Simultaneous Catalysis of Sulfite Oxidation and Uptake of Heavy Metals by Bifunctional Activated Carbon Fiber in Magnesia Desulfurization
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of ACFs
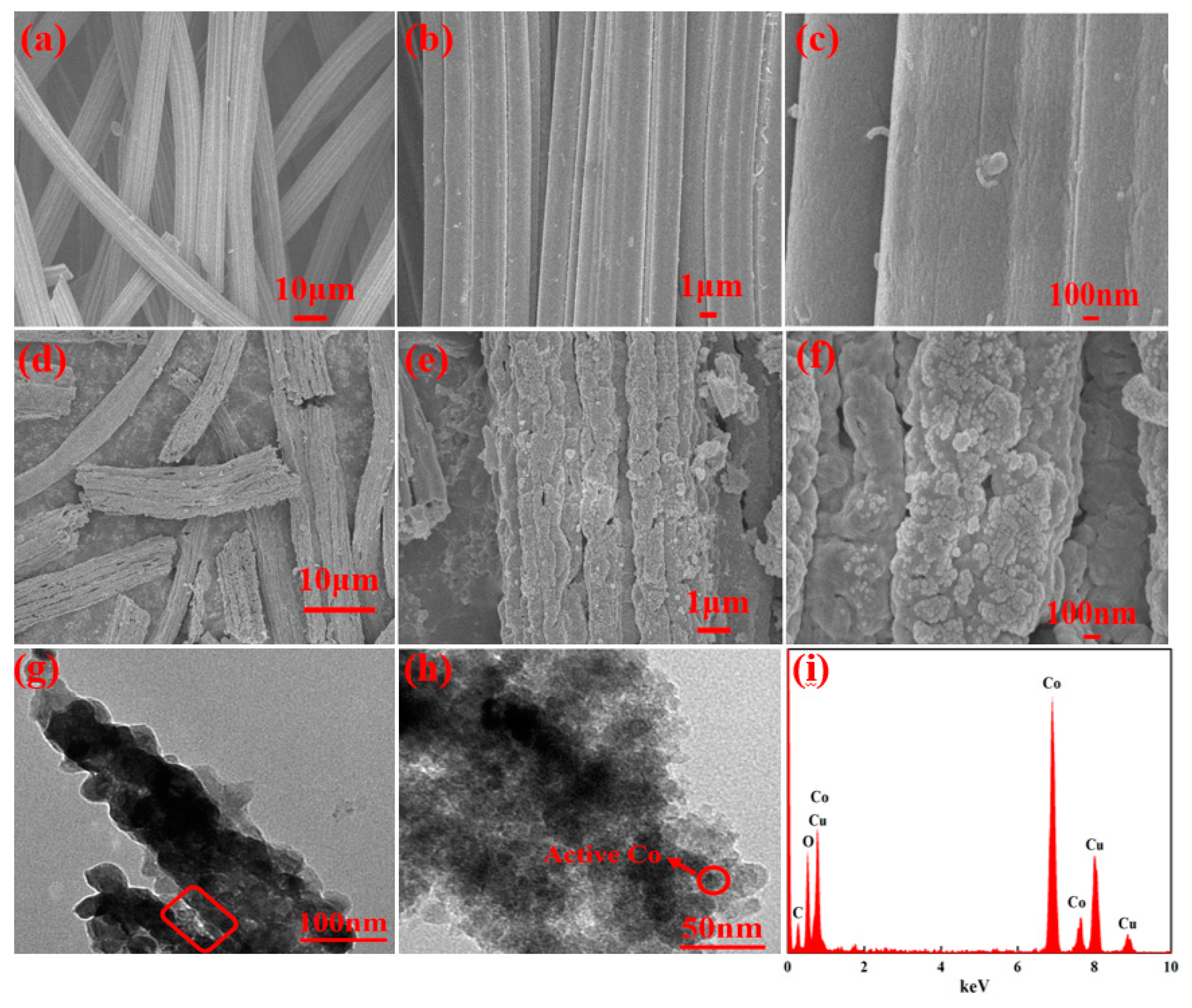
2.1.1. SEM
2.1.2. N2 Adsorption-Desorption
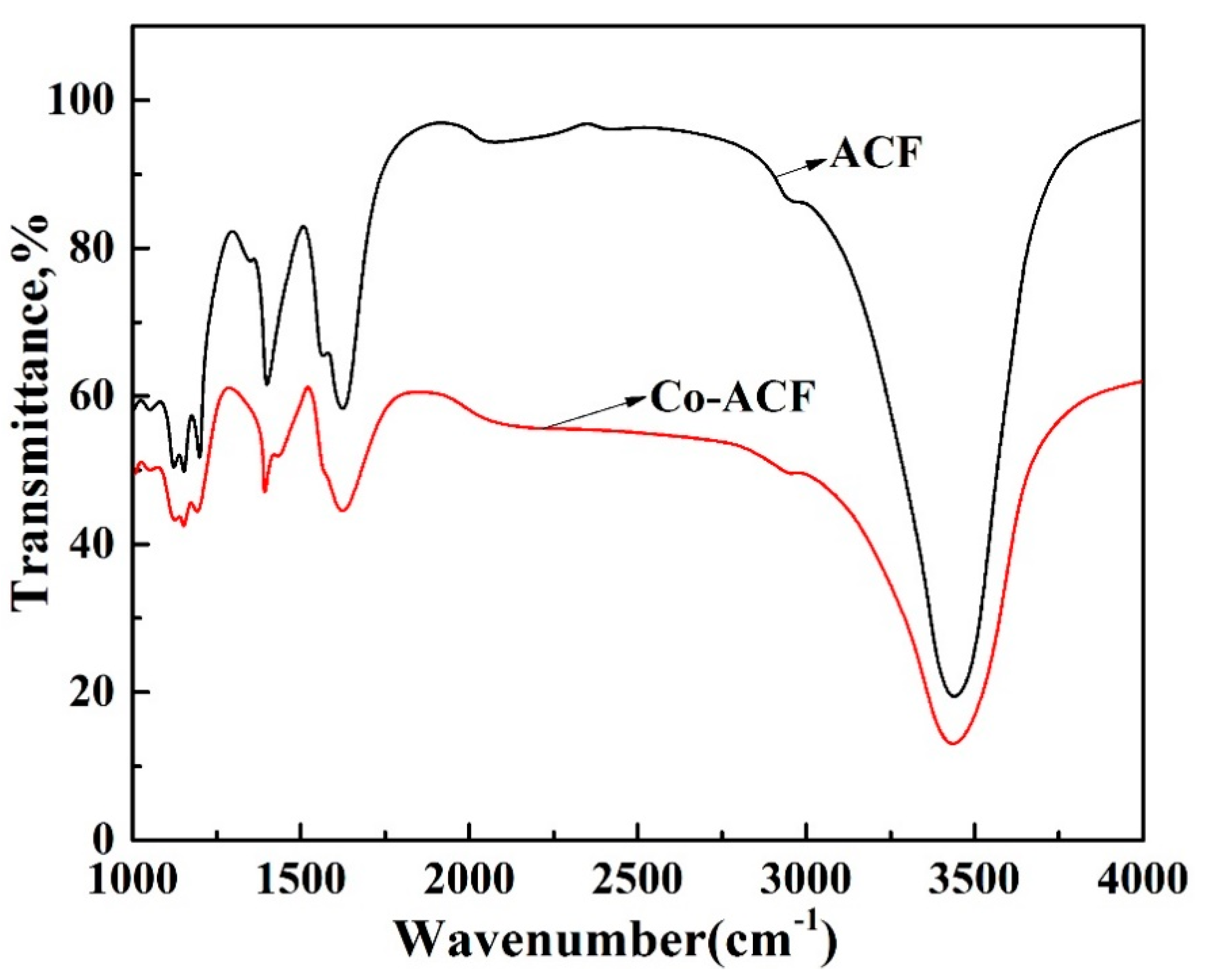
2.1.3. FT-IR
2.1.4. XPS Pattern
2.1.5. Boehm Titration
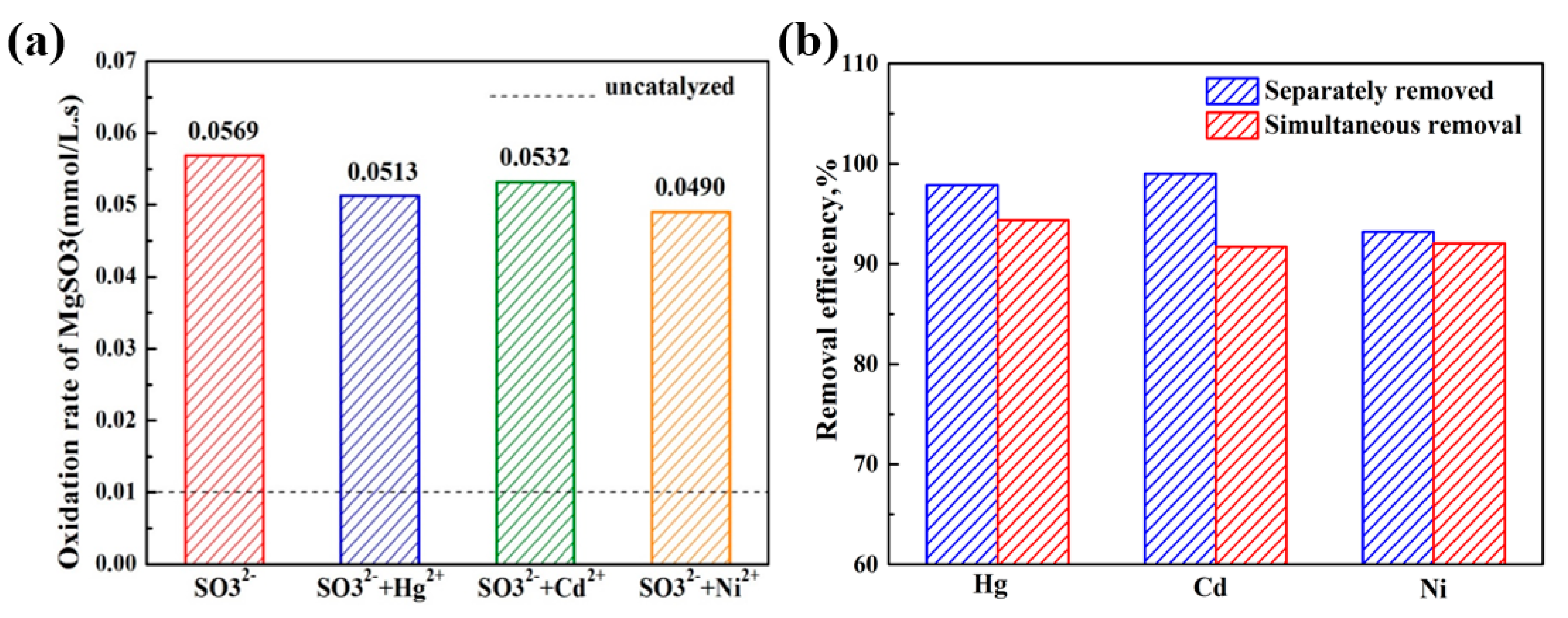
2.2. Catalytic Performance Measurement
2.3. Simultaneous Removal of SO32− and Heavy Metal Ions
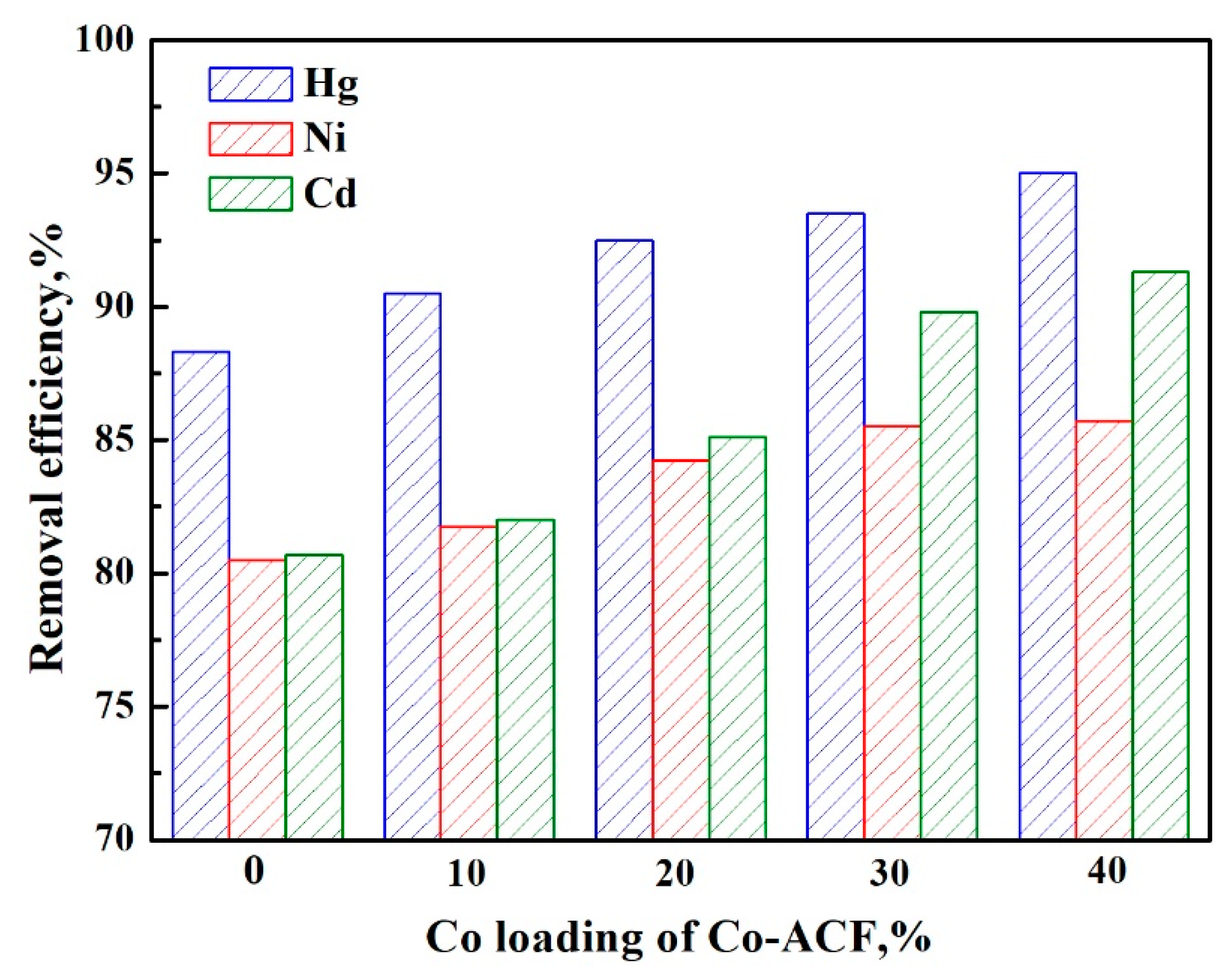
2.4. Adsorption Properties of Co-ACFs for Hg2+, Cd2+, and Ni2+
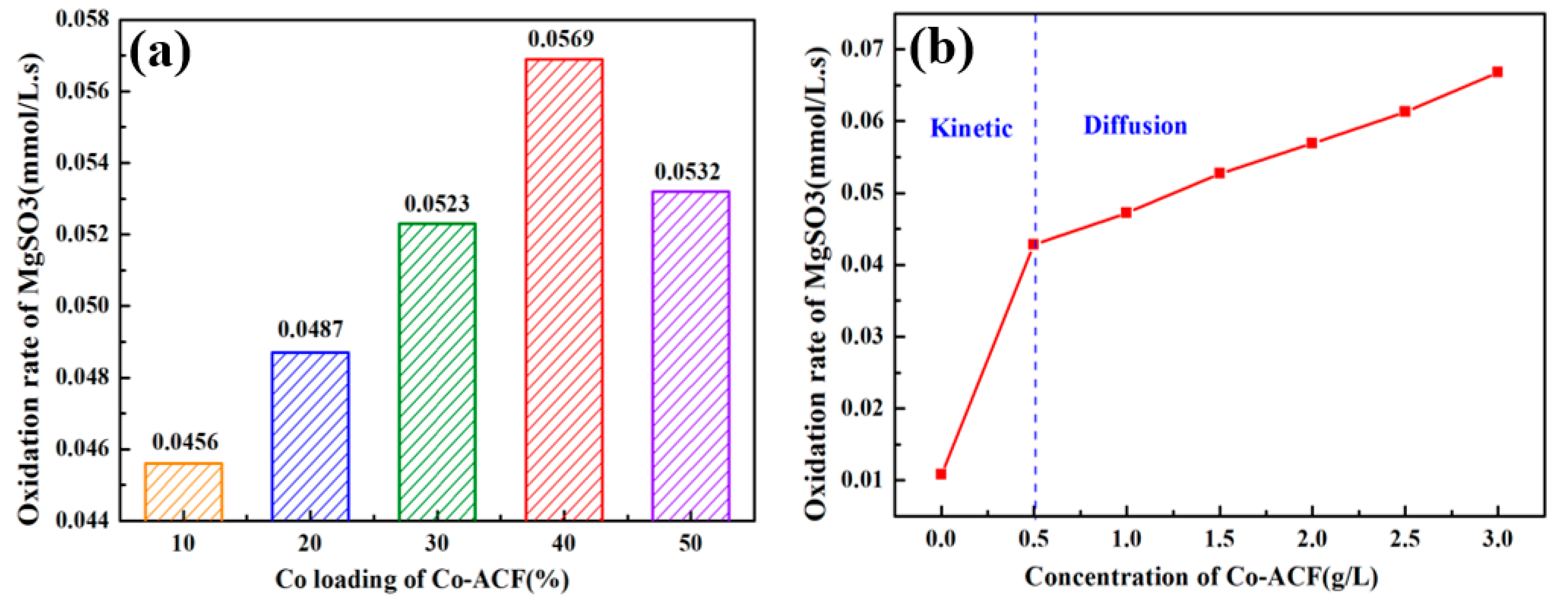
2.4.1. Effect of Cobalt Loading
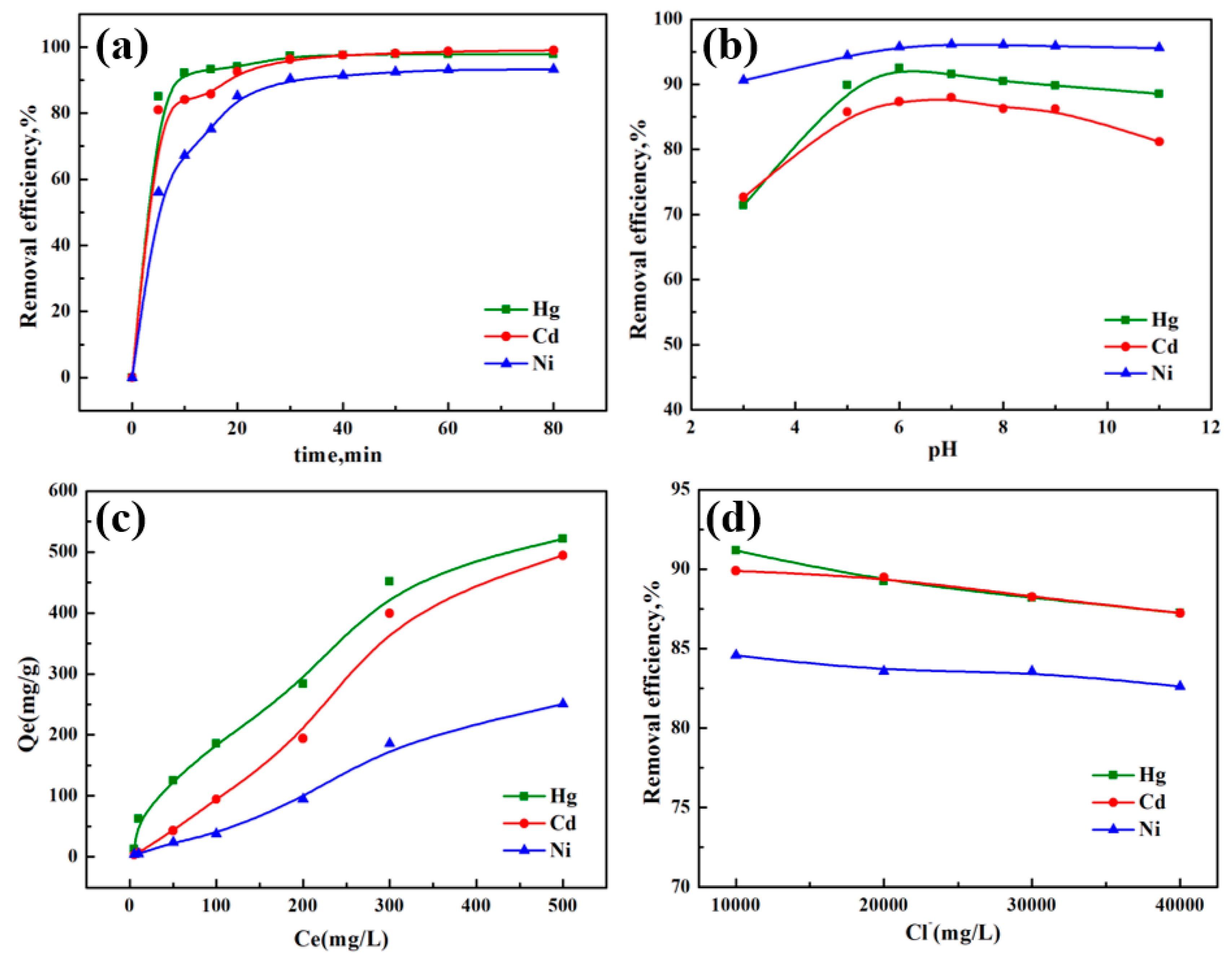
2.4.2. Effect of Adsorption Time
2.4.3. Effect of pH
2.4.4. Effect of the Initial Concentration of Hg2+, Cd2+, and Ni2+ in the Solution
2.4.5. Effect of Cl− Concentration
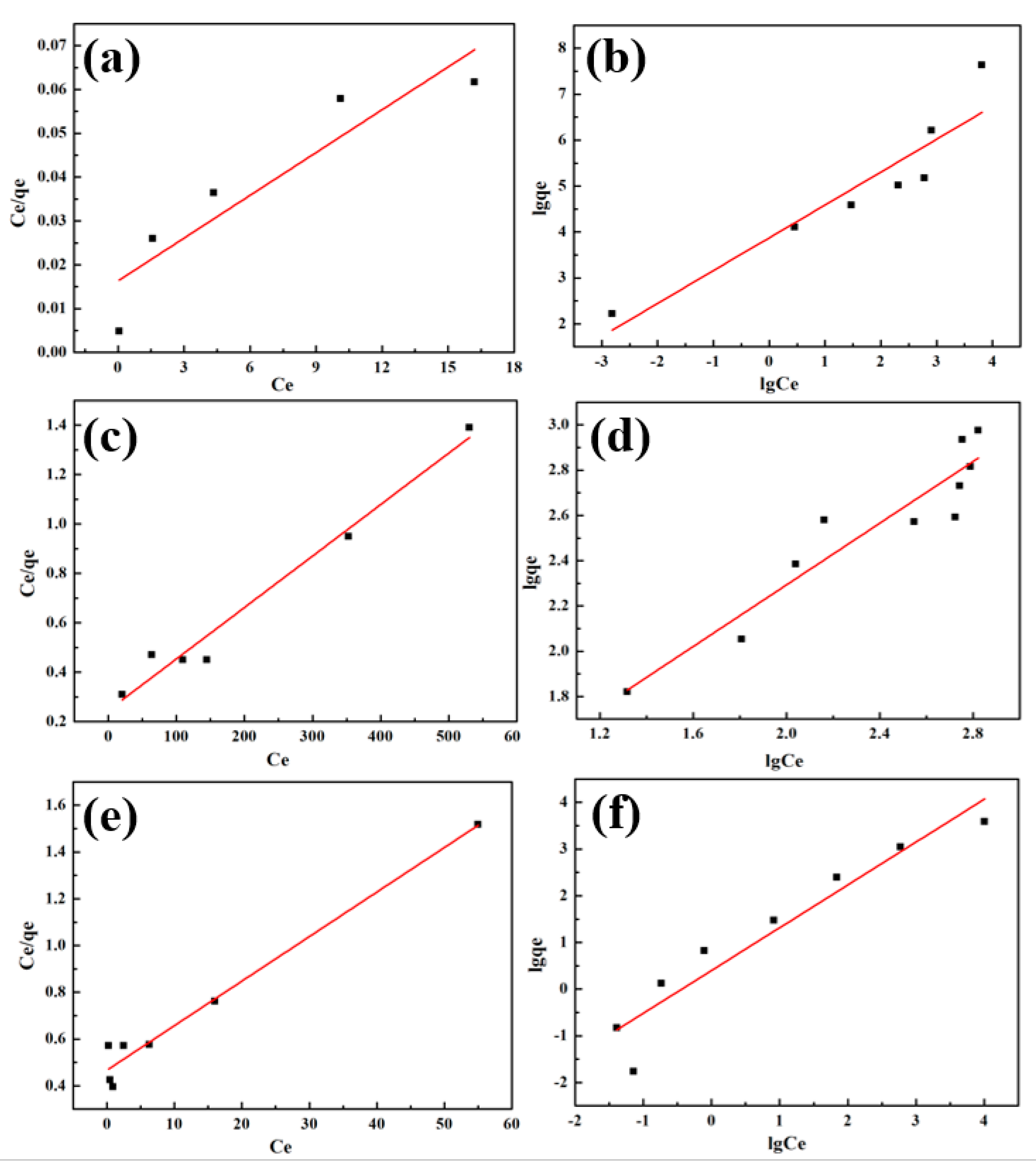
2.5. Adsorption Mechanism
2.5.1. Adsorption Isotherms
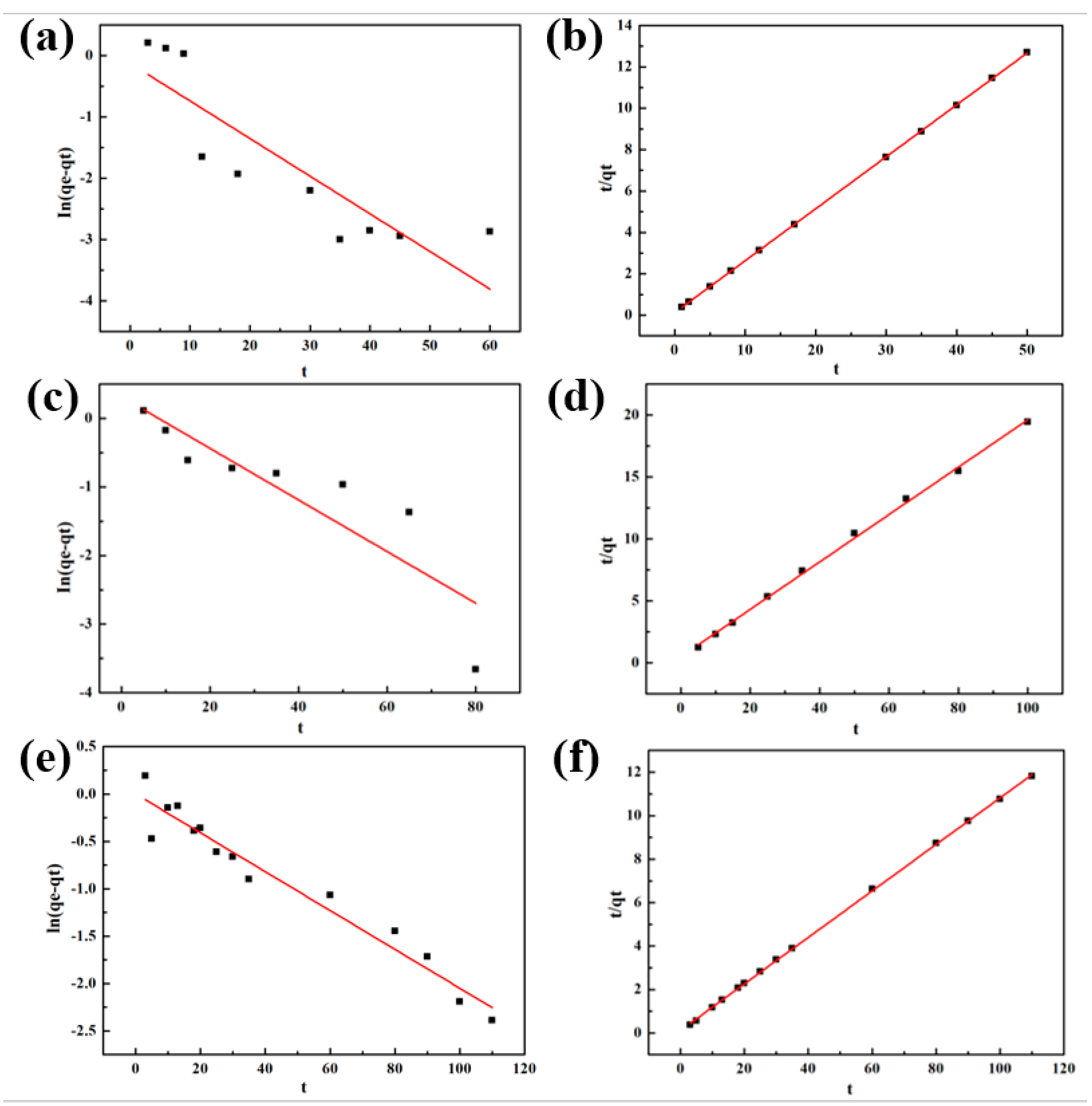
2.5.2. Adsorption Kinetics
3. Materials and Methods
3.1. Chemicals
3.2. Co-ACFs Catalyst-Adsorbent Synthesis
3.3. Catalyst-Adsorbent Characterization
3.4. Experimental Procedure
3.5. Methodology
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| C0 | initial concentration of heavy metals in the solution (mg/L) |
| Ce | equilibrium concentration of heavy metals in the solution (mg/L) |
| V | solution volume (L) |
| m | mass of the catalytic-adsorbent (g) |
| η | removal efficiency (%) |
| qe | adsorption capacity at equilibrium (mg/g) |
| qm | maximum adsorption capacity of the adsorbent (mg/g) |
| kL | Langmuir equilibrium adsorption constant (L/mg) |
| kF | Freundlich empirical constant (mg/g) |
| nF | Freundlich empirical constant (L/mg) |
| qt | adsorption capacity at time t (mg/g) |
| k1 | equilibrium rate constant of the pseudo-first-order adsorption (min−1) |
| k2 | equilibrium rate constant of the pseudo-second-order adsorption (g/(mg.min)) |
References
- Yuan, Y.; Zhang, J.; Li, H.; Li, Y.; Zhao, Y.; Zheng, C. Simultaneous removal of SO2, NO and mercury using TiO2-aluminum silicate fiber by photocatalysis. Chem. Eng. J. 2012, 192, 21–28. [Google Scholar] [CrossRef]
- Ma, Y.; Qu, Z.; Xu, H.; Wang, W.; Yan, N. Investigation on mercury removal method from flue gas in the presence of sulfur dioxide. J. Hazard. Mater. 2014, 279, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhao, Y.; Chang, L.; Zhang, J.; Zheng, C. Mercury Adsorption and Oxidation over Cobalt Oxide Loaded Magnetospheres Catalyst from Fly Ash in Oxyfuel Combustion Flue Gas. Environ. Sci. Technol. 2015, 49, 8210–8218. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, T.; Lv, Q.; Li, Y.; Che, D. Simultaneous removal of NO and SO2 from coal-fired flue gas based on the catalytic decomposition of H2O2 over Fe2(MoO4)3. Chem. Eng. J. 2019, 371, 486–499. [Google Scholar] [CrossRef]
- Shen, Z.; Chen, X.; Tong, M.; Guo, S.; Ni, M.; Lu, J. Studies on magnesium-based wet flue gas desulfurization process with oxidation inhibition of the byproduct. Fuel 2013, 105, 578–584. [Google Scholar] [CrossRef]
- Shen, Z.; Guo, S.; Kang, W.; Zeng, K.; Yin, M.; Tian, J.; Lu, J. Kinetics and Mechanism of Sulfite Oxidation in the Magnesium-Based Wet Flue Gas Desulfurization Process. Ind. Eng. Chem. Res. 2012, 51, 4192–4198. [Google Scholar] [CrossRef]
- Li, Q.; Wang, L.; Zhao, Y.; Ma, Y.; Cui, S.; Liu, S.; Xu, P.; Hao, J. Oxidation Rate of Magnesium Sulfite Catalyzed by Cobalt Ions. Environ. Sci. Technol. 2014, 48, 4145–4152. [Google Scholar]
- Barron, C.; O’Hern, H. Reaction kinetics of sodium sulfite oxidation by the Rapid-mixing method. Chem. Eng. Sci. 1966, 21, 397–404. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Y.; Wang, H.; Weng, X.; Wu, Z. Mercury Re-emission Behaviors in Magnesium-Based Wet Flue Gas Desulfurization Process: The Effects of Oxidation Inhibitors. Energy Fuels 2015, 29, 2610–2615. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.; Mei, R.; Wang, H.; Weng, X.; Wu, Z. Mercury re-emission in flue gas multipollutants simultaneous absorption system. Environ. Sci. Technol. 2014, 48, 14025–14030. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Y.; Yang, Z.; Wang, H.; Weng, X.; Wang, Y.; Wu, Z. Study of mercury re-emission in a simulated WFGD solution containing thiocyanate and sulfide ions. Fuel 2014, 134, 588–594. [Google Scholar] [CrossRef]
- Lidong, W.; Juan, W.; Peiyao, X.; Qiangwei, L.; Wendi, Z.; Shuai, C. Selectivity of transition metal catalysts in promoting the oxidation of solid sulfites in flue gas desulfurization. Appl. Catal. A Gen. 2015, 508, 52–60. [Google Scholar] [CrossRef]
- Wang, L.; Qi, T.; Wu, S.; Zhang, S.; Qi, D.; Xiao, H. A green and robust solid catalyst facilitating the magnesium sulfite oxidation in the magnesia desulfurization process. J. Mater. Chem. A 2017, 5, 8018–8028. [Google Scholar] [CrossRef]
- Wang, L.; Wu, S.; Liu, S.; Cui, S.; Liu, J.; Zhang, S. Cobalt impregnated porous catalyst promoting ammonium sulfate recovery in an ammonia-based desulfurization process. Chem. Eng. J. 2018, 331, 416–424. [Google Scholar] [CrossRef]
- Li, M.; Qi, T.; Yang, R.; Xiao, H.-N.; Fang, Z.; Hodge, S.A.; James, T.D.; Wang, L.; Mao, B. Promoting magnesium sulfite oxidation via partly oxidized metal nanoparticles on graphitic carbon nitride (g-C3N4) in the magnesia desulfurization process. J. Mater. Chem. A 2018, 6, 11296–11305. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Qi, T.; Wang, J.; Zhang, S.; Xiao, H.; Ma, Y. Uniform dispersion of cobalt nanoparticles over nonporous TiO2 with low activation energy for magnesium sulfate recovery in a novel magnesia-based desulfurization process. J. Hazard. Mater. 2018, 342, 579–588. [Google Scholar] [CrossRef]
- Qi, T.; Wang, L.; Wang, Y.; Xing, L.; Zhang, L.; Liu, J.; Xiao, H.; Zhang, S. Suppressing Ammonia Re-Emission with the Aid of the Co3O4-NPs@KIT-6 Catalyst in Ammonia-Based Desulfurization. Environ. Sci. Technol. 2019, 53, 13477–13485. [Google Scholar] [CrossRef]
- Li, M.; Guo, Q.; Xing, L.; Yang, L.; Qi, T.; Xu, P.; Zhang, S.; Wang, L. Cobalt-based metal-organic frameworks promoting magnesium sulfite oxidation with ultrahigh catalytic activity and stability. J. Colloid Interface Sci. 2020, 559, 88–95. [Google Scholar] [CrossRef]
- Li, C.M.; Wang, X.P.; Jiao, Z.H.; Zhang, Y.S.; Yin, X.B.; Cui, X.M.; Wei, Y.Z. Functionalized Porous Silica-Based Nano/Micro Particles for Environmental Remediation of Hazard Ions. Nanomaterials 2019, 9, 247. [Google Scholar] [CrossRef] [Green Version]
- Subramaniam, M.N.; Goh, P.S.; Lau, W.J.; Ismail, A.F. The Roles of Nanomaterials in Conventional and Emerging Technologies for Heavy Metal Removal: A State-of-the-Art Review. Nanomaterials 2019, 9, 625. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.; Wang, Z.; Yang, Q.; Huang, R. Efficient Removal of Lead, Copper and Cadmium Ions from Water by a Porous Calcium Alginate/Graphene Oxide Composite Aerogel. Nanomaterials 2018, 8, 957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhury, T.; Zhang, L.; Zhang, J.; Aggarwal, S. Removal of Arsenic(III) from Aqueous Solution Using Metal Organic Framework-Graphene Oxide Nanocomposite. Nanomaterials 2018, 8, 1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tummino, M.L.; Testa, M.L.; Malandrino, M.; Gamberini, R.; Bianco Prevot, A.; Magnacca, G.; Laurenti, E. Green Waste-Derived Substances Immobilized on SBA-15 Silica: Surface Properties, Adsorbing and Photosensitizing Activities towards Organic and Inorganic Substrates. Nanomaterials 2019, 9, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Xing, X.; Li, J.; Shi, M.; Lin, A.; Xu, C.; Zheng, J.; Li, R. Preparation of thiol-functionalized activated carbon from sewage sludge with coal blending for heavy metal removal from contaminated water. Environ. Pollut. 2018, 234, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Bandaru, N.M.; Reta, N.; Dalal, H.; Ellis, A.V.; Shapter, J.; Voelcker, N.H. Enhanced adsorption of mercury ions on thiol derivatized single wall carbon nanotubes. J. Hazard. Mater. 2013, 261, 534–541. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, G.; Chen, H.; Hu, X.; Niu, Z.; Ma, S. Functionalized metal–organic framework as a new platform for efficient and selective removal of cadmium(ii) from aqueous solution. J. Mater. Chem. A 2015, 3, 15292–15298. [Google Scholar] [CrossRef]
- Liu, D.; Deng, S.; Vakili, M.; Du, R.; Tao, L.; Sun, J.; Wang, B.; Huang, J.; Wang, Y.; Yu, G. Fast and high adsorption of Ni(II) on vermiculite-based nanoscale hydrated zirconium oxides. Chem. Eng. J. 2019, 360, 1150–1157. [Google Scholar] [CrossRef]
- Calderon, J.C.; Rios Rafales, M.; Nieto-Monge, M.J.; Pardo, J.I.; Moliner, R.; Lazaro, M.J. Oxidation of CO and Methanol on Pd-Ni Catalysts Supported on Different Chemically-Treated Carbon Nanofibers. Nanomaterials 2016, 6, 187. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Tao, H.; Yu, D.; Chang, C. Performance Assessment of Ordered Porous Electrospun Honeycomb Fibers for the Removal of Atmospheric Polar Volatile Organic Compounds. Nanomaterials 2018, 8, 350. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Chi, C.; Zhu, B.; Qiao, K.; Cai, X.; Cheng, Y.; Yan, S. High adsorptivity and recycling performance activated carbon fibers for Cu(II) adsorption. Sci. Total. Environ. 2019, 700, 134412. [Google Scholar] [CrossRef]
- Mena Aguilar, K.M.; Amano, Y.; Machida, M. Ammonium persulfate oxidized activated carbon fiber as a high capacity adsorbent for aqueous Pb(II). J. Environ. Chem. Eng. 2016, 4, 4644–4652. [Google Scholar] [CrossRef]
- Huang, C.C.; Su, Y.J. Removal of copper ions from wastewater by adsorption/electrosorption on modified activated carbon cloths. J. Hazard. Mater. 2010, 175, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.A.; Yang, M.T.; Lin, J.T.; Du, Y. Cobalt ferrite nanoparticles supported on electrospun carbon fiber as a magnetic heterogeneous catalyst for activating peroxymonosulfate. Chemosphere 2018, 208, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Beswick, O.; Yuranov, I.; Alexander, D.T.L.; Kiwi-Minsker, L. Iron oxide nanoparticles supported on activated carbon fibers catalyze chemoselective reduction of nitroarenes under mild conditions. Catal. Today 2015, 249, 45–51. [Google Scholar] [CrossRef]
- Hung, C.M. Activity of Cu-activated carbon fiber catalyst in wet oxidation of ammonia solution. J. Hazard. Mater. 2009, 166, 1314–1320. [Google Scholar] [CrossRef]
- Pradhan, B.; Sandle, N.K. Effect of Different Oxidizing Agent Treatments on the Surface Properties of Activated Carbons. Carbon 1999, 37, 1323–1332. [Google Scholar] [CrossRef]
- Terzyk, A. The influence of activated carbon surface chemical composition on the adsorption of acetaminophen (paracetamol) in vitro: Part II. TG, FTIR, and XPS analysis of carbons and the temperature dependence of adsorption kinetics at the neutral pH. Colloid Surf. A 2001, 177, 23–45. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Lopez Ramon, M.V.; Carrasco-Marín, F. Changes in surface chemistry of activated carbons by wet oxidation. Carbon 2000, 38, 1995–2001. [Google Scholar] [CrossRef]
- Castro, E.B.; Gervasi, C.A. Electrodeposited Ni–Co-oxide electrodes:characterization and kinetics of the oxygen evolution reaction. Int. J. Hydrog. Energy 2000, 25, 1163–1170. [Google Scholar] [CrossRef]
- Taghavimoghaddam, J.; Knowles, G.P.; Chaffee, A.L. SBA-15 supported cobalt oxide species: Synthesis, morphology and catalytic oxidation of cyclohexanol using TBHP. J. Mol. Catal. A Chem. 2013, 379, 277–286. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, S.; Guan, N.; Schreier, E.; Richter, M.; Eckelt, R.; Fricke, R. NO SCR with propane and propene on Co-based alumina catalysts prepared by co-precipitation. Appl. Catal. B Environ. 2007, 73, 209–219. [Google Scholar] [CrossRef]
- Jia, C.J.; Schwickardi, M.; Weidenthaler, C.; Schmidt, W.; Korhonen, S.; Weckhuysen, B.M.; Schuth, F. Co3O4-SiO2 nanocomposite: A very active catalyst for CO oxidation with unusual catalytic behavior. J. Am. Chem. Soc. 2011, 133, 11279–11288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varga, E.; Ferencz, Z.; Oszkó, A.; Erdőhelyi, A.; Kiss, J. Oxidation states of active catalytic centers in ethanol steam reforming reaction on ceria based Rh promoted Co catalysts: An XPS study. J. Mol. Catal. A Chem. 2015, 397, 127–133. [Google Scholar] [CrossRef]
- Sexton, B.A.; Hughes, A.E.; Turney, T. An XPS and TPR study of the reduction of promoted cobalt-kieselguhr Fischer-Tropsch catalysts. J. Catal. 1986, 97, 390–406. [Google Scholar] [CrossRef]
- Li, Q.; Yang, Y.; Wang, L.; Xu, P.; Han, Y. Mechanism and kinetics of magnesium sulfite oxidation catalyzed by multiwalled carbon nanotube. Appl. Catal. B Environ. 2017, 203, 851–858. [Google Scholar] [CrossRef]
- Wang, L.; Qi, T.; Hu, M.; Zhang, S.; Xu, P.; Qi, D.; Wu, S.; Xiao, H. Inhibiting Mercury Re-emission and Enhancing Magnesia Recovery by Cobalt-Loaded Carbon Nanotubes in a Novel Magnesia Desulfurization Process. Environ. Sci. Technol. 2017, 51, 11346–11353. [Google Scholar] [CrossRef]
- Sathyamurthy, N.; Degaleesan, T.; Chandrasekharan, K.; Laddha, G. Absorption of oxygen by aqueous sodium sulphite solutions. Can. J. Chem. Eng. 1979, 57, 145–149. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Ai, Y.; Alsaedi, A.; Hayat, T.; Wang, X. Combined experimental and theoretical investigation on selective removal of mercury ions by metal organic frameworks modified with thiol groups. Chem. Eng. J. 2018, 354, 790–801. [Google Scholar] [CrossRef]
- Javadian, H.; Taghavi, M. Application of novel Polypyrrole/thiol-functionalized zeolite Beta/MCM-41 type mesoporous silica nanocomposite for adsorption of Hg2+ from aqueous solution and industrial wastewater: Kinetic, isotherm and thermodynamic studies. Appl. Surf. Sci. 2014, 289, 487–494. [Google Scholar] [CrossRef]
- Boehm, H.P. Some Aspects of the Surface Chemistry of Carbon Black and Other Carbons. Carbon 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Lidong, W.; Shuai, C.; Qiangwei, L.; Juan, W.; Shuang, L. Kinetics and mechanism of magnesium sulphite oxidation promoted by a novel cobalt-based molecular sieve catalyst. Appl. Catal. A Gen. 2016, 511, 16–22. [Google Scholar] [CrossRef]
- Kundu, S.; Gupta, A.K. Arsenic adsorption onto iron oxide-coated cement (IOCC): Regression analysis of equilibrium data with several isotherm models and their optimization. Chem. Eng. J. 2006, 122, 93–106. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, F.; Wei, T.; Zhang, C.; Xiao, H. Hydrophobic modification of bagasse cellulose fibers with cationic latex: Adsorption kinetics and mechanism. Chem. Eng. J. 2016, 302, 33–43. [Google Scholar] [CrossRef]
- Ahmed, M.; Theydan, S. Equilibrium isotherms and kinetics modeling of methylene blue adsorption on agricultural wastes-based activated carbons. Fluid Phase Equilib. 2012, 317, 9–14. [Google Scholar] [CrossRef]
- Ho, Y.-S.; McKay, G. Pseudo-Second Order Model for Sorption Process. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]

| Sample | Specific Surface Area, m2·g−1 | Pore Volume, cm3·g−1 | Average Pore Size, nm |
|---|---|---|---|
| ACFs | 1035 | 0.57 | 2.102 |
| 40% Co-ACFs | 780 | 0.42 | 3.312 |
| Material | Acidic Group (mmoL/g) |
|---|---|
| Untreated ACFs | 0.7861 |
| 0%Co-ACFs | 2.4903 |
| 10%Co-ACFs | 2.5010 |
| 20%Co-ACFs | 2.5443 |
| 30%Co-ACFs | 2.5809 |
| 40%Co-ACFs | 2.5909 |
| Metals | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| KL | Qm | R2 | KF | n | R2 | |
| Hg2+ | 6.25 | 333.3 | 0.905 | 55.92 | 6.25 | 0.851 |
| Cd2+ | 3.98 | 500.0 | 0.970 | 1.22 | 8.40 | 0.881 |
| Ni2+ | 2.14 | 52.6 | 0.967 | 1.49 | 1.39 | 0.902 |
| Metals | Pseudo-first-order Model | Pseudo-second-order | ||||
|---|---|---|---|---|---|---|
| k1 | qe | R2 | k2 | qe | R2 | |
| Hg2+ | 0.06 | 1.005 | 0.774 | 0.46 | 4.00 | 0.999 |
| Cd2+ | 0.035 | 1.361 | 0.743 | 0.07 | 5.32 | 0.998 |
| Ni2+ | 0.02 | 1.010 | 0.947 | 0.09 | 9.34 | 0.999 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Qi, T.; Hu, M.; Yang, Y.; Xing, L.; Wang, L. Simultaneous Catalysis of Sulfite Oxidation and Uptake of Heavy Metals by Bifunctional Activated Carbon Fiber in Magnesia Desulfurization. Catalysts 2020, 10, 244. https://doi.org/10.3390/catal10020244
Wang Y, Qi T, Hu M, Yang Y, Xing L, Wang L. Simultaneous Catalysis of Sulfite Oxidation and Uptake of Heavy Metals by Bifunctional Activated Carbon Fiber in Magnesia Desulfurization. Catalysts. 2020; 10(2):244. https://doi.org/10.3390/catal10020244
Chicago/Turabian StyleWang, Yuguo, Tieyue Qi, Mengxuan Hu, Yu Yang, Lei Xing, and Lidong Wang. 2020. "Simultaneous Catalysis of Sulfite Oxidation and Uptake of Heavy Metals by Bifunctional Activated Carbon Fiber in Magnesia Desulfurization" Catalysts 10, no. 2: 244. https://doi.org/10.3390/catal10020244
APA StyleWang, Y., Qi, T., Hu, M., Yang, Y., Xing, L., & Wang, L. (2020). Simultaneous Catalysis of Sulfite Oxidation and Uptake of Heavy Metals by Bifunctional Activated Carbon Fiber in Magnesia Desulfurization. Catalysts, 10(2), 244. https://doi.org/10.3390/catal10020244