Encapsulation of HRP Enzyme onto a Magnetic Fe3O4 Np–PMMA Film via Casting with Sustainable Biocatalytic Activity
Abstract
:1. Introduction
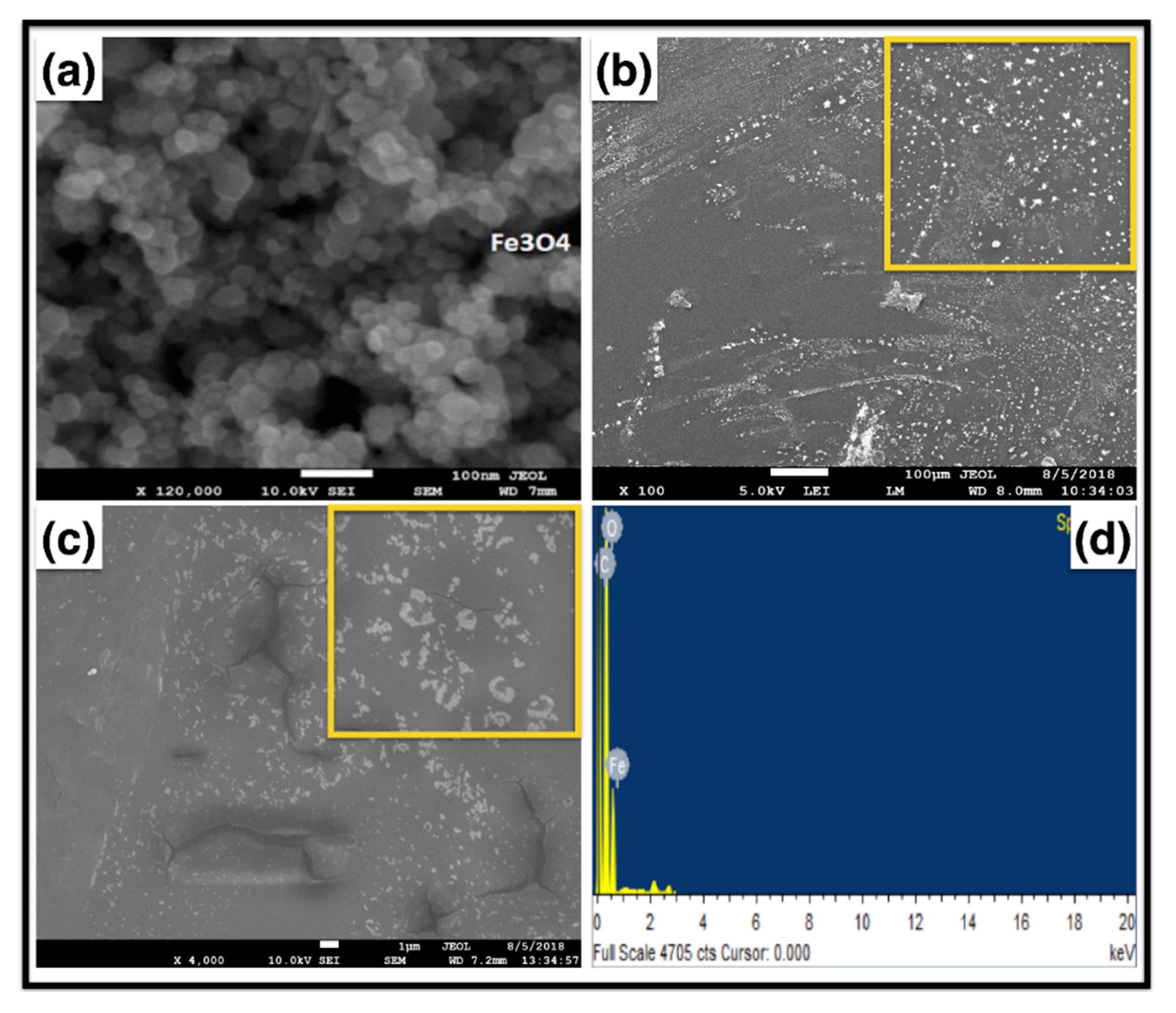
2. Results and Discussion
3. Experimental Section
3.1. Materials
3.2. Peroxidase Assay
3.3. Procedure for Immobilization
3.4. Characterization
3.5. Reusability of Immobilized Enzymes
3.6. Physicochemical Characterization of the Enzyme
3.6.1. Optimal Temperature and pH
3.6.2. Thermal Stability
3.6.3. Kinetic Constant
3.6.4. Substrate specificity
3.6.5. Effects of Metal Ions
3.6.6. Effects of Different Organic Compounds
3.6.7. Phenol Removal Determination
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B.D. Immobilization strategies to develop enzymatic vbiosensors. Biotechnol. Adv. 2012, 30, 489–511. [Google Scholar] [CrossRef]
- Asgher, M.; Shahid, M.; Kamal, S.; Iqbal, H.M.N. Recent trends and valorization of immobilization strategies and ligninolytic enzymes by industrial biotechnology. J. Mol. Cataly. B-Enzym. 2014, 101, 56–66. [Google Scholar] [CrossRef]
- Klebanoff, S.J. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J. Bacteriol. 1968, 95, 2131–2138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamocky, M.; Obinger, C. Molecular phylogeny of heme peroxidases. In Biocatalysis Based on Heme Peroxidases; Torres, E., Ayala, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 7–35. [Google Scholar]
- Hammel, K.E.; Kalyanaraman, B.; Kirk, T.K. Oxidation of polycyclic aromatic hydrocarbons and dibenzo [p]-dioxins by Phanerochaete chrysosporium ligninase. J. Biol. Chem. 1986, 261, 16948–16952. [Google Scholar] [PubMed]
- Klibanov, A.M.; Tu, T.M.; Scott, K.P. Peroxidase-catalyzed removal of phenols from coal-conversion waste waters. Science 1983, 221, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Aitken, M.D. Waste treatment applications of enzymes: opportunities and obstacles. Chem. Eng. J. 1993, 52, 49–58. [Google Scholar] [CrossRef]
- Patel, M.; Day, B.J. Metalloporphyrin class of therapeutic catalytic antioxidants. Trends Pharmacol. Sci. 1999, 20, 359–364. [Google Scholar] [CrossRef]
- Ingram, D.T.; Lamichhane, C.M.; Rollins, D.M.; Carr, L.E.; Mallinson, E.T.; Joseph, S.W. Development of a colony lift immunoassay to facilitate rapid detection and quantification of Escherichia coli O157: H7 from agar plates and filter monitor membranes. Clin. Diagn. Lab. Immunol. 1998, 5, 567–573. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, G.; Wang, Z.; Wang, Y.; Huang, R. Purification and characterization of peroxidase from zucchini (Cucurbita pepo L.). J. Food Process. Preserv. 2019, 43, e13977. [Google Scholar] [CrossRef]
- Liu, W.; Kumar, J.; Tripathy, S.; Senecal, K.J.; Samuelson, L. Enzymatically synthesized conducting polyaniline. J. Amer. Chem. Soc. 1999, 121, 71–78. [Google Scholar] [CrossRef]
- Gaspar, S.; Popescu, I.C.; Gazaryan, I.G.; Bautista, A.G.; Sakharov, I.Y.; Mattiasson, B.; Csöregi, E. Biosensors based on novel plant peroxidases: A comparative study. Electrochim. Acta 2000, 46, 255–264. [Google Scholar] [CrossRef]
- Valderrama, B.; Ayala, M.; Vazquez-Duhalt, R. Suicide inactivation of peroxidases and the challenge of engineering more robust enzymes. Chem. Biol. 2002, 9, 555–565. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X.; et al. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.W.; Zeng, G.M.; Gong, J.L.; Liang, J.; Xu, P.; Zhang, C.; Huang, B.B. Impact of humic/fulvic acid on the removal of heavy metals from aqueous solutions using nanomaterials: A review. Sci. Environ. 2014, 468, 1014–1027. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jia, S.; Wu, Q.; Ran, J.; Zhang, W.; Wu, S. Studies of Fe3O4-chitosan nanoparticles prepared by co-precipitation under the magnetic field for lipase immobilization. Catal. Commun. 2011, 12, 717–720. [Google Scholar] [CrossRef]
- Gao, J.; Gu, H.; Xu, B. Multifunctional magnetic nanoparticles: Design, synthesis, and biomedical applications. Acc. Chem. Res. 2009, 42, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, N.; Akgöl, S.; Arısoy, M.; Denizli, A. Reversible adsorption of lipase on novel hydrophobic nanospheres. Sep. Purif. Technol. 2007, 58, 83–90. [Google Scholar] [CrossRef]
- Almulaiky, Y.Q.; Al-Harbi, S.A. A novel peroxidase from Arabian balsam (Commiphora gileadensis) stems: Its purification, characterization and immobilization on a carboxymethylcellulose/Fe3O4 magnetic hybrid material. Int. J. Boil. Macromol. 2019, 133, 767–774. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Al-Harbi, M.H.; Almulaiky, Y.Q.; Ibrahim, I.H.; El-Shishtawy, R.M. Immobilization of horseradish peroxidase on Fe3O4 magnetic nanoparticles. Electron. J. Biotechnol. 2017, 27, 84–90. [Google Scholar] [CrossRef]
- Jun, L.Y.; Mubarak, N.M.; Yon, L.S.; Bing, C.H.; Khalid, M.; Jagadish, P.; Abdullah, E.C. Immobilization of peroxidase on functionalized MWCNTs-buckypaper/polyvinyl alcohol nanocomposite membrane. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, S.A.; Al-Ghamdi, S.S.; El-Shishtawy, R.M. Immobilization of horseradish peroxidase on amidoximated acrylic polymer activated by cyanuric chloride. Int. J. Boil. Macromol. 2016, 91, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.; Al-Harbi, M.H.; Almulaiky, Y.Q.; Ibrahim, I.H.; Salah, H.A.; El-Badry, M.O.; El-Shishtawy, R.M. Immobilization of Trichoderma harzianum α-amylase on PPyAgNp/Fe3O4-nanocomposite: Chemical and physical properties. Artif. Cell Nanomed. Biotechnol. 2018, 46, 201–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, P.; Xu, Z.K.; Wu, J.; Innocent, C.; Seta, P. Nanofibrous poly (acrylonitrile-co-maleic acid) membranes functionalized with gelatin and chitosan for lipase immobilization. Biomaterials 2006, 27, 4169–4176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cai, X. Immobilization of horseradish peroxidase on Fe3O4/nanotubes composites for Biocatalysis-degradation of phenol. Compos. Interfaces 2019, 26, 379–396. [Google Scholar] [CrossRef]
- Yu, B.; Cheng, H.; Zhuang, W.; Zhu, C.; Wu, J.; Niu, H.; Ying, H. Stability and repeatability improvement of horseradish peroxidase by immobilization on amino-functionalized bacterial cellulose. Process Biochem. 2019, 79, 40–48. [Google Scholar] [CrossRef]
- Darwesh, O.M.; Matter, I.A.; Eida, M.F. Development of peroxidase enzyme immobilized magnetic nanoparticles for bioremediation of textile wastewater dye. J. Environ. Chem. Eng. 2019, 7, 102805. [Google Scholar] [CrossRef]
- Sahin, F.; Demirel, G.; Tümtürk, H. A novel matrix for the immobilization of acetylcholinesterase. Int. J. Biol. Macromol. 2005, 37, 148–153. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Akgol, S.; Bulut, A.; Denizli, A.; Arica, M.Y. Covalent immobilization of invertase onto a reactive film composed of 2-hydroxyethylmethacrylate and glycidylmethacrylate: Properties and application in a continuous flow system. Biochem. Eng. J. 2003, 14, 117–126. [Google Scholar] [CrossRef]
- Yahsi, A.; Sahin, F.; Demirel, G.; Tumturk, H. Binary immobilization of tyrosinase by using alginate gel beads and poly(acrylamide-co-acrylic acid) hydrogels. Int. J. Biol. Macromol. 2005, 36, 253–258. [Google Scholar] [CrossRef]
- Kahraman, M.V.; Bayramoglu, G.; Kayaman-Apohan, N.; Gungor, A. UV curable methacrylated/fumaric acid modified epoxy as a potential support for enzyme immobilization. React. Funct. Polym. 2007, 67, 97–103. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Darwish, A.A.; El-Shishtawy, R.M. Immobilization of horseradish peroxidase on activated wool. Process Biochem. 2013, 48, 649–655. [Google Scholar] [CrossRef]
- Arica, M.Y.; Alaeddinoğlu, N.G.; Hasirci, V. Immobilization of glucoamylase onto activated pHEMA/EGDMA microspheres: Properties and application to a packed-bed reactor. Enzyme Microb. Technol. 1998, 22, 152–157. [Google Scholar] [CrossRef]
- Erginer, R.; Toppare, L.; Alkan, S.; Bakir, U. Immobilization of invertase in functionalized copolymermatrices. React. Funct. Polym. 2000, 45, 227–233. [Google Scholar] [CrossRef]
- Monier, M.; Ayad, D.M.; Wei, Y.; Sarhan, A.A. Immobilization of horseradish peroxidase on modified chitosan beads. Int. J. Biol. Macromol. 2010, 46, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Emregul, E.; Sungur, S.; Akbulut, U. Polyacrylamide-gelatine carrier system used for invertase immobilization. Food Chem. 2006, 97, 591–597. [Google Scholar] [CrossRef]
- Martinek, K.; Kilbanov, A.M.; Goldmacher, V.S.; Berezin, I.V. The principles of enzyme stabilization. Biochim. Biophys. Acta. 1977, 485, 1–12. [Google Scholar] [CrossRef]
- Lobarzewsk, J.; Brzyska, M.; Wojcik, A. The influence of metal ions on the soluble and immobilized cytoplasmic cabbage peroxidase activity and its kinetics. J. Mol. Catal. 1990, 59, 373–383. [Google Scholar] [CrossRef]
- Ashraf, H.; Husain, Q. Stabilization of DEAE cellulose adsorbed and glutaraldehyde crosslinked white radish (Raphanus sativus) peroxidase. J. Sci. Ind. Res. 2010, 69, 613–620. [Google Scholar]
- Tanford, C. Theoretical models for the mechanismof denaturation. Adv. Protein Chem. 1970, 24, 1–95. [Google Scholar]
- Mohamed, S.A.; Aly, A.S.; Mohamed, T.M.; Salah, H.A. Immobilization of horseradish peroxidase on non-woven polyester fabric coated with chitosan. Appl. Biochem. Biotechnol. 2008, 144, 169–179. [Google Scholar] [CrossRef]
- Kulshrestha, Y.; Husain, Q. Direct immobilization of peroxidase on DEAE cellulose from ammonium sulphate fractionated proteins of bitter gourd (Momordica charantia). Enzyme Microb. Technol. 2006, 38, 470–477. [Google Scholar] [CrossRef]
- Jamal, F.; Qidwai, T.; Singh, D.; Pandey, P.K. Biocatalytic activity of immobilized poited gourd (Trichosanthes dioica) peroxidase-concanavalin. A complex on calcium alginate pectin gel. J. Mol. Catal. B Enzym. 2012, 74, 125–131. [Google Scholar] [CrossRef]
- Bayramoğlu, G.; Arıca, M.Y. Enzymatic removal of phenol and p-chlorophenol in enzyme reactor: horseradish peroxidase immobilized on magnetic beads. J. Hazard. Mater. 2008, 156, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.; Abulnaja, K.O.; Ads, A.S.; Khan, J.A.; Kumosani, T.A. Characterization of an anionic peroxidase from horseradish cv. Balady. Food Chem. 2011, 128, 725–730. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Jiang, T.J. Horseradish peroxidase. In Handbook of Food Enzymology; Whitaker, J.R., Voragen, A., Wong, D.W.S., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2003; pp. 403–411. [Google Scholar]
- Bhunia, A.; Durant, S.; Wangikar, P.P. Horseradish peroxidase catalyzed degradation of industrially important dyes. Biotechnol. Bioeng. 2001, 72, 562–567. [Google Scholar] [CrossRef]
- Gomez, J.L.; Bodalo, A.; Gomez, E.; Bastida, J.; Hidalgo, A.M.; Gomez, M. Immobilization of peroxidases on glass beads: An improved alternative for phenol removal. Enzyme Microb. Technol. 2006, 39, 1016–1022. [Google Scholar] [CrossRef]







| Substrate | Relative Activity % | |
|---|---|---|
| Free HRP | HRP–Immobilized on 0.5% Fe3O4Np–PMMA | |
| o-Dianisidine | 40 ± 1.55 | 87 ± 1.64 |
| o-Phenylenediamine | 73 ± 1.12 | 98 ± 1.71 |
| Pyrogallol | 18 ± 0.54 | 30 ± 0.22 |
| p-Aminoantipyrine | 31 ± 0.71 | 64 ± 0.95 |
| ABTS | 7 ± 0.13 | 22 ± 0.50 |
| Parameter | Free HRP | HRP Immobilized on 0.5% Fe3O4Np–PMMA | ||
|---|---|---|---|---|
| Guaiacol | H2O2 | Guaiacol | H2O2 | |
| Km (mM) | 30 | 4.4 | 41 | 5.84 |
| Vmax (units/mL) | 1.10 | 1.76 | 0.89 | 0.66 |
| Km / Vmax | 27.27 | 2.5 | 46 | 8.84 |
| Metal ion (5mM) | Relative Activity % | |
|---|---|---|
| Free HRP | HRP–Immobilized on 0.5% Fe3O4Np–PMMA | |
| Control | 100 ±1.24 | 100 ± 1.56 |
| Fe3+ | 92 ± 1.15 | 175 ± 1.83 |
| Cu2+ | 89 ± 1.51 | 119 ± 1.79 |
| Cd2+ | 26 ± 0.56 | 83 ± 1.21 |
| Co2+ | 75 ± 1.37 | 88 ± 0.89 |
| Ni2+ | 57 ± 0.32 | 64 ± 1.13 |
| Hg2+ | 11 ± 0.18 | 42 ± 0.54 |
| Zn2+ | 3 ± 0.12 | 24 ± 0.47 |
| Pb2+ | 31 ± 0.22 | 55 ± 0.34 |
| Chemical | Relative Activity% | ||
|---|---|---|---|
| Concentration | Free HRP | HRP-Immobilized on 0.5% Fe3O4Np–PMMA | |
| Control | - | 100 ± 1.89 | 100 ± 1.95 |
| Triton X-100 | 5% | 64 ± 1.32 | 82 ± 1.61 |
| 10% | 30 ± 0.86 | 78 ± 1.59 | |
| Isopropanol | 5% | 78 ± 1.47 | 87 ± 1.59 |
| 10% | 63 ± 1.42 | 76 ± 1.48 | |
| Urea | 2 M | 64 ±1.59 | 89 ± 1.52 |
| 4 M | 28 ± 1.74 | 58 ± 1.74 | |
| Parameter | HRP-Immobilized on 0.5% Fe3O4 Np–PMMA | HRP-Immobilized on Non-Modified Fe3O4Np |
|---|---|---|
| Number of times reused, remaining activity (78.5%) | 10 | ~5 |
| Effect of metals | Highly resistant | Highly resistant |
| Affinity for guaiacol (km) | 41 | 45 |
| Affinity for H2O2 (km) | 5.84 | 7 |
| Effect of urea (4 mM) | 58 | 33 |
| Effect of isopropanol (10%) | 76 | 75 |
| Effect of Triton X-100 (10%) | 78 | 77 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdulaal, W.H.; Almulaiky, Y.Q.; El-Shishtawy, R.M. Encapsulation of HRP Enzyme onto a Magnetic Fe3O4 Np–PMMA Film via Casting with Sustainable Biocatalytic Activity. Catalysts 2020, 10, 181. https://doi.org/10.3390/catal10020181
Abdulaal WH, Almulaiky YQ, El-Shishtawy RM. Encapsulation of HRP Enzyme onto a Magnetic Fe3O4 Np–PMMA Film via Casting with Sustainable Biocatalytic Activity. Catalysts. 2020; 10(2):181. https://doi.org/10.3390/catal10020181
Chicago/Turabian StyleAbdulaal, Wesam H., Yaaser Q. Almulaiky, and Reda M. El-Shishtawy. 2020. "Encapsulation of HRP Enzyme onto a Magnetic Fe3O4 Np–PMMA Film via Casting with Sustainable Biocatalytic Activity" Catalysts 10, no. 2: 181. https://doi.org/10.3390/catal10020181
APA StyleAbdulaal, W. H., Almulaiky, Y. Q., & El-Shishtawy, R. M. (2020). Encapsulation of HRP Enzyme onto a Magnetic Fe3O4 Np–PMMA Film via Casting with Sustainable Biocatalytic Activity. Catalysts, 10(2), 181. https://doi.org/10.3390/catal10020181






