High-Performance Vapor-Phase Selective Oxidation of Ethyl Lactate to Ethyl Pyruvate over SiO2 Supported PMoVNb Oxides
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Properties
2.2. Redox Properties
2.3. Acidic Properties
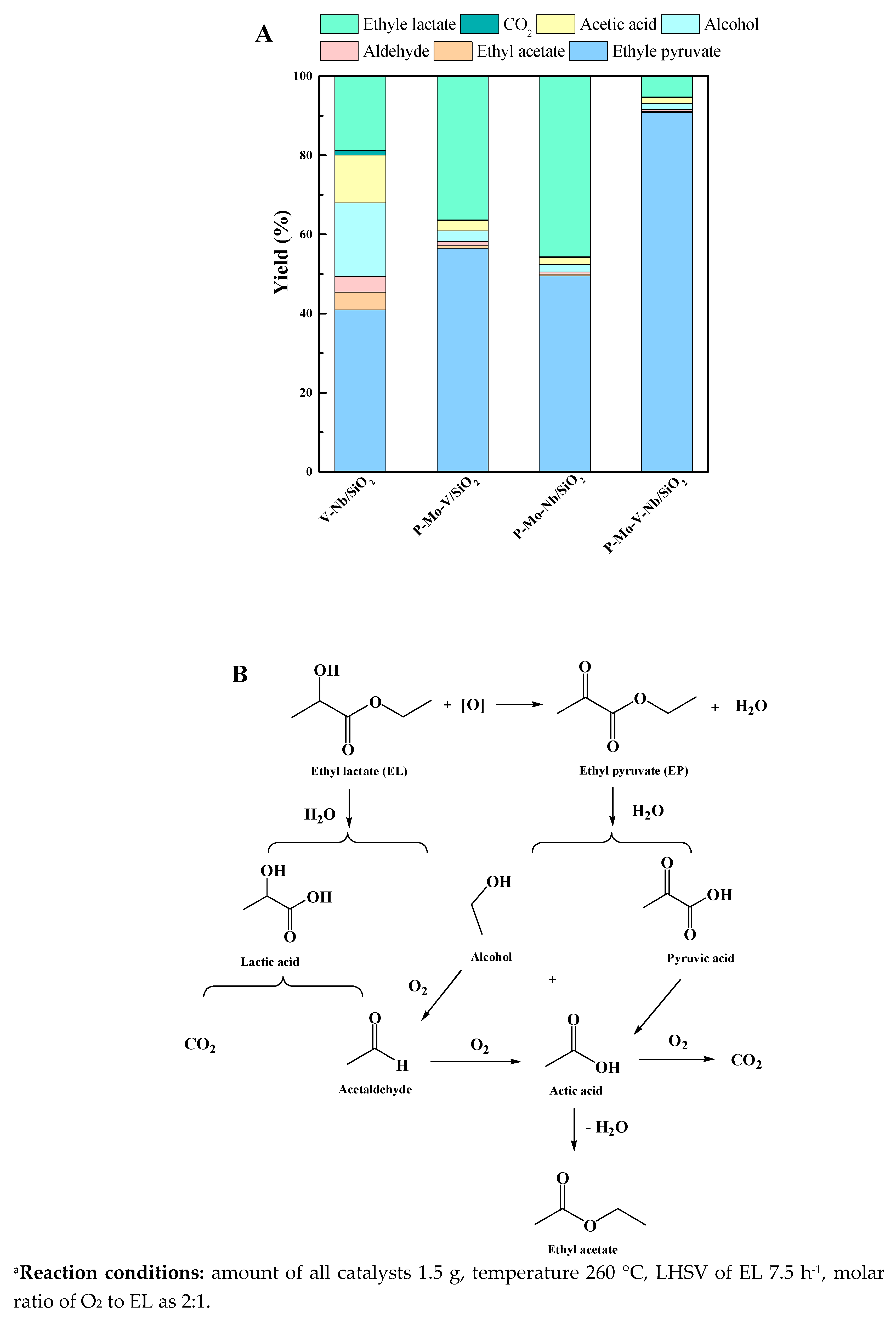
2.4. Catalytic Performance
2.5. Relationship between Catalyst Structure and Catalytic Performance
3. Experimental
3.1. Preparation of the Catalyst
3.2. Catalyst Characterization
3.3. Catalytic Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dusselier, M.; Van Wouwe, P.; Dewaele, A.; Makshina, E.; Sels, B.F. Lactic acid as a platform chemical in the biobased economy: the role of chemocatalysis. Energy Environ. Sci. 2013, 6, 1415. [Google Scholar] [CrossRef]
- Murlykina, M.V.; Sakhno, Y.I.; Desenko, S.M.; Shishkina, S.V.; Shishkin, O.V.; Sysoiev, D.O.; Kornet, M.N.; Schols, D.; Goeman, J.L.; Van der Eycken, J.; et al. Study of the Chemoselectivity of Multicomponent Heterocyclizations Involving 3-Amino-1,2,4-triazole and Pyruvic Acids as Key Reagents, and Biological Activity of the Reaction Products. Eur. J. Org. Chem. 2015, 2015, 4481–4492. [Google Scholar] [CrossRef]
- Dennig, A.; Busto, E.; Kroutil, W.; Faber, K. Biocatalytic One-Pot Synthesis of l-Tyrosine Derivatives from Monosubstituted Benzenes, Pyruvate, and Ammonia. ACS Catal. 2015, 5, 1–5. [Google Scholar] [CrossRef]
- Muller, A.J.; Duhadaway, J.B.; Daniel, J.; Peter, C.; Richard, M.; Prendergast, G.C. Immunotherapeutic suppression of indoleamine 2,3-dioxygenase and tumor growth with ethyl pyruvate. Cancer Res. 2010, 70, 1845–1853. [Google Scholar] [CrossRef] [Green Version]
- Sugiyama, S.; Fukunaga, S.; Ito, K.; Ohigashi, S.; Hayashi, H. Catalysts for vapor-phase dehydration of ethylene glycol and their application to pyruvic acid synthesis. J. Catal. 1991, 129, 12–18. [Google Scholar] [CrossRef]
- Shigeru Sugiyama, S.F.K.K.H.H. Catalytic Conversion of Diethyl Tartrate into Pyruvate over Silica-Supported Potassium Disulfate. Bull. Chem. Soc. JPN 2006, 65, 2083–2085. [Google Scholar] [CrossRef]
- Miyazaki, M.; Shibue, M.; Ogino, K.; Nakamura, H.; Maeda, H. ChemInform Abstract: Enzymatic Synthesis of Pyruvic Acid from Acetaldehyde and Carbon Dioxide. Chem. Commun. 2001, 33, 1800–1801. [Google Scholar] [CrossRef]
- MIYATA; YONEHARA. Improvement of fermentative production of pyruvate from glucose by Torulopsis glabrata IFO 0005. J. Ferment. Bioeng. 1996, 82, 475–479. [Google Scholar] [CrossRef]
- Miyata, R.; Yonehara, T. Breeding of high-pyruvate-producing Torulopsis glabrata and amino acid auxotrophic mutants. J. Biosci. Bioeng. 2000, 90, 137–141. [Google Scholar] [CrossRef]
- Sugiyama, S.; Shigemoto, N.; Masaoka, N.; Suetoh, S.; Hayashi, H. Vapor-Phase Oxidation of Ethyl Lactate to Pyruvate over Various Oxide Catalysts. Cheminform 1993, 66, 1542–1547. [Google Scholar] [CrossRef]
- Ai, M. Catalytic activity of palladium-doped iron phosphate in the oxidative dehydrogenation of lactic acid to pyruvic acid. Appl. Catal. A Gen. 2002, 232, 1–6. [Google Scholar] [CrossRef]
- Chen, Z.; Tao, W.; Ding, Y. Oxidative dehydrogenation of lactic acid to pyruvic acid over Pb-Pt bimetallic supported on carbon materials. Appl. Catal. A Gen. 2017, 533, 59–65. [Google Scholar]
- Liu, K.; Huang, X.; Pidko, E.A.; Hensen, E.J.M. MoO3–TiO2 synergy in oxidative dehydrogenation of lactic acid to pyruvic acid. Green Chem. 2017, 19, 3014–3022. [Google Scholar] [CrossRef] [Green Version]
- Lomate, S.; Bonnotte, T.; Paul, S.; Dumeignil, F.; Katryniok, B. Synthesis of pyruvic acid by vapour phase catalytic oxidative dehydrogenation of lactic acid. J. Mol. Catal. A Chem. 2013, 377, 123–128. [Google Scholar] [CrossRef]
- Ramos-Fernandez, E.V.; Geels, N.J.; Shiju, N.R.; Rothenberg, G. Titania-catalysed oxidative dehydrogenation of ethyl lactate: effective yet selective free-radical oxidation. Green Chem. 2014, 16, 3358–3363. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Innocenti, G.; Oulego, P.; Gitis, V.; Wu, H.; Ensing, B.; Cavani, F.; Rothenberg, G.; Shiju, N.R. Highly Selective Oxidation of Ethyl Lactate to Ethyl Pyruvate Catalyzed by Mesoporous Vanadia-Titania. ACS Catal. 2018, 8, 2365–2374. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.; Zou, J.; Zhan, Y.; Yang, X.; Wen, Y.; Wang, X.; Zhou, L.; Xu, J. Highly Efficient Oxidation of Ethyl Lactate to Ethyl Pyruvate Catalyzed by TS-1 Under Mild Conditions. ACS Catal. 2018, 8, 1287–1296. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, C.; Xu, C.; Li, H.; Huang, H.; Song, L.; Li, X. Kinetics study for the oxidative dehydrogenation of ethyl lactate to ethyl pyruvate over MoVNbOx based catalysts. Chem. Eng. J. 2016, 296, 217–224. [Google Scholar] [CrossRef]
- Jo, B.Y.; Kum, S.S.; Moon, S.H. Performance of WOx-added Mo–V–Te–Nb–O catalysts in the partial oxidation of propane to acrylic acid. Appl. Catal. A Gen. 2010, 378, 76–82. [Google Scholar] [CrossRef]
- Ramli, I.; Botella, P.; Ivars, F.; Pei Meng, W.; Zawawi, S.M.M.; Ahangar, H.A.; Hernández, S.; Nieto, J.M.L. Reflux method as a novel route for the synthesis of MoVTeNbOx catalysts for selective oxidation of propane to acrylic acid. J. Mole. Catal. A Chem. 2011, 342–343, 50–57. [Google Scholar] [CrossRef]
- Botella, P.; Dejoz, A.; Lopeznieto, J.; Concepcion, P.; Vazquez, M. Selective oxidative dehydrogenation of ethane over MoVSbO mixed oxide catalysts. Appl. Catal. A Gen. 2006, 298, 16–23. [Google Scholar] [CrossRef]
- Baek, M.; Lee, J.K.; Kang, H.J.; Kwon, B.J.; Lee, J.H.; Song, I.K. Ammoxidation of propane to acrylonitrile over Mo-V-P-Oy/Al2O3 catalysts: Effect of phosphorus content. Catal. Commun. 2017, 92, 27–30. [Google Scholar] [CrossRef]
- Shiju, N.R.; Guliants, V.V. Microwave-assisted hydrothermal synthesis of monophasic Mo-V-Te-Nb-O mixed oxide catalyst for the selective ammoxidation of propane. Chemphyschem 2007, 8, 1615–1617. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Iglesia, E. Support and promoter effects in the selective oxidation of ethane to acetic acid catalyzed by Mo-V-Nb oxides. Appl. Catal. A Gen. 2008, 334, 339–347. [Google Scholar] [CrossRef]
- Li, X.; Iglesia, E. Synergistic Effects of TiO2 and Palladium-Based Cocatalysts on the Selective Oxidation of Ethene to Acetic Acid on Mo–V–Nb Oxide Domains. Angew. Chem. 2007, 119, 8803–8806. [Google Scholar] [CrossRef]
- Li, X.; Iglesia, E. Selective catalytic oxidation of ethanol to acetic acid on dispersed Mo-V-Nb mixed oxides. Chemistry 2007, 13, 9324–9330. [Google Scholar] [CrossRef]
- Cassiers, K.; L, T.; Mathieu, M.; Benjelloun, M.; Schrijnemakers, K.; van der Voort, P.; Cool, P.; Vansant, E.F. A Detailed Study of Thermal, Hydrothermal, and Mechanical Stabilities of a Wide Range of Surfactant Assembled Mesoporous Silicas. Chem. Mater. 2002, 14, 2317–2324. [Google Scholar] [CrossRef]
- Fang, K.; Liu, L.; Zhang, M.; Zhao, L.; Zhou, J.; Li, W.; Mu, X.; Yang, C. Synthesis of Three-Dimensionally Ordered Macroporous NiCe Catalysts for Oxidative Dehydrogenation of Propane to Propene. Catalysts 2018, 8, 19. [Google Scholar] [CrossRef] [Green Version]
- Jeziorowski, H.K. Raman spectra of cobalt molybdenum oxide supported on silica. J. Phys. Chem. 1980, 84, 1825–1829. [Google Scholar] [CrossRef]
- Pei, S.; Yue, B.; Qian, L.; Yan, S.; Cheng, J.; Zhou, Y.; Xie, S.; He, H. Preparation and characterization of P–Mo–V mixed oxide-incorporating mesoporous silica catalysts for selective oxidation of methane to formaldehyde. Appl. Catal. A Gen. 2007, 329, 148–155. [Google Scholar] [CrossRef]
- Kim, T.; Wachs, I. CH3OH oxidation over well-defined supported V2O5/Al2O3 catalysts: Influence of vanadium oxide loading and surface vanadium–oxygen functionalities. J. Catal. 2008, 255, 197–205. [Google Scholar] [CrossRef]
- Zhao, C.; Wachs, I. Selective oxidation of propylene over model supported V2O5 catalysts: Influence of surface vanadia coverage and oxide support. J. Catal. 2008, 257, 181–189. [Google Scholar] [CrossRef]
- Grasselli, R.K.; Lugmair, C.G.; Volpe Jr, A.F.; Andersson, A.; Burrington, J.D. Enhancement of acrylic acid yields in propane and propylene oxidation by selective P Doping of MoV(Nb)TeO-based M1 and M2 catalysts. Catal. Today 2010, 157, 33–38. [Google Scholar] [CrossRef]
- Jeziorowski, H.; Knoezinger, H. Raman and ultraviolet spectroscopic characterization of molybdena on alumina catalysts. Cheminform 1979, 10, 1166–1173. [Google Scholar] [CrossRef]
- Yang, S.; Iglesia, E.; Bell, A.T. Oxidative dehydrogenation of propane over V2O5/MoO3/Al2O3 and V2O5/Cr2O3/Al2O3: structural characterization and catalytic function. J. Phys. Chem. B 2005, 109, 8987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solsona, B.; Dejoz, A.; Garcia, T.; Concepción, P.; Nieto, J.M.L.; Vázquez, M.I.; Navarro, M.T. Molybdenum–vanadium supported on mesoporous alumina catalysts for the oxidative dehydrogenation of ethane. Catal. Today 2006, 117, 228–233. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, F.; Chen, S.; Chen, F.; Lu, W.M. Performance of Mo-V-Te-P catalysts supported on the SiC for propane selective oxidation to acrolein. Chin. Chem. Lett. 2011, 22, 1321–1325. [Google Scholar] [CrossRef]
- Voge, H.H.; Adams, C.R. Catalytic Oxidation of Olefins. Adv. Catal. 1967, 17, 151–221. [Google Scholar]
- Thomas, R.; Oers, E.M.V.; Beer, V.H.J.D.; Medema, J.; Moulijn, J.A. Characterization of γ-alumina-supported Molybdenum oxide and tungsten oxide; reducibility of the oxidic state versus hydrodesulfurization activity of the sulfided state. J. Catal. 1984, 84, 275–287. [Google Scholar] [CrossRef] [Green Version]
- Pernicone, N.; Lazzerin, F.; Liberti, G.; Lanzavecchia, G. On the mechanism of CH3OH oxidation to CH2O over MoO3Fe2(MoO4)3 catalyst. J. Catal. 1969, 14, 293–302. [Google Scholar] [CrossRef]
- Li, J.; Zhu, H.; Razzaq, R.; Zhu, M.; Li, C.; Li, Z. Effect of zirconium addition on the structure and properties of CuO/CeO2 catalysts for high-temperature water–gas shift in an IGCC system. Int. J. Hydrogen Energ. 2012, 37, 15914–15924. [Google Scholar]
- Kang, M.; Park, E.D.; Kim, J.M.; Yie, J.E. Manganese oxide catalysts for NOx reduction with NH3 at low temperatures. Appl. Catal. A Gen. 2007, 327, 261–269. [Google Scholar] [CrossRef]
- Lee, K.J.; Kumar, P.A.; Maqbool, M.S.; Rao, K.N.; Song, K.H.; Ha, H.P. Ceria added Sb-V2O5/TiO2 catalysts for low temperature NH3 SCR: Physico-chemical properties and catalytic activity. Appl. Catal. B Environl. 2013, 142–143, 705–717. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, T.; Chen, S.; Zhao, Y.; Ma, X.; Gong, J. Selective oxidation of methanol to dimethoxymethane on V2O5–MoO3/γ-Al2O3 catalysts. Appl. Catal. B Environ. 2014, 160–161, 161–172. [Google Scholar] [CrossRef]










| Samples | Molar Ratio | |||
|---|---|---|---|---|
| P/Si | Mo/Si | V/Si | Nb/Si | |
| SiO2 | - | - | - | - |
| V-Nb/SiO2 | - | - | 0.17 | 0.02 |
| P-Mo-Nb/SiO2 | 0.91 | 0.75 | 0.18 | - |
| P-Mo-V/SiO2 | 0.89 | 0.73 | - | 0.02 |
| P-Mo-V-Nb/SiO2 | 0.88 | 0.74 | 0.15 | 0.03 |
| Samples | SBET (m2/g) a | VP (cm3/g) a | DP (nm) a |
|---|---|---|---|
| SiO2 | 208 | 1.2 | 11.6 |
| V-Nb/SiO2 | 127 | 1.2 | 18.3 |
| P-Mo-Nb/SiO2 | 17.2 | 0.2 | 18.3 |
| P-Mo-V/SiO2 | 9.1 | 0.1 | 17.1 |
| P-Mo-V-Nb/SiO2 | 9.7 | 0.1 | 15.1 |
| Samples | Surface Atomic Ratio (%) | Mo5+/(Mo5++Mo6+) | V4+/(V4++V5+) | |
|---|---|---|---|---|
| Mo/Si | V/Si | |||
| SiO2 | - | - | - | - |
| V-Nb/SiO2 | - | 0.02 | - | 0.24 |
| P-Mo-V/SiO2 | 0.38 | 0.05 | - | 0.48 |
| P-Mo-Nb/SiO2 | 0.23 | - | 0.33 | - |
| P-Mo-V-Nb/SiO2 | 0.71 | 0.09 | - | 0.68 |
| Samples | H2 Consumption (mmol·g−1) (Temperature before 700 °C) |
|---|---|
| SiO2 | 0.04 |
| V-Nb/SiO2 | 1.06 |
| P-Mo-V/SiO2 | 1.53 |
| P-Mo-Nb/SiO2 | 1.48 |
| P-Mo-V-Nb/SiO2 | 1.72 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wang, Q.; Liu, C.; Wang, X.; Peng, C.; Liu, R.; Yang, C. High-Performance Vapor-Phase Selective Oxidation of Ethyl Lactate to Ethyl Pyruvate over SiO2 Supported PMoVNb Oxides. Catalysts 2020, 10, 197. https://doi.org/10.3390/catal10020197
Liu X, Wang Q, Liu C, Wang X, Peng C, Liu R, Yang C. High-Performance Vapor-Phase Selective Oxidation of Ethyl Lactate to Ethyl Pyruvate over SiO2 Supported PMoVNb Oxides. Catalysts. 2020; 10(2):197. https://doi.org/10.3390/catal10020197
Chicago/Turabian StyleLiu, Xiaoyu, Qi Wang, Chenghao Liu, Xiaolong Wang, Cuina Peng, Rong Liu, and Cheng Yang. 2020. "High-Performance Vapor-Phase Selective Oxidation of Ethyl Lactate to Ethyl Pyruvate over SiO2 Supported PMoVNb Oxides" Catalysts 10, no. 2: 197. https://doi.org/10.3390/catal10020197




