Flow Simulations Including Iron Nanoparticle Nucleation, Growth and Evaporation for Floating Catalyst CNT Production
Abstract
1. Introduction
2. Results and Discussion
2.1. Model Comparison
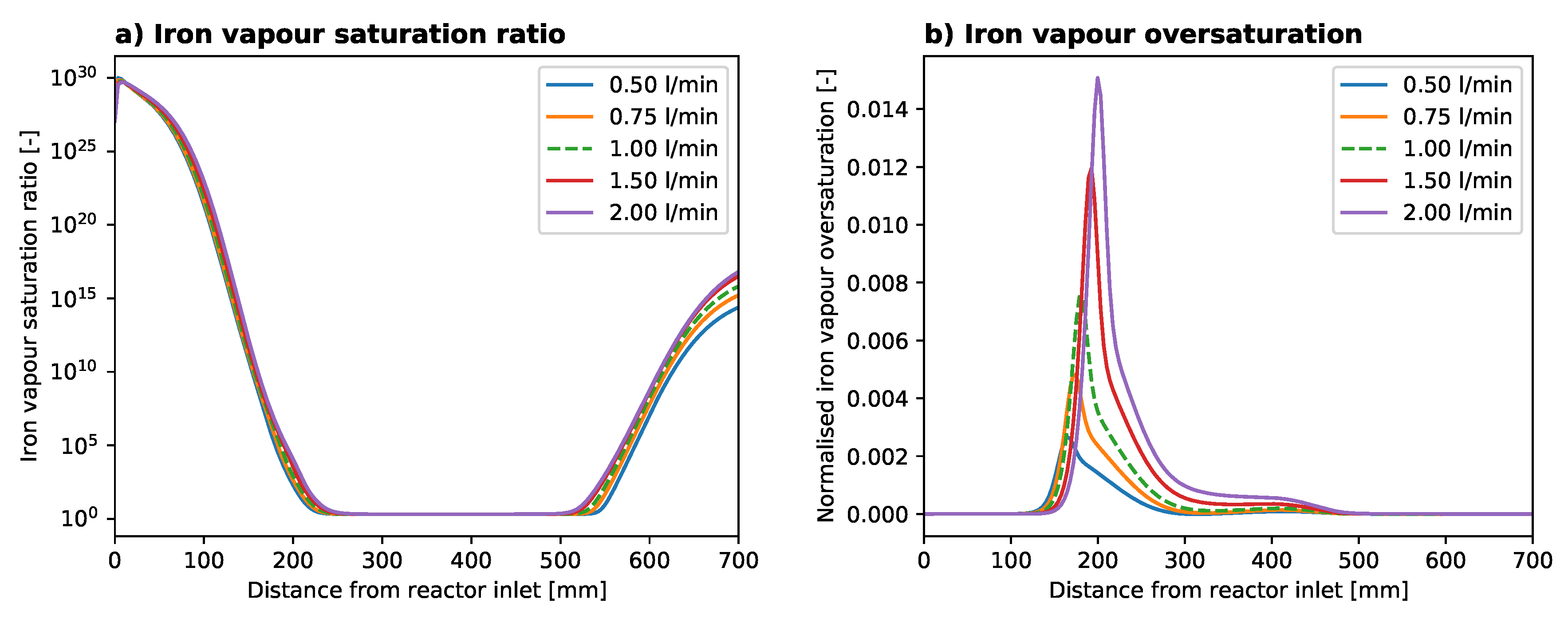
2.2. Overall Results
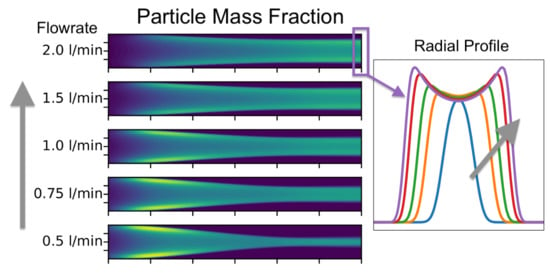
2.3. The Tendency of the Aerogel to Form a Sock
3. Methods
3.1. The Fluid Kinetics and Dynamics
3.2. The Ferrocene Decomposition
3.3. The Particle Formation
3.4. Normalising the Particle Equations
3.5. Iron Vapour and Particle Diffusivity
3.6. Solving the Equations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CNT | Carbon Nanotube |
| FC-CVD | Floating Catalyst Chemical Vapour Deposition |
Appendix A
References
- Li, Y.L.; Kinloch, I.A.; Windle, A.H. Direct Spinning of Carbon Nanotube Fibers from Chemical Vapor Deposition Synthesis. Science 2004, 304, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Weller, L.; Smail, F.R.; Elliott, J.A.; Windle, A.H.; Boies, A.M.; Hochgreb, S. Mapping the parameter space for direct-spun carbon nanotube aerogels. Carbon 2019, 146, 789–812. [Google Scholar] [CrossRef]
- Motta, M.; Kinloch, I.; Moisala, A.; Premnath, V.; Pick, M.; Windle, A. The parameter space for the direct spinning of fibres and films of carbon nanotubes. Phys.-Low-Dimens. Syst. Nanostruct. 2007, 37, 40–43. [Google Scholar] [CrossRef]
- Kaniyoor, A.; Bulmer, J.; Gspann, T.; Mizen, J.; Ryley, J.; Kiley, P.; Terrones, J.; Miranda-Reyes, C.; Divitini, G.; Sparkes, M.; et al. High throughput production of single-wall carbon nanotube fibres independent of sulfur-source. Nanoscale 2019, 11, 18483–18495. [Google Scholar] [CrossRef]
- Bulmer, J.S.; Kaniyoor, A.; Gspann, T.; Mizen, J.; Ryley, J.; Kiley, P.; Ratering, G.; Sparreboom, W.; Bauhuis, G.; Stehr, T.; et al. Forecasting continuous carbon nanotube production in the floating catalyst environment. Chem. Eng. J. 2020, 390, 124497. [Google Scholar] [CrossRef]
- Reguero, V.; Alemán, B.; Mas, B.; Vilatela, J.J. Controlling Carbon Nanotube Type in Macroscopic Fibers Synthesized by the Direct Spinning Process. Chem. Mater. 2014, 26, 3550–3557. [Google Scholar] [CrossRef]
- Kuwana, K.; Endo, H.; Saito, K.; Qian, D.; Andrews, R.; Grulke, E.A. Catalyst deactivation in CVD synthesis of carbon nanotubes. Carbon 2005, 43, 253–260. [Google Scholar] [CrossRef]
- Conroy, D.; Moisala, A.; Cardoso, S.; Windle, A.; Davidson, J. Carbon nanotube reactor: Ferrocene decomposition, iron particle growth, nanotube aggregation and scale-up. Chem. Eng. Sci. 2010, 65, 2965–2977. [Google Scholar] [CrossRef]
- Hoecker, C.; Smail, F.; Bajada, M.; Pick, M.; Boies, A. Catalyst nanoparticle growth dynamics and their influence on product morphology in a CVD process for continuous carbon nanotube synthesis. Carbon 2016, 96, 116–124. [Google Scholar] [CrossRef]
- Rodiles, X.; Reguero, V.; Vila, M.; Alemán, B.; Arévalo, L.; Fresno, F.; O’Shea, V.A.d.l.P.; Vilatela, J.J. Carbon nanotube synthesis and spinning as macroscopic fibers assisted by the ceramic reactor tube. Sci. Rep. 2019, 9, 9239. [Google Scholar] [CrossRef]
- Hou, G.; Ng, V.; Song, Y.; Zhang, L.; Xu, C.; Shanov, V.; Mast, D.; Schulz, M.; Liu, Y. Numerical and Experimental Investigation of Carbon Nanotube Sock Formation. MRS Adv. 2017, 2, 21–26. [Google Scholar] [CrossRef]
- Segré, G.; Silberberg, A. Radial Particle Displacements in Poiseuille Flow of Suspensions. Nature 1961, 189, 209–210. [Google Scholar] [CrossRef]
- Segré, G.; Silberberg, A. Behaviour of macroscopic rigid spheres in Poiseuille flow Part 1. Determination of local concentration by statistical analysis of particle passages through crossed light beams. J. Fluid Mech. 1962, 14, 115–135. [Google Scholar] [CrossRef]
- Segré, G.; Silberberg, A. Behaviour of macroscopic rigid spheres in Poiseuille flow Part 2. Experimental results and interpretation. J. Fluid Mech. 1962, 14, 136–157. [Google Scholar] [CrossRef]
- Hoecker, C.; Smail, F.; Pick, M.; Weller, L.; Boies, A.M. The Dependence of CNT Aerogel Synthesis on Sulfur-driven Catalyst Nucleation Processes and a Critical Catalyst Particle Mass Concentration. Sci. Rep. 2017, 7, 14519. [Google Scholar] [CrossRef]
- Hou, G.; Su, R.; Wang, A.; Ng, V.; Li, W.; Song, Y.; Zhang, L.; Sundaram, M.; Shanov, V.; Mast, D.; et al. The effect of a convection vortex on sock formation in the floating catalyst method for carbon nanotube synthesis. Carbon 2016, 102, 513–519. [Google Scholar] [CrossRef]
- Kuwana, K.; Saito, K. Modeling CVD synthesis of carbon nanotubes: Nanoparticle formation from ferrocene. Carbon 2005, 43, 2088–2095. [Google Scholar] [CrossRef]
- Lewis, K.E.; Smith, G.P. Bond dissociation energies in ferrocene. J. Am. Chem. Soc. 1984, 106, 4650–4651. [Google Scholar] [CrossRef]
- Gspann, T.S.; Smail, F.R.; Windle, A.H. Spinning of carbon nanotube fibres using the floating catalyst high temperature route: Purity issues and the critical role of sulphur. Faraday Discuss. 2014, 173, 47–65. [Google Scholar] [CrossRef]
- Girshick, S.L.; Chiu, C. Kinetic nucleation theory: A new expression for the rate of homogeneous nucleation from an ideal supersaturated vapor. J. Chem. Phys. 1990, 93, 1273–1277. [Google Scholar] [CrossRef]
- Friedlander, S.K. Smoke, Dust, and Haze: Fundamentals of Aerosol Dynamics, 2nd ed.; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Alnæs, M.S.; Blechta, J.; Hake, J.; Johansson, A.; Kehlet, B.; Logg, A.; Richardson, C.; Ring, J.; Rognes, M.E.; Wells, G.N. The FEniCS Project Version 1.5. Arch. Numer. Softw. 2015, 3. [Google Scholar] [CrossRef]
- Geuzaine, C.; Remacle, J.F. Gmsh: A 3-D finite element mesh generator with built-in pre-and post-processing facilities. Int. J. Numer. Methods Eng. 2009, 79, 1309–1331. [Google Scholar] [CrossRef]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gökstorp, F.K.A.; Juniper, M.P. Flow Simulations Including Iron Nanoparticle Nucleation, Growth and Evaporation for Floating Catalyst CNT Production. Catalysts 2020, 10, 1383. https://doi.org/10.3390/catal10121383
Gökstorp FKA, Juniper MP. Flow Simulations Including Iron Nanoparticle Nucleation, Growth and Evaporation for Floating Catalyst CNT Production. Catalysts. 2020; 10(12):1383. https://doi.org/10.3390/catal10121383
Chicago/Turabian StyleGökstorp, Filip K. A., and Matthew P. Juniper. 2020. "Flow Simulations Including Iron Nanoparticle Nucleation, Growth and Evaporation for Floating Catalyst CNT Production" Catalysts 10, no. 12: 1383. https://doi.org/10.3390/catal10121383
APA StyleGökstorp, F. K. A., & Juniper, M. P. (2020). Flow Simulations Including Iron Nanoparticle Nucleation, Growth and Evaporation for Floating Catalyst CNT Production. Catalysts, 10(12), 1383. https://doi.org/10.3390/catal10121383