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2008, 43, 1261–1270. [Google Scholar] [CrossRef]
- Hajkova, P.; Spatenka, P.; Horsky, J.; Horska, I.; Kolouch, A. Photocatalytic Effect of TiO2 Films on Viruses and Bacteria. Plasma Processes Polym. 2007, 4, S397–S401. [Google Scholar] [CrossRef]
- Nakano, R.; Ishiguro, H.; Yao, Y.; Kajioka, J.; Fujishima, A.; Sunada, K.; Minoshima, M.; Hashimoto, K.; Kubota, Y. Photocatalytic inactivation of influenza virus by titanium dioxide thin film. Photochem. Photobiol. Sci. 2012, 11, 1293–1298. [Google Scholar] [CrossRef]
- Guillard, C.; Bui, T.H.; Felix, C.; Moules, V.; Lina, B.; Lejeune, P. Microbiological disinfection of water and air by photocatalysis. C. R. Chim. 2008, 11, 107–113. [Google Scholar] [CrossRef]
- Mazurkova, N.A.; Spitsyna, Y.E.; Shikina, N.V.; Ismagilov, Z.R.; Zagrebel’nyi, S.N.; Ryabchikova, E.I. Interaction of titanium dioxide nanoparticles with influenza virus. Nanotechnol. Russia 2010, 5, 417–420. [Google Scholar] [CrossRef]
- Zheng, X.; Shen, Z.-p.; Cheng, C.; Shi, L.; Cheng, R.; Yuan, D.-H. Photocatalytic disinfection performance in virus and virus/bacteria system by Cu-TiO2 nanofibers under visible light. Environ. Pollut. 2018, 237, 452–459. [Google Scholar] [CrossRef]
- Bintsis, T.; Litopoulou-Tzanetaki, E.; Robinson, R.K. Existing and potential applications of ultraviolet light in the food industry–a critical review. J. Sci. Food Agric. 2000, 80, 637–645. [Google Scholar] [CrossRef]
- Xu, R.; Liu, X.; Zhang, P.; Ma, H.; Liu, G.; Xia, Z. The photodestruction of virus in Nano-TiO2 suspension. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2007, 22, 422–425. [Google Scholar] [CrossRef]
- Liga, M.V.; Maguire-Boyle, S.J.; Jafry, H.R.; Barron, A.R.; Li, Q. Silica decorated TiO2 for virus inactivation in drinking water--simple synthesis method and mechanisms of enhanced inactivation kinetics. Environ. Sci. Technol. 2013, 47, 6463–6470. [Google Scholar] [CrossRef]
- Fiorillo, L.; Cervino, G.; Matarese, M.; D’Amico, C.; Surace, G.; Paduano, V.; Fiorillo, M.T.; Moschella, A.; Bruna, A.; Romano, G.L.; et al. COVID-19 Surface Persistence: A Recent Data Summary and Its Importance for Medical and Dental Settings. Int. J. Environ. Res. Public Health 2020, 17, 3132. [Google Scholar] [CrossRef] [PubMed]
- Khaiboullina, S.; Uppal, T.; Dhabarde, N.; Subramanian, V.R.; Verma, S.C. In Vitro Inactivation of Human Coronavirus by Titania Nanoparticle Coatings and UVC Radiation: Throwing Light on SARS-CoV-2. bioRxiv 2020. [Google Scholar] [CrossRef]
- Seven, O.; Dindar, B.; Aydemir, S.; Metin, D.; Ozinel, M.A.; Icli, S. Solar photocatalytic disinfection of a group of bacteria and fungi aqueous suspensions with TiO2, ZnO and Sahara desert dust. J. Photochem. Photobiol. A 2004, 165, 103–107. [Google Scholar] [CrossRef]
- Maneerat, C.; Hayata, Y. Antifungal activity of TiO2 photocatalysis against Penicillium expansum in vitro and in fruit tests. Int. J. Food. Microbiol. 2006, 107, 99–103. [Google Scholar] [CrossRef]
- Polo-López, M.I.; Fernández-Ibáñez, P.; García-Fernández, I.; Oller, I.; Salgado-Tránsito, I.; Sichel, C. Resistance of Fusarium sp spores to solar TiO2 photocatalysis: Influence of spore type and water (scaling-up results). J. Chem. Technol. Biotechnol. 2010, 85, 1038–1048. [Google Scholar] [CrossRef]
- Sichel, C.; Tello, J.; de Cara, M.; Fernández-Ibáñez, P. Effect of UV solar intensity and dose on the photocatalytic disinfection of bacteria and fungi. Catal. Today 2007, 129, 152–160. [Google Scholar] [CrossRef]
- Sichel, C.; de Cara, M.; Tello, J.; Blanco, J.; Fernández-Ibáñez, P. Solar photocatalytic disinfection of agricultural pathogenic fungi: Fusarium species. Appl. Catal. B 2007, 74, 152–160. [Google Scholar] [CrossRef]
- Hochmannova, L.; Vytrasova, J. Photocatalytic and antimicrobial effects of interior paints. Prog. Org. Coat. 2010, 67, 1–5. [Google Scholar] [CrossRef]
- Muranyi, P.; Schraml, C.; Wunderlich, J. Antimicrobial efficiency of titanium dioxide-coated surfaces. J. Appl. Microbiol 2010, 108, 1966–1973. [Google Scholar] [CrossRef]
- Vucetic, S.B.; Rudic, O.; Markov, S.L.; Bera, O.J.; Vidakovic, A.M.; Skapin, A.S.; Ranogajec, J.G. Antifungal efficiency assessment of the TiO2 coating on facade paints. Environ. Sci. Pollut. Res. Int. 2014, 21, 11228–11237. [Google Scholar] [CrossRef]
- Lonnen, J.; Kilvington, S.; Kehoe, S.C.; Al-Touati, F.; McGuigan, K.G. Solar and photocatalytic disinfection of protozoan, fungal and bacterial microbes in drinking water. Water Res. 2005, 39, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Kühn, K.P.; Chaberny, I.F.; Massholder, K.; Stickler, M.; Benz, V.W.; Sonntag, H.-G.; Erdinger, L. Disinfection of surfaces by photocatalytic oxidation with titanium dioxide and UVA light. Chemosphere 2003, 53, 71–77. [Google Scholar] [CrossRef]
- Ohara, T.; Tsuge, T. FoSTUA, encoding a basic helix-loop-helix protein, differentially regulates development of three kinds of asexual spores, macroconidia, microconidia, and chlamydospores, in the fungal plant pathogen Fusarium oxysporum. Eukaryot. Cell 2004, 3, 1412–1422. [Google Scholar] [CrossRef]
- Negishi, N.; Miyazaki, Y.; Kato, S.; Yang, Y. Effect of HCO3− concentration in groundwater on TiO2 photocatalytic water purification. Appl. Catal. B 2019, 242, 449–459. [Google Scholar] [CrossRef]
- Guillard, C.; Puzenat, E.; Lachheb, H.; Houas, A.; Herrmann, J.-M. Why inorganic salts decrease the TiO2 photocatalytic efficiency. Int. J. Photoenergy 2005, 7, 641208. [Google Scholar] [CrossRef]
- Pratap Reddy, M.; Venugopal, A.; Subrahmanyam, M. Hydroxyapatite-supported Ag-TiO2 as Escherichia coli disinfection photocatalyst. Water Res. 2007, 41, 379–386. [Google Scholar] [CrossRef] [PubMed]
- ISO27447:2019. Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)-Test Method for Antibacterial Activity of Semiconducting Photocatalytic Materials; International Organization for Standardization: Geneva, Switzerland, 2019. [Google Scholar]
- JISR1702:2012. Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Test Method For Antibacterial Activity of Photocatalytic Products under Photoirradiation and Efficacy; Japanese Standards Association, 2012. [Google Scholar]
- ISO18061:2014. Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Determination of Antiviral Activity of Semiconducting Photocatalytic Materials—Test Method Using Bacteriophage Q-Beta; International Organization for Standardization: Geneva, Switzerland, 2014. [Google Scholar]
- JISR1706:2013. Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Determination of Antiviral Activity of Photocatalytic Materials—Test Method Using Bacteriophage Q-Beta; Japanese Standard Association, 2013. [Google Scholar]
- ISO13125:2013. Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Test Method for Antifungal Activity of Semiconducting Photocatalytic Materials; International Organization for Standardization, 2013. [Google Scholar]
- JISR1705:2016. Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Test Method for Antifungal Activity of Photocatalytic Products under Photoirradiation; Japanese Standard Association, 2016. [Google Scholar]
- Karagoz, S.; Kiremitler, N.B.; Sakir, M.; Salem, S.; Onses, M.S.; Sahmetlioglu, E.; Ceylan, A.; Yilmaz, E. Synthesis of Ag and TiO2 modified polycaprolactone electrospun nanofibers (PCL/TiO2-Ag NFs) as a multifunctional material for SERS, photocatalysis and antibacterial applications. Ecotoxicol. Environ. Saf. 2020, 188, 109856. [Google Scholar] [CrossRef]
- Lei, S.; Guo, G.; Xiong, B.; Gong, W.; Mei, G. Disruption of bacterial cells by photocatalysis of montmorillonite supported titanium dioxide. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2009, 24, 557–561. [Google Scholar] [CrossRef]
- Abdelbasir, S.M.; Shalan, A.E. An overview of nanomaterials for industrial wastewater treatment. Korean J. Chem. Eng. 2019, 36, 1209–1225. [Google Scholar] [CrossRef]
- Akpan, U.G.; Hameed, B.H. Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: A review. J. Hazard. Mater. 2009, 170, 520–529. [Google Scholar] [CrossRef]
- Reddy, P.V.; Kim, K.H. A review of photochemical approaches for the treatment of a wide range of pesticides. J. Hazard. Mater. 2015, 285, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Truppi, A.; Petronella, F.; Placido, T.; Margiotta, V.; Lasorella, G.; Giotta, L.; Giannini, C.; Sibillano, T.; Murgolo, S.; Mascolo, G.; et al. Gram-scale synthesis of UV–vis light active plasmonic photocatalytic nanocomposite based on TiO2/Au nanorods for degradation of pollutants in water. Appl. Catal. B 2019, 243, 604–613. [Google Scholar] [CrossRef]
- Tahir, M.B.; Ahmad, A.; Iqbal, T.; Ijaz, M.; Muhammad, S.; Siddeeg, S.M. Advances in photo-catalysis approach for the removal of toxic personal care product in aqueous environment. Environ. Dev. Sustain. 2020, 22, 6029–6052. [Google Scholar] [CrossRef]
- Zhang, G.; Li, W.; Chen, S.; Zhou, W.; Chen, J. Problems of conventional disinfection and new sterilization methods for antibiotic resistance control. Chemosphere 2020, 254, 126831. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, S.; Li, Q.L.; Lyon, D.Y.; Brunet, L.; Alvarez, P.J.J. Nanotechnology-Enabled Water Disinfection and Microbial Control: Merits and Limitations; William Andrew Inc.: Norwich, UK, 2009; pp. 157–166. [Google Scholar]
- Mascolo, G.; Comparelli, R.; Curri, M.L.; Lovecchio, G.; Lopez, A.; Agostiano, A. Photocatalytic degradation of methyl red by TiO2: Comparison of the efficiency of immobilized nanoparticles versus conventional suspended catalyst. J. Hazard. Mater. 2007, 142, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Biancullo, F.; Moreira, N.F.F.; Ribeiro, A.R.; Manaia, C.M.; Faria, J.L.; Nunes, O.C.; Castro-Silva, S.M.; Silva, A.M.T. Heterogeneous photocatalysis using UVA-LEDs for the removal of antibiotics and antibiotic resistant bacteria from urban wastewater treatment plant effluents. Chem. Eng. J. 2019, 367, 304–313. [Google Scholar] [CrossRef]
- Rizzo, L.; Sannino, D.; Vaiano, V.; Sacco, O.; Scarpa, A.; Pietrogiacomi, D. Effect of solar simulated N-doped TiO2 photocatalysis on the inactivation and antibiotic resistance of an E. coli strain in biologically treated urban wastewater. Appl. Catal. B 2014, 144, 369–378. [Google Scholar] [CrossRef]
- Rizzo, L.; Della Sala, A.; Fiorentino, A.; Puma, G.L. Disinfection of urban wastewater by solar driven and UV lamp-TiO2 photocatalysis: Effect on a multi drug resistant Escherichia coli strain. Water Res. 2014, 53, 145–152. [Google Scholar] [CrossRef]
- Li, H.Z.; Shen, L.Y.; Zhang, K.F.; Sun, B.J.; Ren, L.P.; Qiao, P.Z.; Pan, K.; Wang, L.; Zhou, W. Surface plasmon resonance-enhanced solar-driven photocatalytic performance from Ag nanoparticle-decorated self-floating porous black TiO2 foams. Appl. Catal. B 2018, 220, 111–117. [Google Scholar] [CrossRef]
- Negishi, N.; Chawengkijwanich, C.; Pimpha, N.; Larpkiattaworn, S.; Charinpanitkul, T. Performance verification of the photocatalytic solar water purification system for sterilization using actual drinking water in Thailand. J. Water Process Eng. 2019, 31, 100835. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Kuna, E. Photoactive Hybrid Catalysts Based on Natural and Synthetic Polymers: A Comparative Overview. Molecules 2017, 22, 790. [Google Scholar] [CrossRef] [PubMed]
- Castro-Munoz, R. The Role of New Inorganic Materials in Composite Membranes for Water Disinfection. Membranes 2020, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Shen, Z.P.; Shi, L.; Cheng, R.; Yuan, D.H. Photocatalytic Membrane Reactors (PMRs) in Water Treatment: Configurations and Influencing Factors. Catalysts 2017, 7, 224. [Google Scholar] [CrossRef]
- Riaz, S.; Park, S.-J. An overview of TiO2-based photocatalytic membrane reactors for water and wastewater treatments. J. Ind. Eng. Chem. 2020, 84, 23–41. [Google Scholar] [CrossRef]
- Cheng, R.; Shen, L.J.; Wang, Q.; Xiang, S.Y.; Shi, L.; Zheng, X.; Lv, W.Z. Photocatalytic Membrane Reactor (PMR) for Virus Removal in Drinking Water: Effect of Humic Acid. Catalysts 2018, 8, 284. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, X.; Choo, K.-H. Submerged microfiltration-catalysis hybrid reactor treatment: Photocatalytic inactivation of bacteria in secondary wastewater effluent. Sep. Purif. Technol. 2018, 198, 87–92. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Z.Y.; Bai, H.W.; Sun, D.D. Concurrent filtration and solar photocatalytic disinfection/degradation using high-performance Ag/TiO2 nanofiber membrane. Water Res. 2012, 46, 1101–1112. [Google Scholar] [CrossRef]
- Ishikawa, T.; Yamaoka, H.; Harada, Y.; Fujii, T.; Nagasawa, T. A general process for in situ formation of functional surface layers on ceramics. Nature 2002, 416, 64–67. [Google Scholar] [CrossRef]
- Nazerah, A.; Ismail, A.F.; Jaafar, J. Incorporation of bactericidal nanomaterials in development of antibacterial membrane for biofouling mitigation: A mini review. J. Teknol. 2016, 78, 53–61. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Sotto, A.; Boromand, A.; Zhang, R.X.; Luis, P.; Arsuaga, J.M.; Kim, J.; Van der Bruggen, B. Effect of nanoparticle aggregation at low concentrations of TiO2 on the hydrophilicity, morphology, and fouling resistance of PES-TiO2 membranes. J. Colloid Interface Sci. 2011, 363, 540–550. [Google Scholar] [CrossRef]
- Becerra, J.; Zaderenko, A.P.; Gomez-Moron, M.A.; Ortiz, P. Nanoparticles Applied to Stone Buildings. Int. J. Archit. Herit. 2019, 1–16. [Google Scholar] [CrossRef]
- Chen, J.; Poon, C.S. Photocatalytic construction and building materials: From fundamentals to applications. Build. Environ. 2009, 44, 1899–1906. [Google Scholar] [CrossRef]
- Dyshlyuk, L.; Babich, O.; Ivanova, S.; Vasilchenco, N.; Atuchin, V.; Korolkov, I.; Russakov, D.; Prosekov, A. Antimicrobial potential of ZnO, TiO2 and SiO2 nanoparticles in protecting building materials from biodegradation. Int. Biodeterior. Biodegrad. 2020, 146, 8. [Google Scholar] [CrossRef]
- Chen, L.; Pan, H.; Zhuang, C.F.; Peng, M.Y.; Zhang, L. Joint wound healing using polymeric dressing of chitosan/strontium-doped titanium dioxide with high antibacterial activity. Mater. Lett. 2020, 268, 3. [Google Scholar] [CrossRef]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, S467–S479. [Google Scholar] [CrossRef] [PubMed]
- Malmir, S.; Karbalaei, A.; Pourmadadi, M.; Hamedi, J.; Yazdian, F.; Navaee, M. Antibacterial properties of a bacterial cellulose CQD-TiO2 nanocomposite. Carbohydr. Polym. 2020, 234, 10. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.L.; Chen, K.K.; He, X.C.; Li, N.; Huang, J.B.; Tang, K.Y.; Li, Y.J.; Wang, F. Nano-TiO2/collagen-chitosan porous scaffold for wound repairing. Int. J. Biol. Macromol. 2016, 91, 15–22. [Google Scholar] [CrossRef]
- Marulasiddeshwara, R.; Jyothi, M.S.; Soontarapa, K.; Keri, R.S.; Velmurugan, R. Nonwoven fabric supported, chitosan membrane anchored with curcumin/TiO2 complex: Scaffolds for MRSA infected wound skin reconstruction. Int. J. Biol. Macromol. 2020, 144, 85–93. [Google Scholar] [CrossRef]
- Ansarizadeh, M.; Haddadi, S.A.; Amini, M.; Hasany, M.; SaadatAbadi, A.R. Sustained release of CIP from TiO2-PVDF/starch nanocomposite mats with potential application in wound dressing. J. Appl. Polym. Sci. 2020, 137, 11. [Google Scholar] [CrossRef]
- Ashraf, R.; Sofi, H.S.; Akram, T.; Rather, H.A.; Abdal-Hay, A.; Shabir, N.; Vasita, R.; Alrokayan, S.H.; Khan, H.A.; Sheikh, F.A. Fabrication of multifunctional cellulose/TiO2/Ag composite nanofibers scaffold with antibacterial and bioactivity properties for future tissue engineering applications. J. Biomed. Mater. Res. A 2020, 108, 947–962. [Google Scholar] [CrossRef] [PubMed]
- Monmaturapoj, N.; Thepsuwan, W.; Mai-Ngam, K.; Ngernpimai, S.; Klinsukhon, W.; Prahsarn, C. Preparation and properties of hydroxyapatite/titania composite for microbial filtration application. Adv. Appl. Ceram. 2014, 113, 267–274. [Google Scholar] [CrossRef]
- Li, Y.; Leung, P.; Yao, L.; Song, Q.W.; Newton, E. Antimicrobial effect of surgical masks coated with nanoparticles. J. Hosp. Infect. 2006, 62, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Jamróz, E.; Kulawik, P.; Kopel, P. The Effect of Nanofillers on the Functional Properties of Biopolymer-Based Films: A Review. Polymers 2019, 11, 675. [Google Scholar] [CrossRef] [PubMed]
- Nazir, S.; Azad, Z.R.A.A. Food Nanotechnology: An Emerging Technology in Food Processing and Preservation. In Health and Safety Aspects of Food Processing Technologies; Springer: Cham, Switzerland, 2019; pp. 567–576. [Google Scholar]
- Bajpai, V.K.; Kamle, M.; Shukla, S.; Mahato, D.K.; Chandra, P.; Hwang, S.K.; Kumar, P.; Huh, Y.S.; Han, Y.-K. Prospects of using nanotechnology for food preservation, safety, and security. J. Food Drug Anal. 2018, 26, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Hoseinnejad, M.; Jafari, S.M.; Katouzian, I. Inorganic and metal nanoparticles and their antimicrobial activity in food packaging applications. Crit. Rev. Microbiol. 2018, 44, 161–181. [Google Scholar] [CrossRef]
- Sharma, R.; Jafari, S.M.; Sharma, S. Antimicrobial bio-nanocomposites and their potential applications in food packaging. Food Control 2020, 112, 11. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Ruvalcaba-Gomez, J.M.; Maytorena-Verdugo, C.I.; Gonzalez-Silva, N.; Romero-Toledo, R.; Aguilera-Aguirre, S.; Perez-Larios, A.; Montalvo-Gonzalez, E. Chitosan-TiO2: A Versatile Hybrid Composite. Materials 2020, 13, 811. [Google Scholar] [CrossRef]
- Goudarzi, V.; Shahabi-Ghahfarrokhi, I.; Babaei-Ghazvini, A. Preparation of ecofriendly UV-protective food packaging material by starch/TiO2 bio-nanocomposite: Characterization. Int. J. Biol. Macromol. 2017, 95, 306–313. [Google Scholar] [CrossRef]
- Bohmer-Maas, B.W.; Fonseca, L.M.; Otero, D.M.; Zavareze, E.D.; Zambiazi, R.C. Photocatalytic zein-TiO2 nanofibers as ethylene absorbers for storage of cherry tomatoes. Food Packag. Shelf Life 2020, 24, 7. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Sayed, S.M. Bionanocomposites materials for food packaging applications: Concepts and future outlook. Carbohydr. Polym. 2018, 193, 19–27. [Google Scholar] [CrossRef]
- Zhang, X.D.; Xiao, G.; Wang, Y.Q.; Zhao, Y.; Su, H.J.; Tan, T.W. Preparation of chitosan-TiO2 composite film with efficient antimicrobial activities under visible light for food packaging applications. Carbohydr. Polym. 2017, 169, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.R.; Yang, Y.M.; Wang, J.; Yan, W.J.; Wu, Z.J.; Chen, H.; Zhang, Q.; Wu, D.T.; Qin, W.; Tu, Z.C. Preparation and characterization of TiO2-Ag loaded fish gelatin-chitosan antibacterial composite film for food packaging. Int. J. Biol. Macromol. 2020, 154, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Hung, Y.C. UV-A activated TiO2 embedded biodegradable polymer film for antimicrobial food packaging application. LWT-Food Sci. Technol. 2018, 96, 307–314. [Google Scholar] [CrossRef]
- Hong, L.; Luo, S.H.; Yu, C.H.; Xie, Y.; Xia, M.Y.; Chen, G.Y.; Peng, Q. Functional Nanomaterials and Their Potential Applications in Antibacterial Therapy. Pharm. Nanotechnol. 2019, 7, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Teymourpour, S.; Mohammadi Nafchi, A.; Nahidi, F. Functional, thermal, and antimicrobial properties of soluble soybean polysaccharide biocomposites reinforced by nano TiO2. Carbohydr. Polym. 2015, 134, 726–731. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).












