Zeolite Beta Doped with La, Fe, and Pd as a Hydrocarbon Trap
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterisation
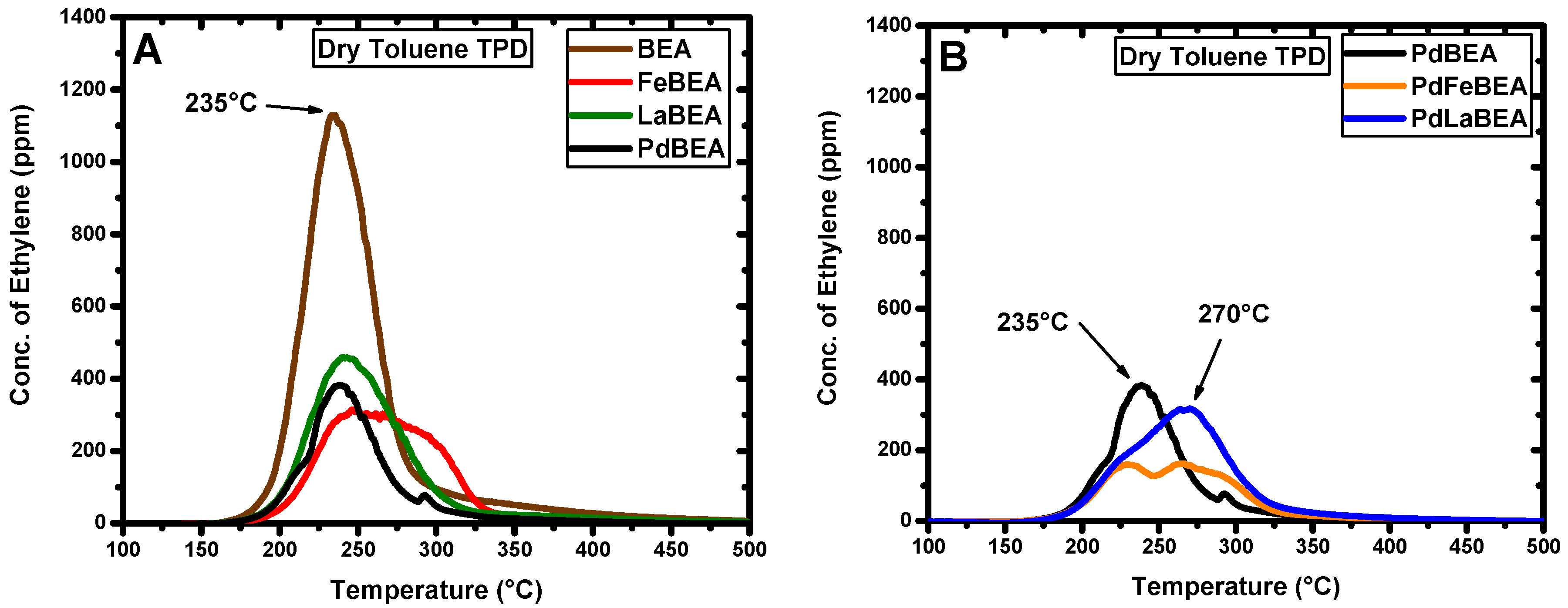
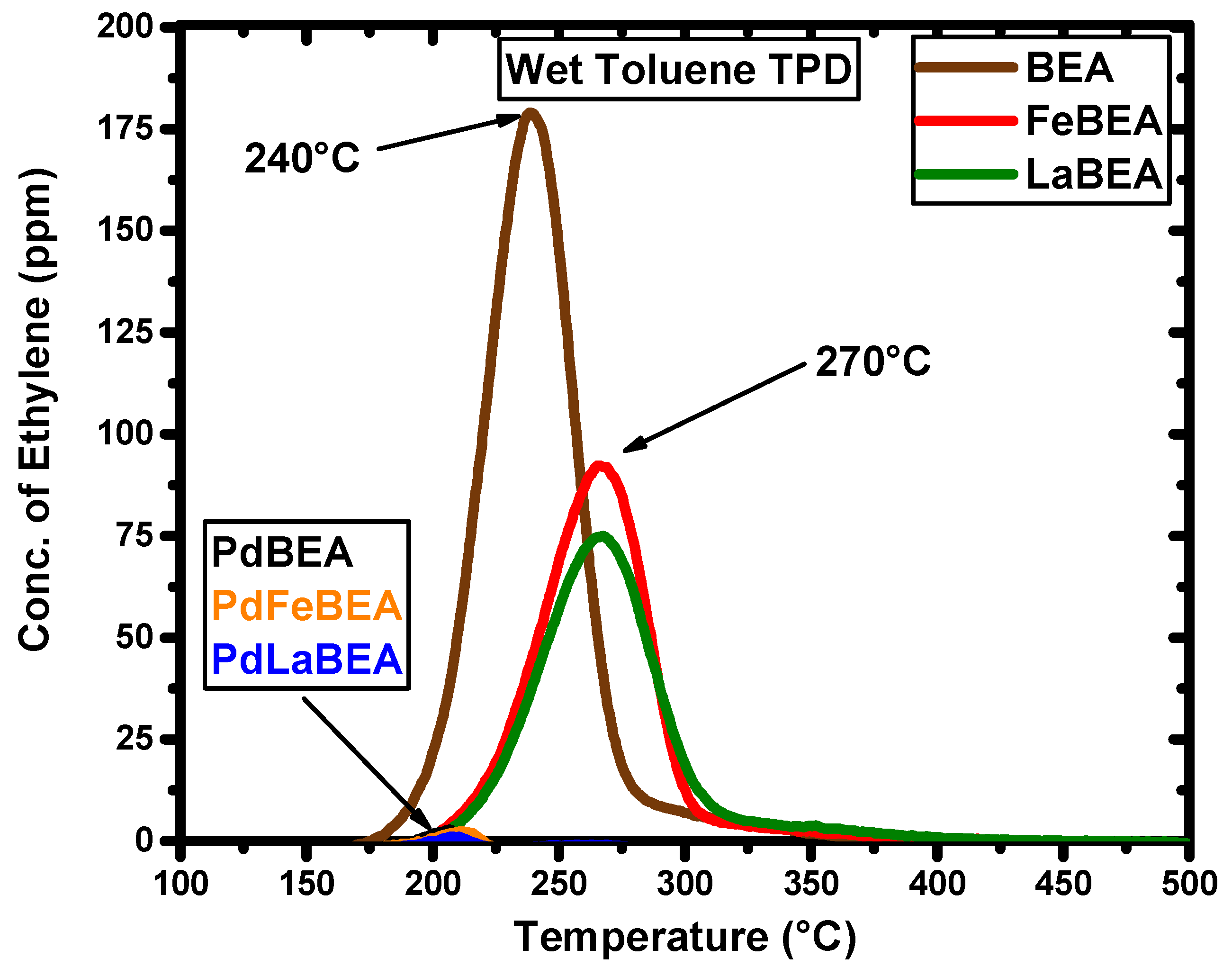
2.2. Temperature-Programmed Desorption of Hydrocarbons
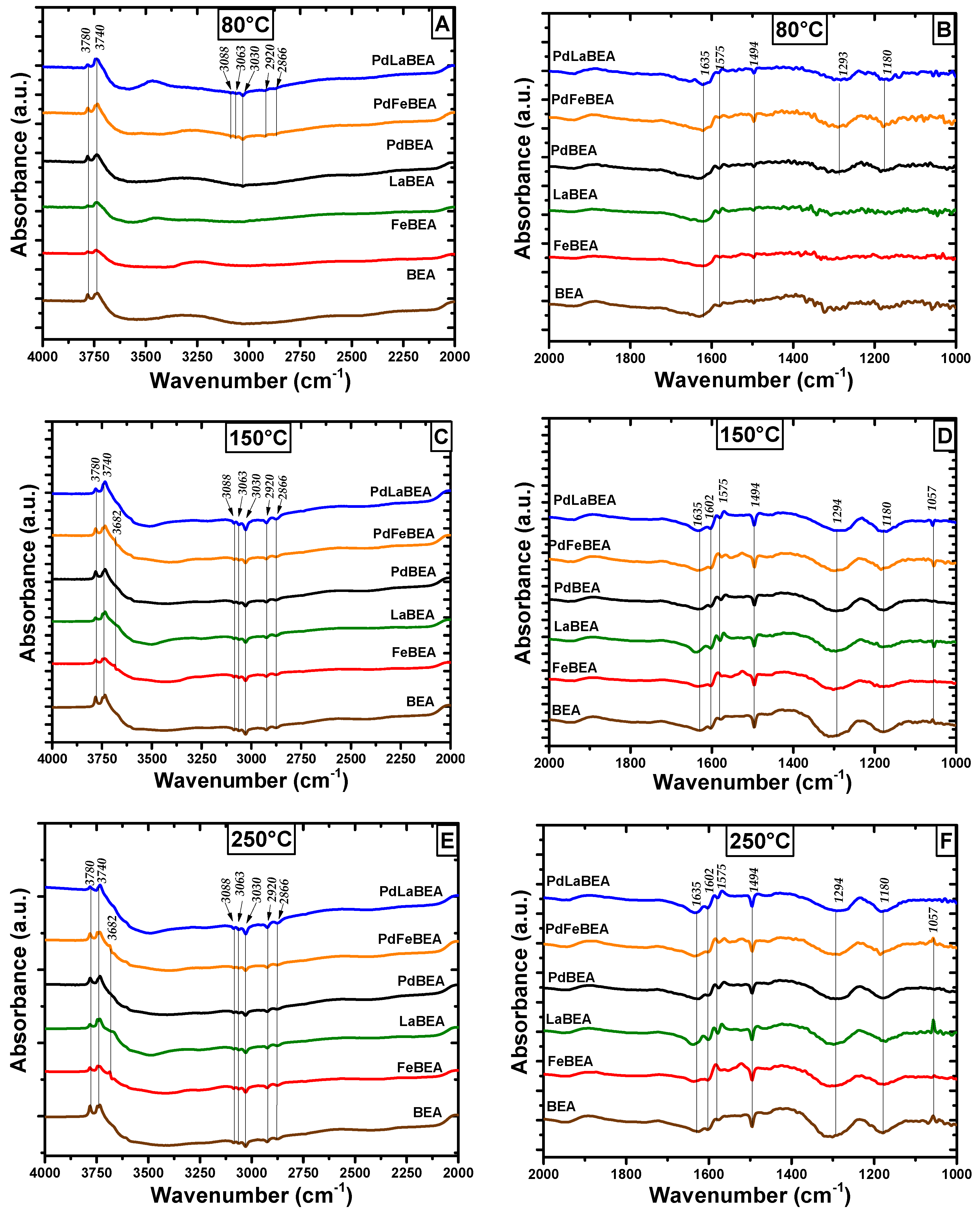
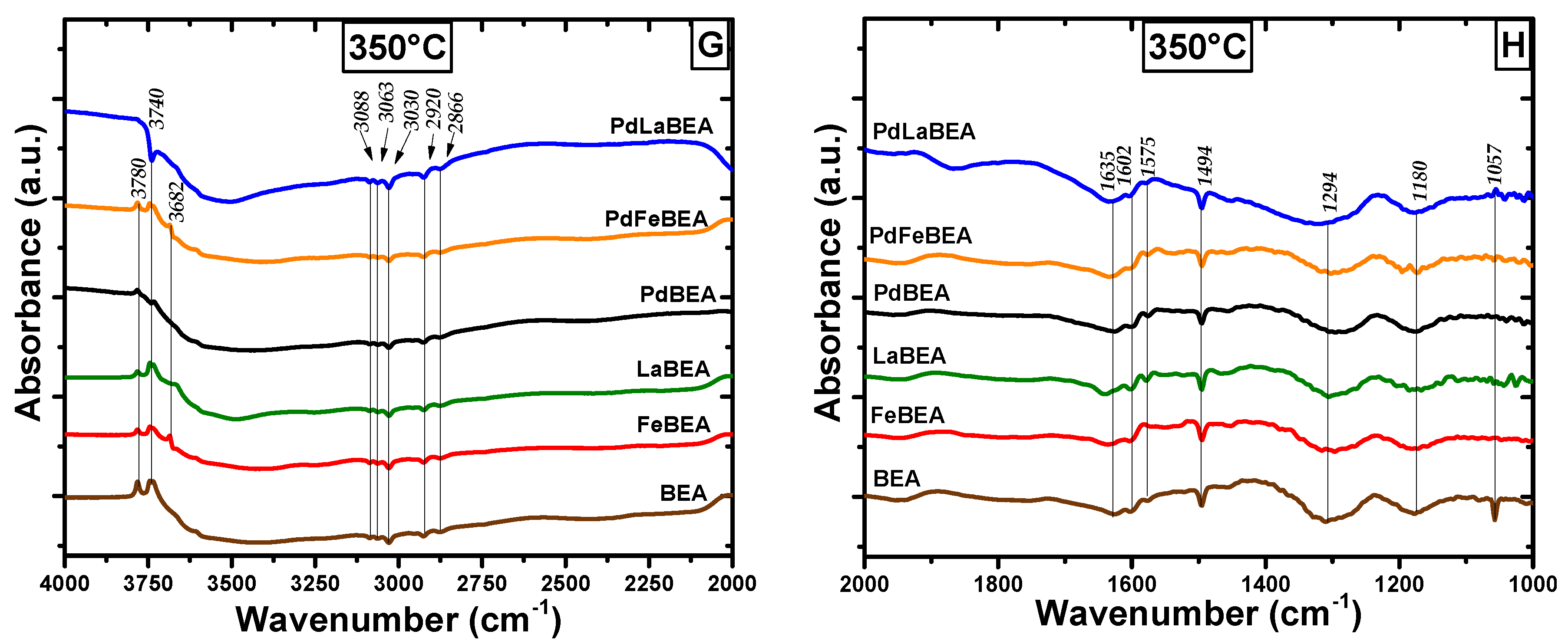
2.3. In Situ DRIFT Spectroscopy of Adsorbed Hydrocarbons during Heating
3. Materials and Methods
3.1. Sample Preparation
3.2. Catalyst Characterisation
3.3. Temperature-Programmed Desorption
3.4. In Situ DRIFT Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Park, J.H.; Park, S.J.; Nam, I.S.; Yeo, G.K.; Kil, J.K.; Youn, Y.K. A fast and quantitative assay for developing zeolite-type hydrocarbon trap catalyst. Microporous Mesoporous Mater. 2007, 101, 264–270. [Google Scholar] [CrossRef]
- Kim, M.Y.; Kyriakidou, E.A.; Choi, J.S.; Toops, T.J.; Binder, A.J.; Thomas, C.; Parks, J.E.; Schwartz, V.; Chen, J.; Hensley, D.K. Enhancing low-temperature activity and durability of Pd-based diesel oxidation catalysts using ZrO2 supports. Appl. Catal. B Environ. 2016, 187, 181–194. [Google Scholar] [CrossRef] [Green Version]
- Du, S.; Tang, W.; Guo, Y.; Binder, A.; Kyriakidou, E.A.; Toops, T.J.; Wang, S.; Ren, Z.; Hoang, S.; Gao, P.X. Understanding low temperature oxidation activity of nanoarray-based monolithic catalysts: From performance observation to structural and chemical insights. Emiss. Control Sci. Technol. 2017, 3, 18–36. [Google Scholar] [CrossRef]
- Hazlett, M.J.; Epling, W.S. Spatially resolving CO and C3H6 oxidation reactions in a Pt/Al2O3 model oxidation catalyst. Catal. Today 2016, 267, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Theis, J.R.; Kyriakidou, E.A. Vehicle emissions trapping materials: Successes, challenges, and the path forward. Appl. Catal. B Environ. 2019, 243, 397–414. [Google Scholar] [CrossRef]
- Wesson, P.J.; Snurr, R.Q. Modified temperature programmed desorption evaluation of hydrocarbon trapping by CsMOR zeolite under cold start conditions. Microporous Mesoporous Mater. 2009, 125, 35–38. [Google Scholar] [CrossRef]
- Yoshimoto, R.; Hara, K.; Okumura, K.; Katada, N.; Niwa, M. Analysis of toluene adsorption on na-form zeolite with a temperature-programmed desorption method. J. Phys. Chem. C 2007, 111, 1474–1479. [Google Scholar] [CrossRef]
- Kanazawa, T. Development of hydrocarbon adsorbents, oxygen storage materials for three-way catalysts and NOx storage-reduction catalyst. Catal. Today 2004, 96, 171–177. [Google Scholar] [CrossRef]
- De Moor, B.A.; Reyniers, M.F.; Gobin, O.C.; Lercher, J.A.; Marin, G.B. Adsorption of C2−C8 n-Alkanes in Zeolites. J. Phys. Chem. C 2011, 115, 1204–1219. [Google Scholar] [CrossRef]
- Sarshar, Z.; Zahedi-Niaki, M.H.; Huang, Q.; Eić, M.; Kaliaguine, S. MTW zeolites for reducing cold-start emissions of automotive exhaust. Appl. Catal. B Environ. 2009, 87, 37–45. [Google Scholar] [CrossRef]
- Daldoul, I.; Auger, S.; Picard, P.; Nohair, B.; Kaliaguine, S. Effect of temperature Ramp on hydrocarbon desorption profiles from zeolite ZSM-12. Can. J. Chem. Eng. 2016, 94, 931–937. [Google Scholar] [CrossRef]
- Dorner, R.W.; Deifalla, M.; Catlow, C.R.A.; Corà, F.; Elangovan, S.P.; Okubo, T.; Sankar, G. Heteroatom-substituted microporous AFI and ATS structured materials for hydrocarbon trap: An insight into the aluminophosphate framework-toluene interaction. J. Phys. Chem. C 2008, 112, 4187–4194. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Liu, X.; Li, X.; Pan, R.; Han, P.; Dou, T. Synthesis of hierarchical mesoporous zeolites based on MOR zeolite: Application in the automobile tailpipe hydrocarbon trap. J. Porous Mater. 2015, 22, 807–815. [Google Scholar] [CrossRef]
- Puértolas, B.; García-Andújar, L.; García, T.; Navarro, M.V.; Mitchell, S.; Pérez-Ramírez, J. Bifunctional Cu/H-ZSM-5 zeolite with hierarchical porosity for hydrocarbon abatement under cold-start conditions. Appl. Catal. B Environ. 2014, 154–155, 161–170. [Google Scholar]
- Nakagawa, S.; Minowa, T.; Katogi, K.; Higashiyama, K.; Nagano, M.; Hamada, I. A New Catalyzed Hydrocarbon Trap Control System for ULEV/SULEV Standard. SAE Tech. Pap. 2003, 2003-01-0567. [Google Scholar] [CrossRef]
- López, J.M.; Navarro, M.V.; García, T.; Murillo, R.; Mastral, A.M.; Varela-Gandía, F.J.; Lozano-Castelló, D.; Bueno-López, A.; Cazorla-Amorós, D. Screening of different zeolites and silicoaluminophosphates for the retention of propene under cold start conditions. Microporous Mesoporous Mater. 2010, 130, 239–247. [Google Scholar] [CrossRef]
- Yoda, E.; Kondo, J.N.; Domen, K. Detailed process of adsorption of alkanes and alkenes on zeolites. J. Phys. Chem. B 2005, 109, 1464–1472. [Google Scholar] [CrossRef]
- Westermann, A.; Azambre, B.; Finqueneisel, G.; Da Costa, P.; Can, F. Evolution of unburnt hydrocarbons under “cold-start” conditions from adsorption/desorption to conversion: On the screening of zeolitic materials. Appl. Catal. B Environ. 2014, 158–159, 48–59. [Google Scholar] [CrossRef]
- Westermann, A.; Azambre, B.; Chebbi, M.; Koch, A. Modification of y Faujasite zeolites for the trapping and elimination of a propene-toluene-decane mixture in the context of cold-start. Microporous Mesoporous Mater. 2016, 230, 76–88. [Google Scholar] [CrossRef]
- Azambre, B.; Westermann, A.; Finqueneisel, G.; Can, F.; Comparot, J.D. Adsorption and desorption of a model hydrocarbon mixture over HY zeolite under dry and wet conditions. J. Phys. Chem. C 2015, 119, 315–331. [Google Scholar] [CrossRef]
- Burke, N.R.; Trimm, D.L.; Howe, R.F. The effect of silica:alumina ratio and hydrothermal ageing on the adsorption characteristics of BEA zeolites for cold start emission control. Appl. Catal. B Environ. 2003, 46, 97–104. [Google Scholar] [CrossRef]
- Park, J.H.; Park, S.J.; Ahn, H.A.; Nam, I.S.; Yeo, G.K.; Kil, J.K.; Youn, Y.K. Promising zeolite-type hydrocarbon trap catalyst by a knowledge-based combinatorial approach. Microporous Mesoporous Mater. 2009, 117, 178–184. [Google Scholar] [CrossRef]
- Liu, X.; Lampert, J.K.; Arendarskiia, D.A.; Farrauto, R.J. FT-IR spectroscopic studies of hydrocarbon trapping in Ag+-ZSM-5 for gasoline engines under cold-start conditions. Appl. Catal. B Environ. 2001, 35, 125–136. [Google Scholar] [CrossRef]
- Kang, S.B.; Kalamaras, C.; Balakotaiah, V.; Epling, W. Hydrocarbon Trapping over Ag-Beta Zeolite for Cold-Start Emission Control. Catal. Lett. 2017, 147, 1355–1362. [Google Scholar] [CrossRef]
- Takamitsu, Y.; Ariga, K.; Yoshida, S.; Ogawa, H.; Sano, T. Adsorption of toluene on alkali metal ion-exchanged zsm-5 and β-zeolites under humid conditions. Bull. Chem. Soc. Jpn. 2012, 85, 869–876. [Google Scholar] [CrossRef]
- Serra, R.M.; Miró, E.E.; Bolcatto, P.; Boix, A.V. Experimental and theoretical studies about the adsorption of toluene on ZSM5 and mordenite zeolites modified with Cs. Microporous Mesoporous Mater. 2012, 147, 17–29. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Srinivasan, K.R.; Singh, A.P. Temperature-programmed desorption of aromatic hydrocarbons on silicalite-I and ZSM-5-type zeolites. Zeolites 1990, 10, 16–20. [Google Scholar] [CrossRef]
- Kobatake, Y.; Momma, K.; Elangovan, S.P.; Itabashi, K.; Okubo, T.; Ogura, M. “Super Hydrocarbon Reformer Trap” for the Complete Oxidation of Toluene Using Iron-Exchanged β-Zeolite with a Low Silicon/Aluminum Ratio. ChemCatChem 2016, 8, 2516–2524. [Google Scholar] [CrossRef]
- Xu, L.; Lupescu, J.; Cavataio, G.; Guo, K.; Jen, H. The Impacts of Pd in BEA Zeolite on Decreasing Cold-Start NMOG Emission of an E85 Fuel Vehicle. SAE Int. J. Fuels Lubr. 2018, 11, 239–246. [Google Scholar] [CrossRef]
- Lupescu, J.; Xu, L.; Jen, H.W.; Harwell, A.; Nunan, J.; Alltizer, C.; Denison, G. A New Catalyzed HC Trap Technology that Enhances the Conversion of Gasoline Fuel Cold-Start Emissions. SAE Int. J. Fuels Lubr. 2018, 11, 411–426. [Google Scholar] [CrossRef]
- Mihai, O.; Trandafilović, L.; Wentworth, T.; Torres, F.F.; Olsson, L. The Effect of Si/Al Ratio for Pd/BEA and Pd/SSZ-13 Used as Passive NOx Adsorbers. Top. Catal. 2018, 61, 2007–2020. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Mccabe, R.W.; Dearth, M.A.; Gorte, R.J. Transient Adsorption Studies of Automotive Hydrocarbon Traps. AICHE J. 2014, 60, 2875–2881. [Google Scholar] [CrossRef]
- Huber, G.W.; Corma, A. Synergies between bio- and oil refineries for the production of fuels from biomass. Angew. Chem.-Int. Ed. 2007, 46, 7184–7201. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.N.; Kim, M.; Song, J.; Na, S.; Sik, H.; Heesung, H. Cold-Start Hydrocarbon Speciation and Trap Materials for Gasoline Engines. SAE Tech. Pap. 2018, 2018-01-0940. [Google Scholar] [CrossRef]
- Westermann, A.; Azambre, B. Impact of the Zeolite Structure and Acidity on the Adsorption of Unburnt Hydrocarbons Relevant to Cold Start Conditions. J. Phys. Chem. C 2016, 120, 25903–25914. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Z.; Zhao, Q.; Wang, L. Photocatalytic degradation of gaseous toluene over ZnAl2O4 prepared by different methods: A comparative study. J. Hazard. Mater. 2011, 186, 2089–2096. [Google Scholar] [CrossRef]
- Sanati, M.; Andersson, A. DRIFT study of the oxidation and the ammoxidation of toluene over a TiO2(B)-supported vanadia catalyst. J. Mol. Catal. 1993, 81, 51–62. [Google Scholar] [CrossRef]
- Du, J.; Qu, Z.; Dong, C.; Song, L.; Qin, Y.; Huang, N. Low-temperature abatement of toluene over Mn-Ce oxides catalysts synthesized by a modified hydrothermal approach. Appl. Surf. Sci. 2018, 433, 1025–1035. [Google Scholar] [CrossRef]
- Nagao, M.; Suda, Y. Adsorption of Benzene, Toluene, and Chlorobenzene on Titanium Dioxide. Langmuir 1989, 5, 42–47. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, F.; Zhu, X.; Qi, Z.; Hong, B.; Ding, J.; Bao, J.; Sun, S.; Gao, C. DRIFTS evidence for facet-dependent adsorption of gaseous toluene on TiO2 with relative photocatalytic properties. Langmuir 2015, 31, 1730–1736. [Google Scholar] [CrossRef]
- Liao, Y.; Zhang, X.; Peng, R.; Zhao, M.; Ye, D. Catalytic properties of manganese oxide polyhedra with hollow and solid morphologies in toluene removal. Appl. Surf. Sci. 2017, 405, 20–28. [Google Scholar] [CrossRef]
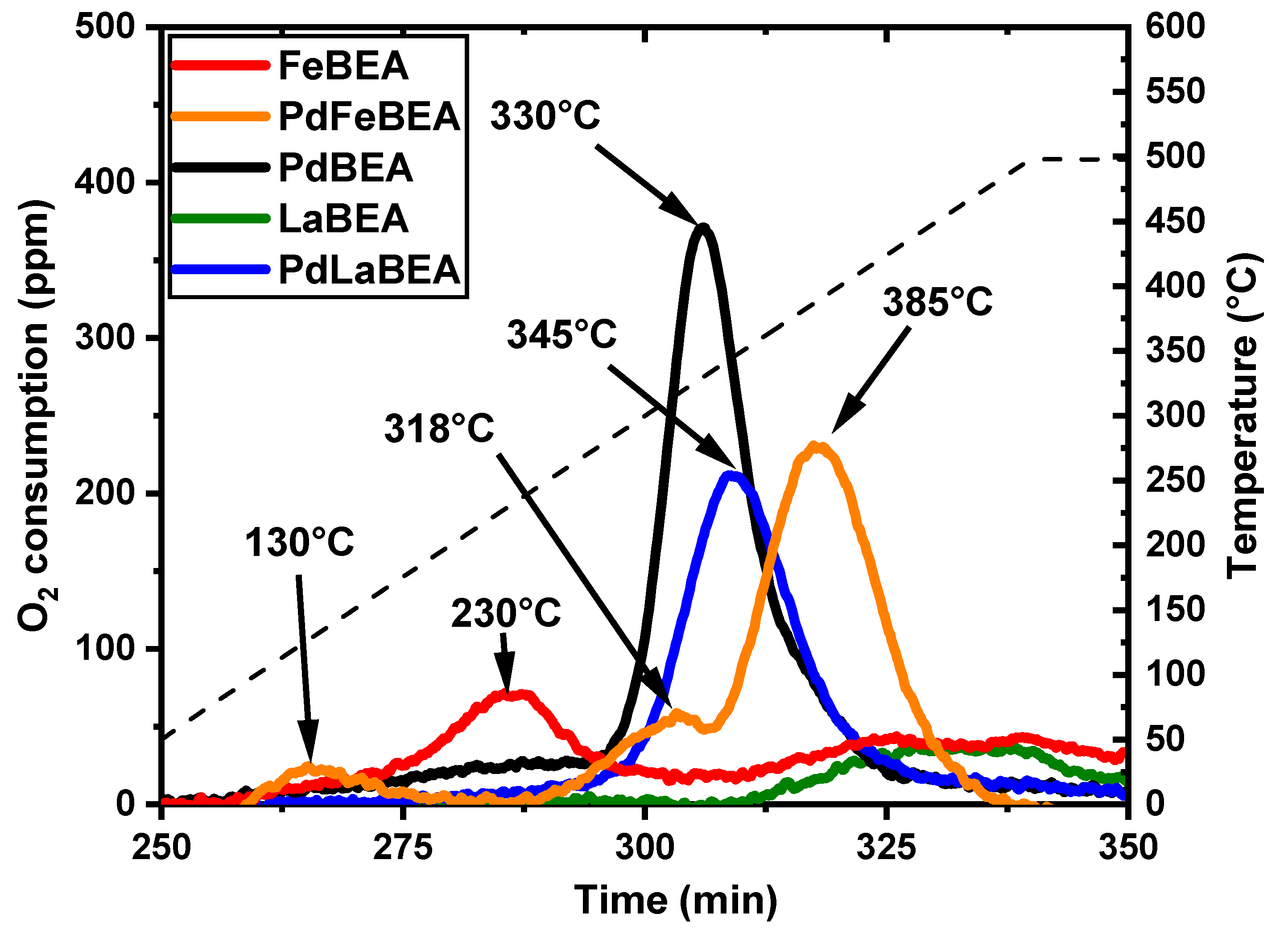
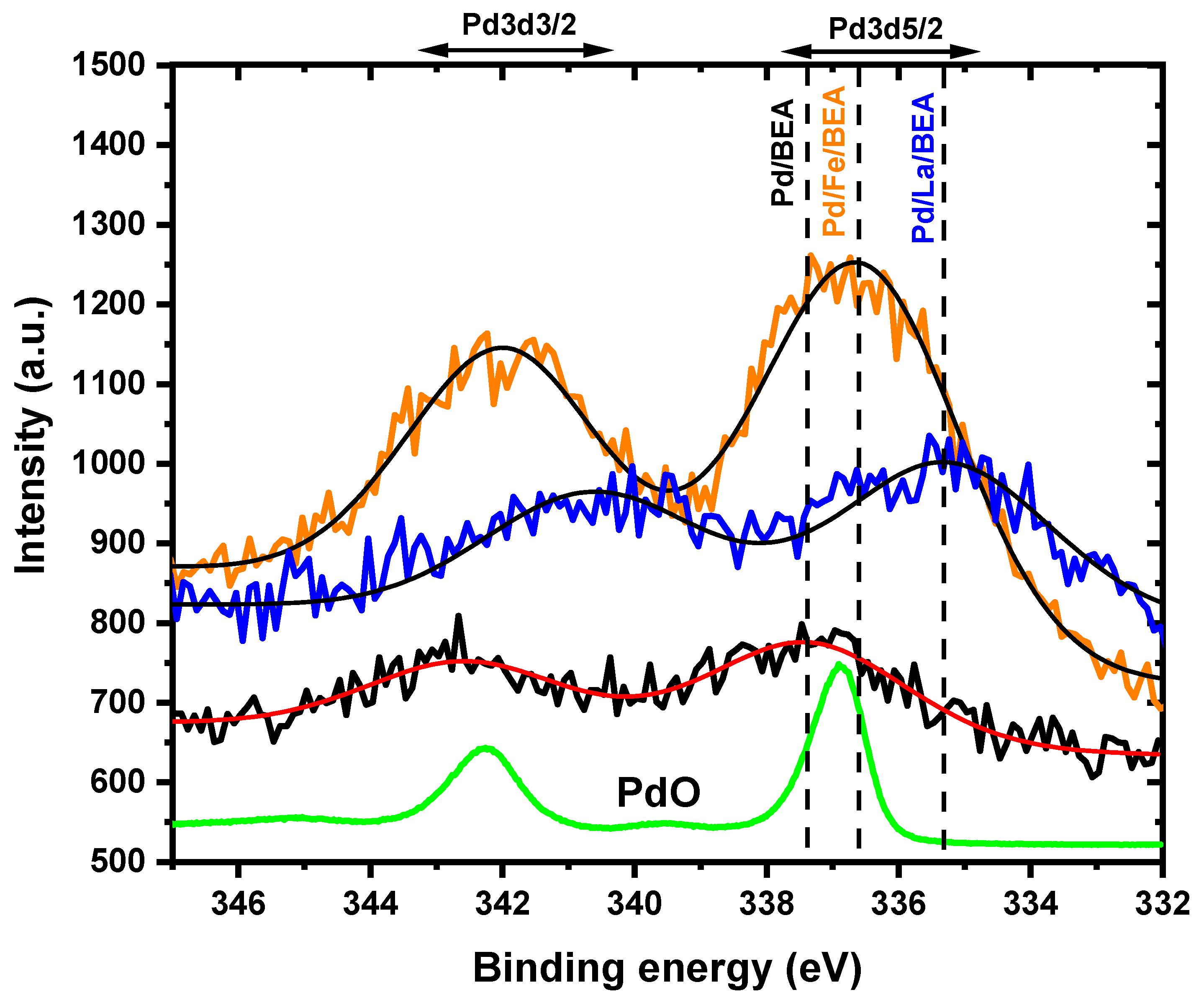
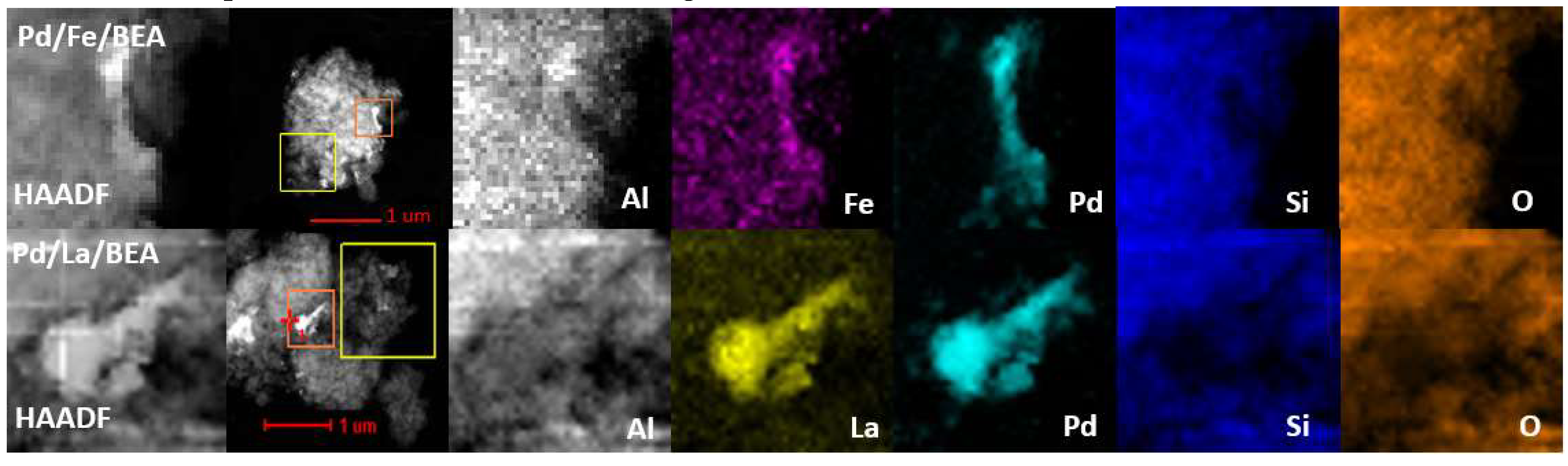
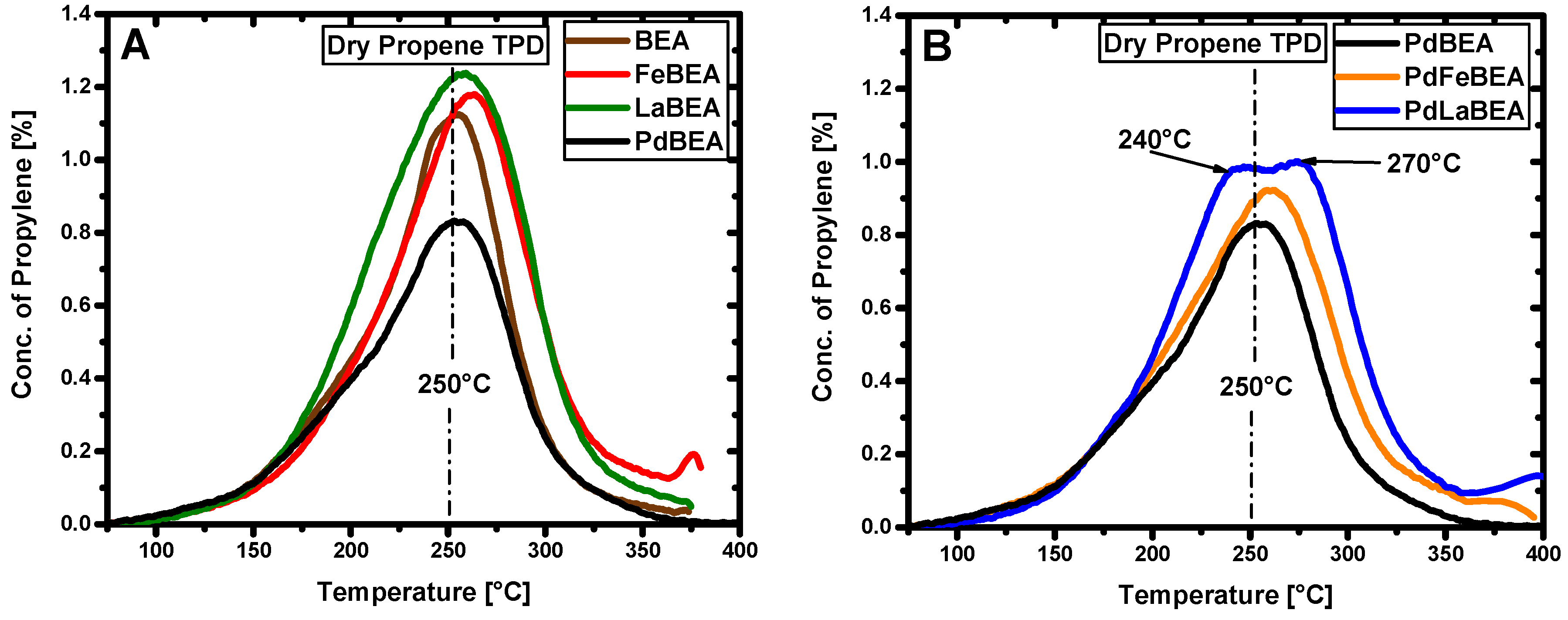




| Fe/BEA | Pd/Fe/BEA | Pd/BEA | Pd/La/BEA | La/BEA | |
|---|---|---|---|---|---|
| Fe (wt-%) | 1.1 | 1.1 | - | - | - |
| La (wt-%) | - | - | - | 2.6 | 2.6 |
| Pd (wt-%) | - | 1.9 | 2.1 | 1.9 | - |
| SiO2/Al2O3 | 21.7 | 23.6 | 22.3 | 22.1 | 23 |
| Pd/Al2 | - | 0.31 | 0.34 | 0.33 | - |
| BEA | FeBEA | LaBEA | PdBEA | PdFeBEA | PdLaBEA | |
|---|---|---|---|---|---|---|
| CO (%) | 0.63 | 0.55 | 1.56 | 0.11 | 0.07 | 0.33 |
| CO2 (%) | 1.07 | 2.52 | 3.15 | 4.19 | 2.42 | 2.68 |
| C3H6 (%) | 98.3 | 96.9 | 95.3 | 95.9 | 97.5 | 70.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jonsson, R.; Woo, J.; Skoglundh, M.; Olsson, L. Zeolite Beta Doped with La, Fe, and Pd as a Hydrocarbon Trap. Catalysts 2020, 10, 173. https://doi.org/10.3390/catal10020173
Jonsson R, Woo J, Skoglundh M, Olsson L. Zeolite Beta Doped with La, Fe, and Pd as a Hydrocarbon Trap. Catalysts. 2020; 10(2):173. https://doi.org/10.3390/catal10020173
Chicago/Turabian StyleJonsson, Rasmus, Jungwon Woo, Magnus Skoglundh, and Louise Olsson. 2020. "Zeolite Beta Doped with La, Fe, and Pd as a Hydrocarbon Trap" Catalysts 10, no. 2: 173. https://doi.org/10.3390/catal10020173





