Friedel–Crafts-Type Alkylation of Indoles in Water Using Amphiphilic Resin-Supported 1,10-Phenanthroline–Palladium Complex under Aerobic Conditions
Abstract
1. Introduction
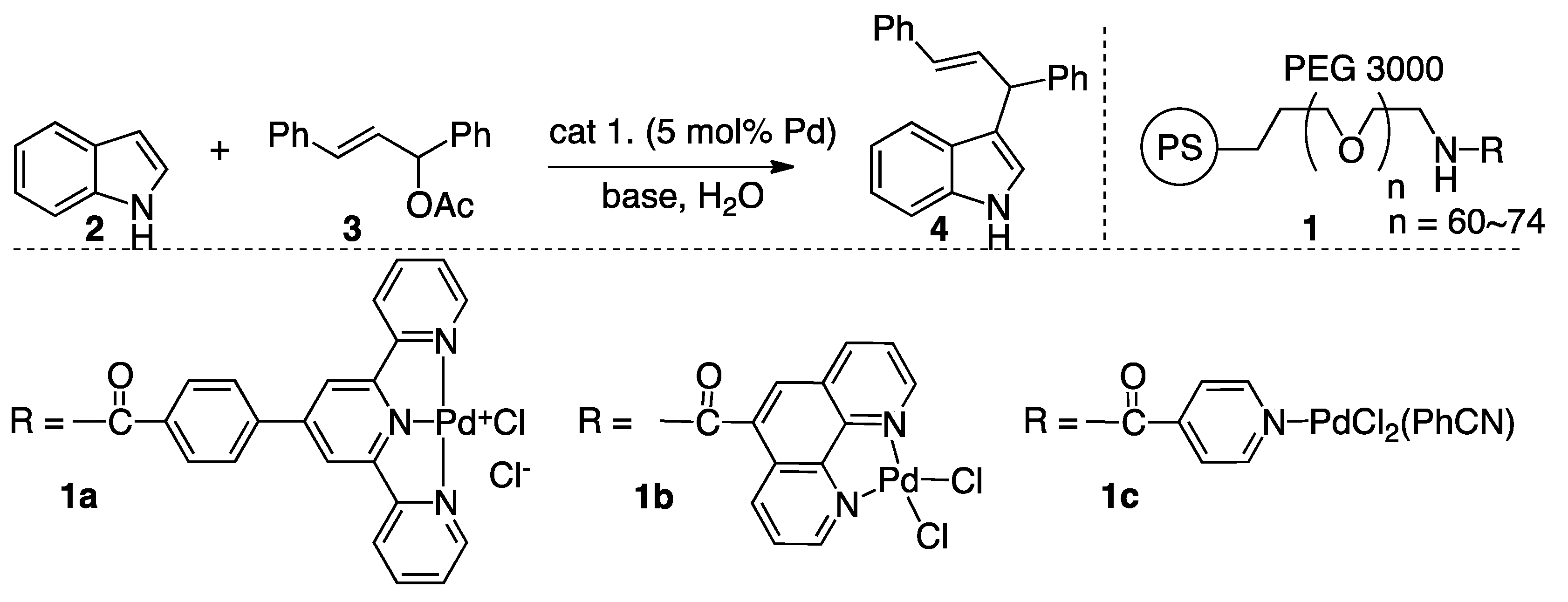
2. Results
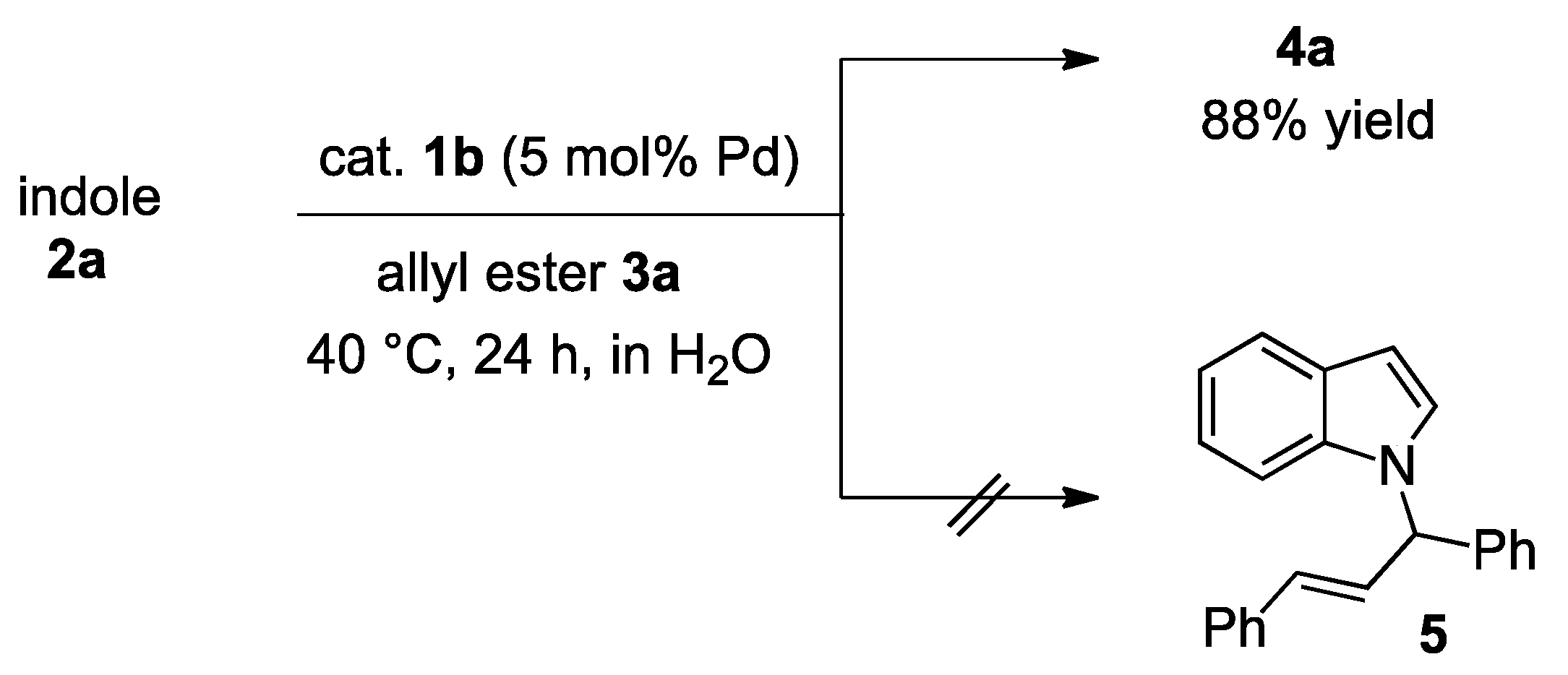
Alkylation Reaction
3. Materials and Methods
3.1. General Methods
3.2. Materials
3.3. Synthesis of Polymer-Supported Ligand
3.4. Preparation of PS–PEG Resin-Supported Phenanthroline–Palladium Complex 1b
3.5. Palladium-Catalyzed Friedel-Crafts-Type Alkylation of Indoles with Allyl Esters
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Calloway, N.O. The Friedel-Crafts Synthesis. Chem. Rev. 1935, 17, 327–392. [Google Scholar] [CrossRef]
- Friedel, C.; Crafts, J.M. A new general synthetical method of producing hydrocarbons. Hebd. Seances Acad. Sci. 1877, 84, 1392–1395. [Google Scholar]
- Ohal, G.A.; Krishnamurti, R.; Prakash, G.K.S. Comprehensive Organic Synthesis; Trost, B.M., Prakash, G.K.S., Eds.; Pergamon: Oxford, UK, 1991; Volume 3, p. 293. [Google Scholar]
- Bandini, M.; Melloni, A.; Umani-Ronchi, A. New catalytic approaches in the stereoselectitive Friedel-Crafts alkylation reaction. Angew. Chem. Int. Ed. 2004, 43, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Van Vranken, D.L. Asynmetric transition metal-catalyzed allylic alkylations. Chem. Rev. 1996, 96, 395–422. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M. On Inventing Reaction for Atom Economy. Acc. Chem. Res. 2002, 35, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Crawly, M.L. Asymmetric Transition-metal-catalyzed allylic alkylations: Applications in total synthesis. Chem. Rev. 2003, 103, 2921–2944. [Google Scholar] [CrossRef]
- Malkov, A.V.; Davis, S.L.; Baxendale, I.R.; Mitchell, W.L.; Kočovský, P. Molybdenum(II)-catalyzed allylation of electron-rich aromatics and heteroaromatics. J. Org. Chem. 1999, 64, 2751–2764. [Google Scholar] [CrossRef]
- Bandini, M.; Melloni, A.; Umani-Ronchi, A. New versatile Pd-catalyzed alkylation of indoles via nucleophilic allylic substitution: Controlling the regioselectivity. Org. Lett. 2004, 6, 3199–3202. [Google Scholar] [CrossRef]
- Bandini, M.; Melloni, A.; Piccinelli, F.; Sinisi, R.; Tommasi, S.; Umani-Ronchi, A. Highly enantioselective synthesis of tetrahydro-ß-carbolines and tetrahydro-γ-carbolines via Pd-catalyzed intramolecular allylic alkylation. J. Am. Chem. Soc. 2006, 128, 1424–1425. [Google Scholar] [CrossRef]
- Ma, S.; Yu, S.; Peng, Z.; Guo, H. Palladium-catalyzed functionalization of indoles with 2-acetoxymethyl-substitued electron-deficient alkenes. J. Org. Chem. 2006, 71, 9865–9868. [Google Scholar] [CrossRef]
- Cheung, H.Y.; Yu, W.-Y.; Lam, F.L.; Au-Yeung, T.T.-L.; Zhou, Z.; Chan, T.H.; Chan, A.S.C. Enantioselective Pd-catalyzed allylic alkylation of indoles by a new class of chiral ferrocenyl P/S ligands. Org. Lett. 2007, 9, 4295–4298. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Futamata, M.; Mukai, R.; Tamaru, Y. Palladium-catalyzed enantioselective C-3 allylation of 3-substituted-1H-indoles using trialkylboranes. J. Am. Chem. Soc. 2006, 128, 6314–6315. [Google Scholar]
- Cao, Z.; Liu, Y.; Liu, Z.; Feng, X.; Zhuang, M.; Du, H. Pd-catalyzed asymmetric allylic alkylation of indoles and pyrroles by chiral alkene-phosphine ligands. Org. Lett. 2011, 13, 2164–2167. [Google Scholar] [CrossRef] [PubMed]
- Trillo, P.; Baeza, A.; Nájera, C. Fluorinated alcohols as promoters for the metal-free direct substitution reaction of allylic alcohols with nitrogenated, silylated, and carbon nucleophiles. J. Org. Chem. 2012, 77, 7344–7354. [Google Scholar] [CrossRef]
- Peng, B.J.; Hsueh, W.T.; Fülöp, F.; Yang, S.C. Platinum-catalyzed selective N-allylation of 2,3-disubstitued indoles with allylic acetates in water. New. J. Chem. 2019, 43, 58–62. [Google Scholar] [CrossRef]
- Peng, B.J.; Huang, Y.T.; Fülöp, F.; Lin, I.L.; Yang, S.C. Palladium-catalyzed selective N-allylation of indoles assiseted by PEG-water system. New J. Chem. 2019, 29, 11549–11553. [Google Scholar] [CrossRef]
- Suzuka, T.; Okada, Y.; Ooshiro, K.; Uozumi, Y. Copper-free Sonogashira coupling in water with an amphiphilic resin-supported palldium complex. Tetrahedron 2010, 66, 1064–1069. [Google Scholar] [CrossRef]
- Suzuka, T.; Kawahara, Y.; Ooshiro, K.; Nagamine, T.; Ogihara, K.; Higa, M. Reusable polymer-Ssupported 2,2′-biarylpyridine–copper complexes for Huisgen [3+2] cycloaddition in water. Heterocycles 2012, 3, 615–626. [Google Scholar] [CrossRef]
- Suzuka, T.; Nagamine, T.; Ogihara, K.; Higa, M. Suzuki-Miyaura Cross coupling reaction in water with polymer-supported terpyridine palladium complex under aerobic conditions. Catal. Lett. 2010, 139, 85–89. [Google Scholar] [CrossRef]
- Suzuka, T.; Nagamine, T.; Ogihara, K.; Higa, M. Mizoroki-Heck Reaction in water with polymer-supported terpyridine palladium complex under aerobic conditions. Trans. Mater. Res. Soc. Jpn. 2010, 35, 889–892. [Google Scholar] [CrossRef][Green Version]
- Suzuka, T.; Kimura, K.; Nagamine, T. Reusable polymer-supported terpyridine palladium complex for the Suzuki-Miyaura, Mizoroki-Heck, Sonogashira, Tsuji-Trost coupling reaction in Water. Polymers 2011, 3, 621–639. [Google Scholar] [CrossRef]
- Suzuka, T.; Adachi, M.; Yang, Z.-S.; Ogihara, K.; Higa, M. Suzuki-Miyaura cross coupling reaction in water with polymer-supported terpyridine palladium complex and application for the synthesis of 2,6-disubstitued pyrimidines. Trans. Mater. Res. Soc. Jpn. 2013, 38, 119–122. [Google Scholar] [CrossRef]
- Suzuka, T.; Adachi, M.; Nakamoto, Y.; Ogihara, K. Use of polymer-supported terpyridine—Palladium complex for Mizoroki-Heck reaction in water under aerobic conditions. Trans. Mater. Res. Soc. Jpn. 2015, 40, 77–80. [Google Scholar] [CrossRef]
- Suzuka, T.; Adachi, M.; Ogihara, K. Sonogashira coupling reaction in water with polymer-supported terpyridine-palladium complex under aerobic conditions. Trans. Mater. Res. Soc. Jpn. 2015, 40, 103–106. [Google Scholar] [CrossRef]
- Suzuka, T.; Sueyoshi, H.; Maehara, S.; Ogasawara, H. Reactivity of aryl halides for transfer reduction in (sea) water using polymer-supported terpyridine palladium complex. Molecules 2015, 20, 9906–9914. [Google Scholar] [CrossRef]
- Suzuka, T.; Sueyoshi, H.; Ogiahra, K. Polymer-supported terpyridine–palladium complex for the aminocarbonylation in water of aryl iodides using methoxylamine hydrochloride as an ammonia equivalent. Trans. Mater. Res. Soc. Jpn. 2016, 41, 225–228. [Google Scholar] [CrossRef]
- Suzuka, T.; Sueyoshi, H.; Ogihara, K. Recyclable polymer supported terpyridine palladium complex for the tandem aminocarbonylation of aryl iodides using NaN3 as an ammonia equivalent. Catalysts 2017, 7, 107–115. [Google Scholar] [CrossRef]
- Li, C.-J.; Chan, T.-H. Organic Reactions in Aqueous Media; Wiley-VCH: New York, NY, USA, 1997. [Google Scholar]
- Grieco, P.A. Organic Synthesis in Water; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1997. [Google Scholar]
- Herrmann, W.A.; Kohlpaintner, C.W. Water-soluble ligands, metal complexes, and catalysts: Synergism of homogeneous and heterogeneous catalyst. Angew. Chem. Int. Ed. Engl. 1993, 32, 1524–1544. [Google Scholar] [CrossRef]
- Lindström, U.M. Stereoselective organic reaction in water. Chem. Rev. 2002, 102, 2751–2772. [Google Scholar] [CrossRef]
- Bailey, D.C.; Langer, S.H. Immobilized transition-metal carbonyls and related catalysts. Chem. Rev. 1981, 81, 109–148. [Google Scholar] [CrossRef]
- Dörwald, F.Z. Organic Synthesis on Solid Phase; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar]
- Leadbeater, N.E.; Marco, M. Preparation of polymer-supported ligands and metal complexes for use in catalysis. Chem. Rev. 2002, 102, 3217–3274. [Google Scholar] [CrossRef] [PubMed]
- McNamara, C.A.; Dixon, M.J.; Bradley, M. Recoverable catalysts and reagents using recyclable polystyrene-based supports. Chem. Rev. 2002, 102, 3275–3300. [Google Scholar] [CrossRef] [PubMed]

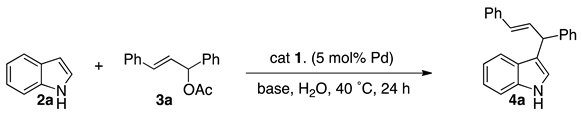
| Entry | Catalyst | Bases (3 equiv.) | Yield of 4a (%) |
|---|---|---|---|
| 1 | 1a | Et3N | 43 |
| 2 | 1b | Et3N | 88 |
| 3 | 1c | Et3N | 53 |
| 4 | 1b | Li2CO3 | 27 |
| 5 | 1b | Na2CO3 | 12 |
| 6 | 1b | K2CO3 | 13 |
| 7 | 1b | Cs2CO3 | 16 |
| 8 | 1b | NaHCO3 | 28 |
| 9 | 1b | DBU | 30 |
| 10 b | 1b | Et3N | 94 c |
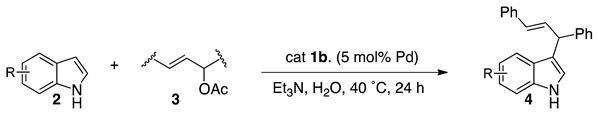
| Entry | 2 | 4 | Yield (%) a |
|---|---|---|---|
| 1 | 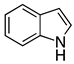 2a | 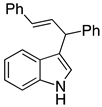 4a | 88 |
| 2 | 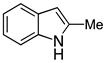 2b |  4b | 78 |
| 3 | 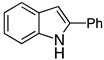 2c |  4c | 27 |
| 4 | 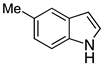 2d | 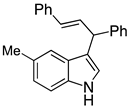 4d | 91 |
| 5 | 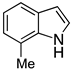 2e | 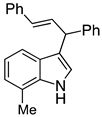 4e | 77 |
| 6 |  2f | 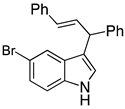 4f | 74 |
| 7 | 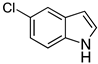 2g | 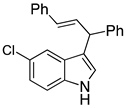 4g | 64 |
| 8 | 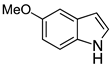 2h | 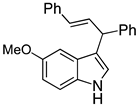 4h | 52 |
| 9 | 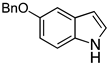 2i |  4i | 33 |
| 10 |  2j |  4j | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuka, T.; Ooshiro, Y.; Ogihara, K. Friedel–Crafts-Type Alkylation of Indoles in Water Using Amphiphilic Resin-Supported 1,10-Phenanthroline–Palladium Complex under Aerobic Conditions. Catalysts 2020, 10, 193. https://doi.org/10.3390/catal10020193
Suzuka T, Ooshiro Y, Ogihara K. Friedel–Crafts-Type Alkylation of Indoles in Water Using Amphiphilic Resin-Supported 1,10-Phenanthroline–Palladium Complex under Aerobic Conditions. Catalysts. 2020; 10(2):193. https://doi.org/10.3390/catal10020193
Chicago/Turabian StyleSuzuka, Toshimasa, Yuto Ooshiro, and Kazuhito Ogihara. 2020. "Friedel–Crafts-Type Alkylation of Indoles in Water Using Amphiphilic Resin-Supported 1,10-Phenanthroline–Palladium Complex under Aerobic Conditions" Catalysts 10, no. 2: 193. https://doi.org/10.3390/catal10020193
APA StyleSuzuka, T., Ooshiro, Y., & Ogihara, K. (2020). Friedel–Crafts-Type Alkylation of Indoles in Water Using Amphiphilic Resin-Supported 1,10-Phenanthroline–Palladium Complex under Aerobic Conditions. Catalysts, 10(2), 193. https://doi.org/10.3390/catal10020193




