Direct Hydroxylation of Phenol to Dihydroxybenzenes by H2O2 and Fe-based Metal-Organic Framework Catalyst at Room Temperature
Abstract
:1. Introduction
2. Results and discussion
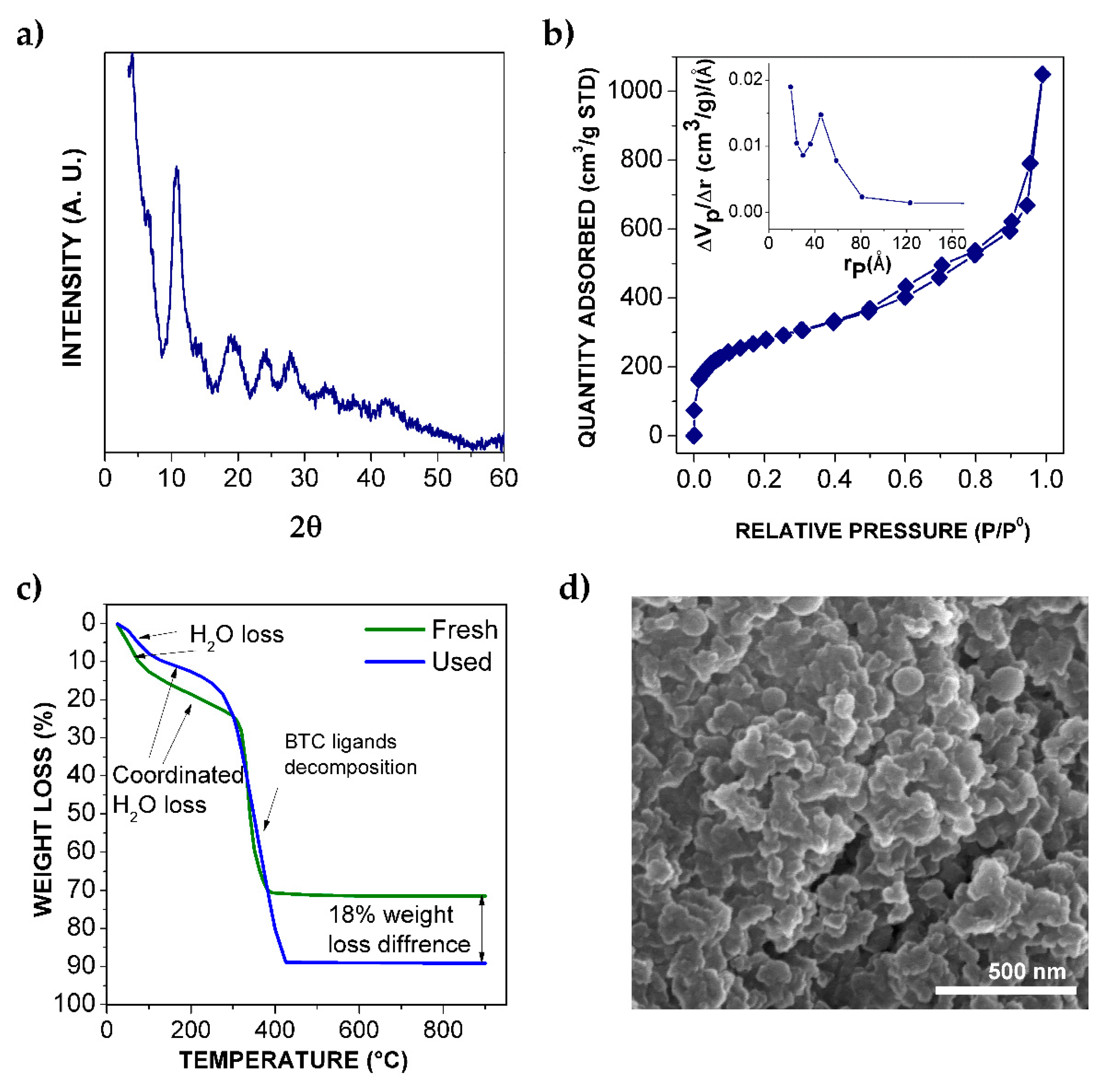
2.1. Catalyst Characterization
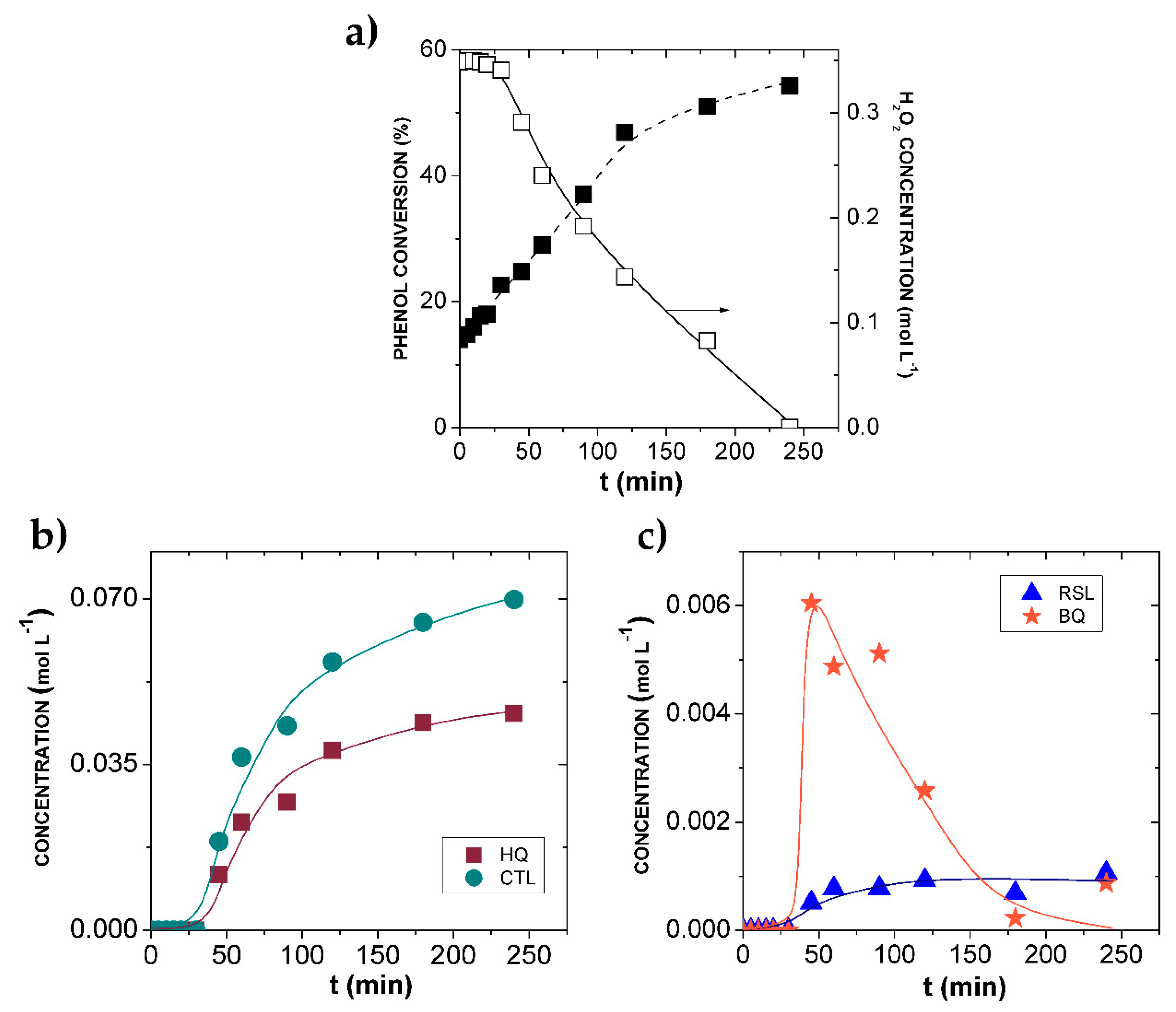
2.2. Hydroxylation of Phenol
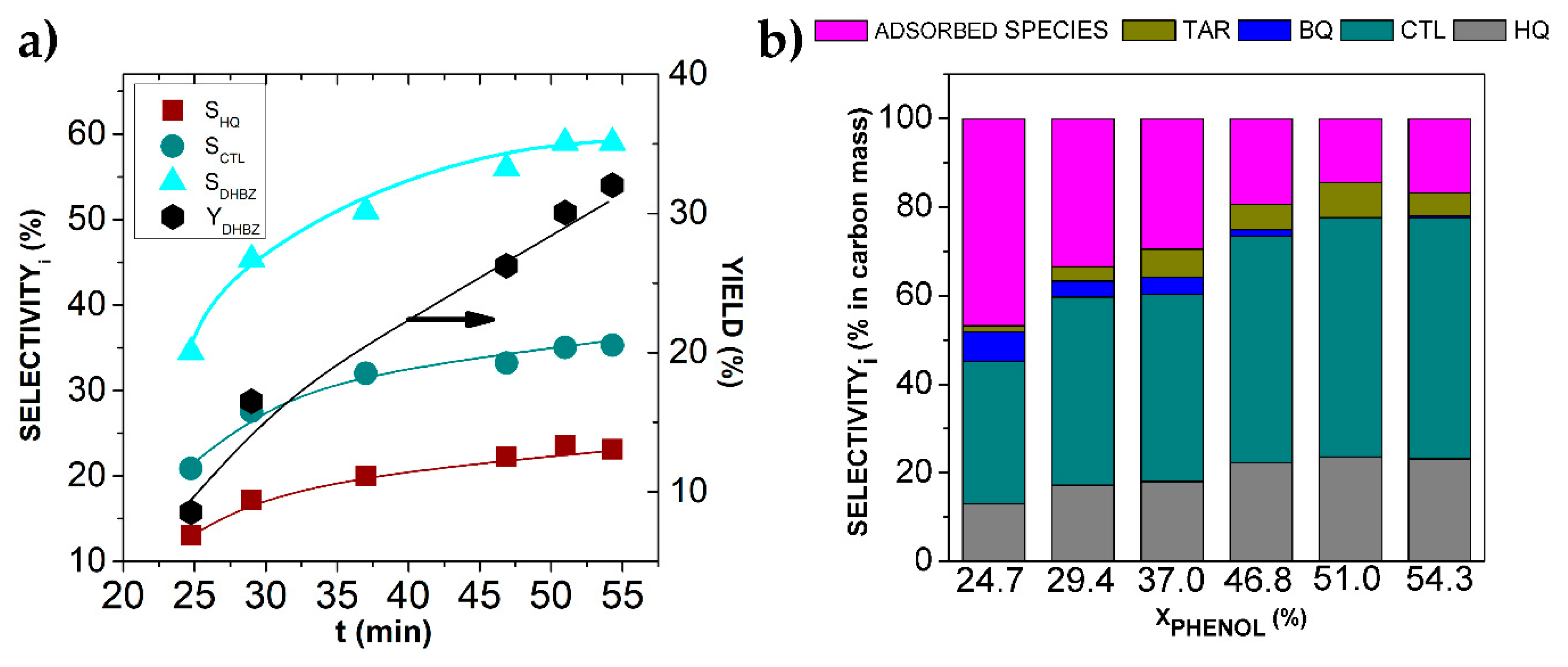
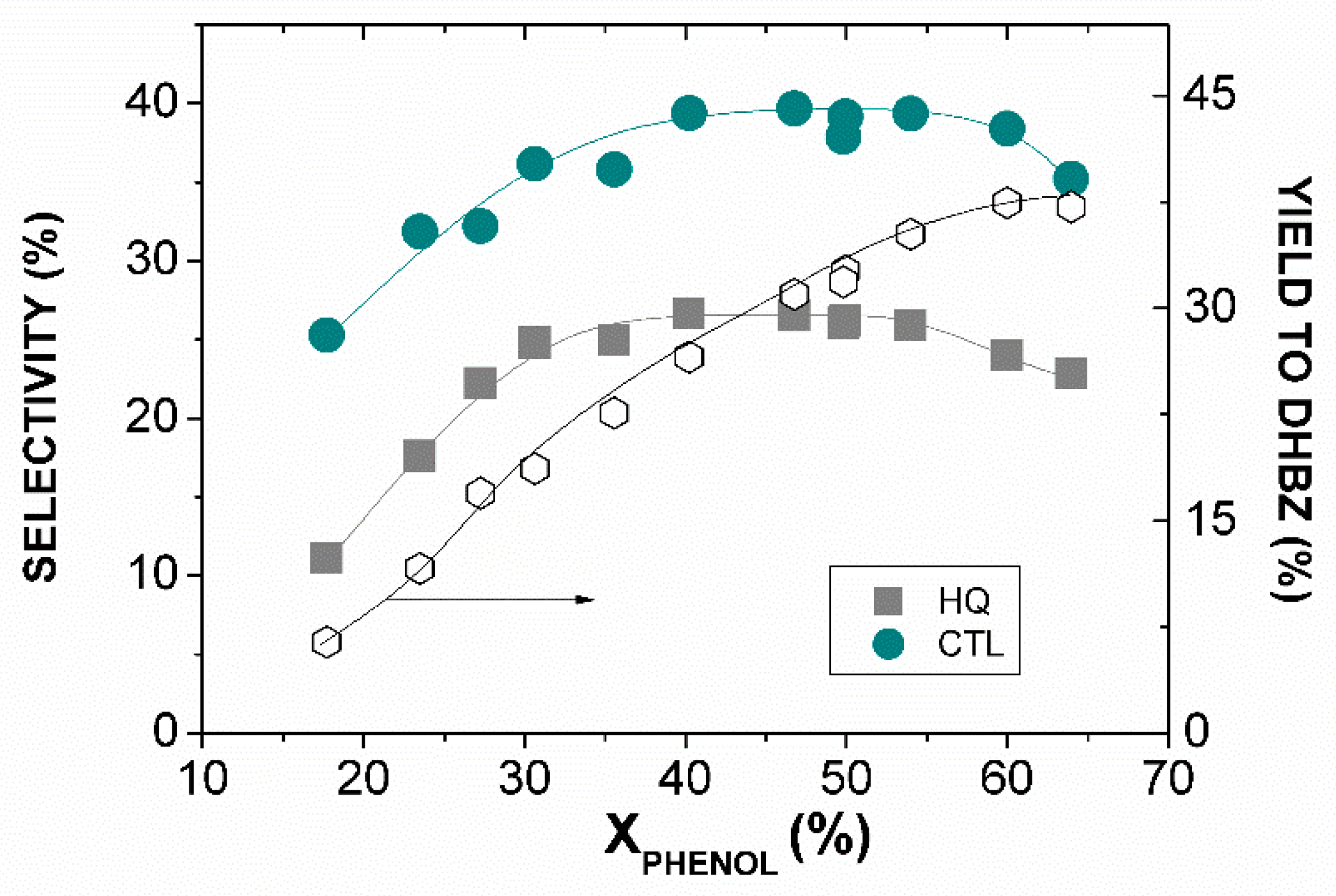
2.2.1. Influence of Operating Conditions
2.2.2. Stability and performance in continuous fixed-bed reactor
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Catalyst
3.3. Characterization of Catalyst
3.4. Hydroxylation Performance
3.5. Analytical Methods
- The conversion (X) of phenol and H2O2,
- The phenol selectivity (S),
- The phenol yield (Y),
- The effective conversion of H2O2 is expressed by,
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Romano, U.; Ricci, M. Industrial Applications. In Liquid Phase Oxidation via Heterogeneous Catalysis: Organic Synthesis and Industrial Applications; Clerici, M.G., Kholdeeva, O.A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 451–506. ISBN 9780470915523. [Google Scholar]
- Karakhanov, E.A.; Maximov, A.L.; Kardasheva, Y.S.; Skorkin, V.A.; Kardashev, S.V.; Ivanova, E.A.; Lurie-Luke, E.; Seeley, J.A.; Cron, S.L. Hydroxylation of Phenol by Hydrogen Peroxide Catalyzed by Copper(II) and Iron(III) Complexes: The Structure of the Ligand and the Selectivity of ortho-Hydroxylation. Ind. Eng. Chem. Res. 2010, 49, 4607–4613. [Google Scholar] [CrossRef]
- Li, S.; Li, G.; Li, G.; Wu, G.; Hu, C. Microporous carbon molecular sieve as a novel catalyst for the hydroxylation of phenol. Microporous Mesoporous Mater. 2011, 143, 22–29. [Google Scholar] [CrossRef]
- Bellussi, G.; Perego, C. Phenol Hydroxylation and Related Oxidations. In Handbook of Heterogeneous Catalysis; Major Reference Works; Wiley-VCH verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; pp. 3538–3547. ISBN 9783527610044. [Google Scholar]
- Perego, C.; Millini, R. Porous materials in catalysis: challenges for mesoporous materials. Chem. Soc. Rev. 2013, 42, 3956–3976. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lu, G.; Guo, Y.; Guo, Y.; Wang, J. Deactivation and regeneration of TS-1/diatomite catalyst for hydroxylation of phenol in fixed-bed reactor. Chem. Eng. J. 2005, 108, 187–192. [Google Scholar] [CrossRef]
- Poza-Nogueiras, V.; Rosales, E.; Pazos, M.; Sanroman, M.A. Current advances and trends in electro-Fenton process using heterogeneous catalysts – A review. Chemosphere 2018, 201, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla, A.; Casas, J.A.; Rodriguez, J. Hydrogen peroxide-promoted-CWAO of phenol with activated carbon. Appl. Catal. B: Environ. 2010, 93, 339–345. [Google Scholar] [CrossRef]
- Kavitha, V.; Palanivelu, K. The role of ferrous ion in Fenton and photo-Fenton processes for the degradation of phenol. Chemosphere 2004, 55, 1235–1243. [Google Scholar] [CrossRef]
- Choi, J.-S.; Yoon, S.-S.; Jang, S.-H.; Ahn, W.-S. Phenol hydroxylation using Fe-MCM-41 catalysts. Catal. Today 2006, 111, 280–287. [Google Scholar] [CrossRef]
- Martínez, F.; Leo, P.; Orcajo, G.; Díaz-García, M.; Sanchez-Sanchez, M.; Calleja, G. Sustainable Fe-BTC catalyst for efficient removal of mehylene blue by advanced fenton oxidation. Catal. Today 2018, 313, 6–11. [Google Scholar] [CrossRef]
- Li, X.; Wang, B.; Cao, Y.; Zhao, S.; Wang, H.; Feng, X.; Zhou, J.; Ma, X. Water Contaminant Elimination Based on Metal–Organic Frameworks and Perspective on Their Industrial Applications. ACS Sustain. Chem. Eng. 2019, 7, 4548–4563. [Google Scholar] [CrossRef]
- Bhattacharjee, S. Hydroxylation of benzene and toluene by heterogeneous iron metal-organic framework (Fe-BTC). Indian J. Chem. -Section A 2018, 57, 778–783. [Google Scholar]
- Zhen, W.; Li, B.; Lu, G.; Ma, J. Enhancing catalytic activity and stability for CO 2 methanation on Ni@MOF-5 via control of active species dispersion. Chem. Commun. 2015, 51, 1728–1731. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Zhao, H.; Cao, T.; Qian, L.; Wang, Y.; Zhao, G. Efficient degradation of high concentration azo-dye wastewater by heterogeneous Fenton process with iron-based metal-organic framework. J. Mol. Catal. A: Chem. 2015, 400, 81–89. [Google Scholar] [CrossRef]
- Gao, Y.; Li, S.; Li, Y.; Yao, L.; Zhang, H. Accelerated photocatalytic degradation of organic pollutant over metal-organic framework MIL-53(Fe) under visible LED light mediated by persulfate. Appl. Catal. B: Environ. 2017, 202, 165–174. [Google Scholar] [CrossRef]
- Tsai, F.-C.; Xia, Y.; Ma, N.; Shi, J.-J.; Jiang, T.; Chiang, T.-C.; Zhang, Z.-C.; Tsen, W.-C. Adsorptive removal of acid orange 7 from aqueous solution with metal–organic framework material, iron (III) trimesate. Desalin. Water Treat. 2016, 57, 3218–3226. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, M.; Li, K.; Zuo, Y.; Han, Y.; Wang, J.; Song, C.; Zhang, G.; Guo, X. Facile synthesis of Fe-containing metal–organic frameworks as highly efficient catalysts for degradation of phenol at neutral pH and ambient temperature. CrystEngComm 2015, 17, 7160–7168. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Alvaro, M.; Horcajada, P.; Gibson, E.; Vishnuvarthan, M.; Vimont, A.; Greneche, J.-M.; Serre, C.; Daturi, M.; Garcia, H. Comparison of Porous Iron Trimesates Basolite F300 and MIL-100(Fe) As Heterogeneous Catalysts for Lewis Acid and Oxidation Reactions: Roles of Structural Defects and Stability. ACS Catal. 2012, 2, 2060–2065. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Li, Z.; Garcia, H. Catalysis and photocatalysis by metal organic frameworks. Chem. Soc. Rev. 2018, 47, 8134–8172. [Google Scholar] [CrossRef]
- Kempahanumakkagari, S.; Vellingiri, K.; Deep, A.; Kwon, E.E.; Bolan, N.; Kim, K.-H. Metal–organic framework composites as electrocatalysts for electrochemical sensing applications. Co-ord. Chem. Rev. 2018, 357, 105–129. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Li, D.-S.; Bu, X.; Feng, P. Metal-Organic Frameworks for Separation. Adv. Mater. 2018, 30, 1705189. [Google Scholar] [CrossRef]
- Li, H.; Wang, K.; Sun, Y.; Lollar, C.T.; Li, J.; Zhou, H.-C. Recent advances in gas storage and separation using metal–organic frameworks. Mater. Today 2018, 21, 108–121. [Google Scholar] [CrossRef]
- Cheng, M.; Lai, C.; Liu, Y.; Zeng, G.; Huang, D.; Zhang, C.; Qin, L.; Hu, L.; Zhou, C.; Xiong, W. Metal-organic frameworks for highly efficient heterogeneous Fenton-like catalysis. Co-ord. Chem. Rev. 2018, 368, 80–92. [Google Scholar] [CrossRef]
- Jian, L.; Chen, C.; Lan, F.; Deng, S.; Xiao, W.; Zhang, N. Catalytic activity of unsaturated coordinated Cu-MOF to the hydroxylation of phenol. Solid State Sci. 2011, 13, 1127–1131. [Google Scholar] [CrossRef]
- Xiang, B.-L.; Fu, L.; Li, Y.; Liu, Y. A New Fe(III)/MOF-5(Ni) Catalyst for Highly Selective Synthesis of Catechol from Phenol and Hydrogen Peroxide. Chem. 2019, 4, 1502–1509. [Google Scholar] [CrossRef]
- Xiang, B.-L.; Fu, L.; Li, Y.; Liu, Y. Preparation of Fe(II)/MOF-5 Catalyst for Highly Selective Catalytic Hydroxylation of Phenol by Equivalent Loading at Room Temperature. J. Chem. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Choi, J.-S.; Yang, S.-T.; Choi, S.B.; Kim, J.; Ahn, W.-S. Solvothermal synthesis of Fe-MOF-74 and its catalytic properties in phenol hydroxylation. J. Nanosci. Nanotechnol. 2010, 10, 135–141. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, M.; De Asua, I.; Ruano, D.; Diaz, K. Direct Synthesis, Structural Features, and Enhanced Catalytic Activity of the Basolite F300-like Semiamorphous Fe-BTC Framework. Cryst. Growth Des. 2015, 15, 4498–4506. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodríguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Dorosti, F.; Alizadehdakhel, A. Chemical Engineering Research and Design Fabrication and investigation of PEBAX / Fe-BTC, a high permeable and CO 2 selective mixed matrix membrane. Chem. Eng. Res. Des. 2017, 136, 119–128. [Google Scholar] [CrossRef]
- Quintanilla, A.; Casas, J.; Miranzo, P.; Osendi, M.; Belmonte, M. 3D-Printed Fe-doped silicon carbide monolithic catalysts for wet peroxide oxidation processes. Appl. Catal. B: Environ. 2018, 235, 246–255. [Google Scholar] [CrossRef]
- Centi, G.; Cavani, F.; Trifirò, F. Selective Oxidation by Heterogeneous Catalysis; Springer Science & Business Media: New York, NY, USA, 2001; ISBN 1461541751. [Google Scholar]
- Liu, H.; Lu, G.; Guo, Y.; Guo, Y.; Wang, J. Chemical kinetics of hydroxylation of phenol catalyzed by TS-1/diatomite in fixed-bed reactor. Chem. Eng. J. 2006, 116, 179–186. [Google Scholar] [CrossRef]
- Gao, C.; Chen, S.; Quan, X.; Yu, H.; Zhang, Y. Enhanced Fenton-like catalysis by iron-based metal organic frameworks for degradation of organic pollutants. J. Catal. 2017, 356, 125–132. [Google Scholar] [CrossRef]
- Eisenberg, G. Colorimetric Determination of Hydrogen Peroxide. Ind. Eng. Chem. Anal. Ed. 1943, 15, 327–328. [Google Scholar] [CrossRef]


| Entry | T (°C) | PHENOL :H2O2 | WCAT (g) | tINDUCTION (min) | CONVERSION (%) | SELECT.(%) | YIELD (%) | Felix (ppm) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| tR (min) | PHENOL | H2O2 | HQ | CTL | HQ+CTL | ||||||
| 1 | 50 | 1:1 | 0.01 | 0 | 10 | 52.3 | 100 | 33.0 | 41.7 | 39.7 | 26 |
| 2 | 25 | 1:1 | 0.01 | 25 | 60 | 51.0 | 68.5 | 20.3 | 37.4 | 29.4 | 17 |
| 3 | 20 | 1:1 | 0.01 | 25 | 60 | 29.4 | 31.4 | 17.4 | 27.5 | 13.2 | 0.4 |
| 4 | 20 | 1:1.8 | 0.01 | 25 | 60 | 37.0 | 14.8 | 18.0 | 29.5 | 17.6 | 1.2 |
| 5 | 20 | 1:0.5 | 0.01 | 25 | 60 | 26.0 | 34.2 | 25.0 | 32.7 | 15.0 | 0 |
| 6 | 20 | 1:1.8 | 0.01 | 25 | 180 | 61.7 | 55.0 | 24.7 | 36.7 | 37.9 | 10 |
| 7 | 20 | 1:1 | 0.01 | 25 | 180 | 50.7 | 78.6 | 23.6 | 35.0 | 29.7 | 1.6 |
| 8 | 20 | 1:0.5 | 0.01 | 25 | 180 | 37.8 | 93.9 | 25.2 | 39.0 | 24.3 | 2.0 |
| 9 | 20 | 1:1 | 0.02 | 20 | 60 | 34.1 | 46.2 | 30.2 | 44.2 | 25.4 | 2.0 |
| 10 | 20 | 1:1 | 0.05 | 15 | 60 | 52.2 | 95.2 | 35.0 | 44.3 | 41.4 | 24 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar-Aguilar, A.D.; Vega, G.; Casas, J.A.; Vega-Díaz, S.M.; Tristan, F.; Meneses-Rodríguez, D.; Belmonte, M.; Quintanilla, A. Direct Hydroxylation of Phenol to Dihydroxybenzenes by H2O2 and Fe-based Metal-Organic Framework Catalyst at Room Temperature. Catalysts 2020, 10, 172. https://doi.org/10.3390/catal10020172
Salazar-Aguilar AD, Vega G, Casas JA, Vega-Díaz SM, Tristan F, Meneses-Rodríguez D, Belmonte M, Quintanilla A. Direct Hydroxylation of Phenol to Dihydroxybenzenes by H2O2 and Fe-based Metal-Organic Framework Catalyst at Room Temperature. Catalysts. 2020; 10(2):172. https://doi.org/10.3390/catal10020172
Chicago/Turabian StyleSalazar-Aguilar, Alma D., Gonzalo Vega, Jose A. Casas, Sofía Magdalena Vega-Díaz, Ferdinando Tristan, David Meneses-Rodríguez, Manuel Belmonte, and Asunción Quintanilla. 2020. "Direct Hydroxylation of Phenol to Dihydroxybenzenes by H2O2 and Fe-based Metal-Organic Framework Catalyst at Room Temperature" Catalysts 10, no. 2: 172. https://doi.org/10.3390/catal10020172
APA StyleSalazar-Aguilar, A. D., Vega, G., Casas, J. A., Vega-Díaz, S. M., Tristan, F., Meneses-Rodríguez, D., Belmonte, M., & Quintanilla, A. (2020). Direct Hydroxylation of Phenol to Dihydroxybenzenes by H2O2 and Fe-based Metal-Organic Framework Catalyst at Room Temperature. Catalysts, 10(2), 172. https://doi.org/10.3390/catal10020172








