Activated Carbon/MnO2 Composites as Electrode for High Performance Supercapacitors
Abstract
:1. Introduction
2. Results and Discussion
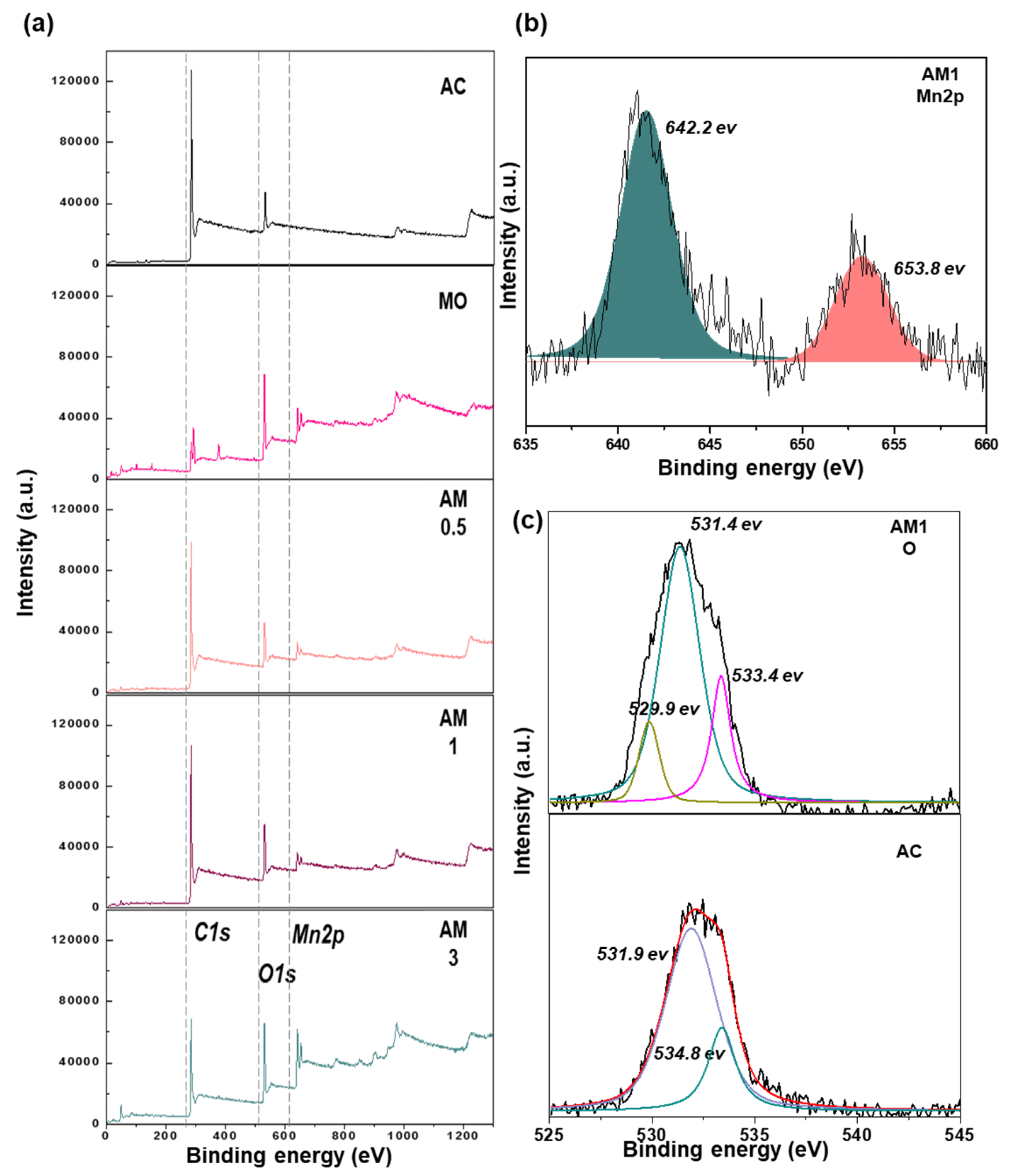
2.1. Structural Properties
2.2. Electrochemical Performance of AM Composites
3. Chemicals and Synthesis
3.1. Chemicals and Composites Synthesis
3.2. Structural Characterizations
3.3. Electrode Preparation and Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sun, Y.; Sills, R.B.; Hu, X.; Seh, Z.W.; Xiao, X.; Xu, H.; Luo, W.; Jin, H.; Xin, Y.; Li, T. A bamboo-inspired nanostructure design for flexible, foldable, and twistable energy storage devices. Nano Lett. 2015, 15, 3899–3906. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.-Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’with high volumetric capacitance. Nature 2014, 516, 78. [Google Scholar] [CrossRef] [PubMed]
- Janoschka, T.; Martin, N.; Martin, U.; Friebe, C.; Morgenstern, S.; Hiller, H.; Hager, M.D.; Schubert, U.S. An aqueous, polymer-based redox-flow battery using non-corrosive, safe, and low-cost materials. Nature 2015, 527, 78. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.R.; Simon, P. Electrochemical capacitors for energy management. Science 2008, 321, 651–652. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.J.; Park, H.W.; Park, S.; Song, I.K. Electrochemical properties of Mn-doped activated carbon aerogel as electrode material for supercapacitor. Curr. Appl. Phys. 2012, 12, 233–237. [Google Scholar] [CrossRef]
- Yang, G.; Park, S.-J. Facile hydrothermal synthesis of NiCo2O4-decorated filter carbon as electrodes for high performance asymmetric supercapacitors. Electrochim. Acta 2018, 285, 405–414. [Google Scholar] [CrossRef]
- Yaglikci, S.; Gokce, Y.; Yagmur, E.; Aktas, Z. The performance of sulphur doped activated carbon supercapacitors prepared from waste tea. Environ. Technol. 2020, 41, 36–48. [Google Scholar] [CrossRef]
- Huang, Y.; Shi, Y.; Gong, Q.; Weng, M.; Li, Y.; Gan, J.; Wang, D.; Shao, Y.; Zhao, M.; Zhuang, D. Scalable preparation of hierarchical porous activated carbon/graphene composites for high-performance supercapacitors. J. Mater. Chem. A 2019, 7, 10058–10066. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, L.; Li, Y.; Wang, Y.; Zhang, J.; Guan, G.; Pan, Z.; Zheng, G.; Peng, H. Nitrogen-Doped Core-Sheath Carbon Nanotube Array for Highly Stretchable Supercapacitor. Adv. Energy Mater. 2017, 7, 1601814. [Google Scholar] [CrossRef]
- Liu, J.-h.; Xu, X.-y.; Yu, J.; Hong, J.-l.; Liu, C.; Ouyang, X.; Lei, S.; Meng, X.; Tang, J.-N.; Chen, D.-Z. Facile construction of 3D porous carbon nanotubes/polypyrrole and reduced graphene oxide on carbon nanotube fiber for high-performance asymmetric supercapacitors. Electrochim. Acta 2019, 314, 9–19. [Google Scholar] [CrossRef]
- Chang, W.-M.; Wang, C.-C.; Chen, C.-Y. Fabrication of ultra-thin carbon nanofibers by centrifuged-electrospinning for application in high-rate supercapacitors. Electrochim. Acta 2019, 296, 268–275. [Google Scholar] [CrossRef]
- Yang, G.; Park, M.; Park, S.-J. Recent progresses of fabrication and characterization of fibers-reinforced composites: A review. Compos. Commun. 2019, 14, 34–42. [Google Scholar] [CrossRef]
- Ke, Q.; Wang, J. Graphene-based materials for supercapacitor electrodes—A review. J. Mater. 2016, 2, 37–54. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Tao, Y.; Zheng, X.; Luo, J.; Kang, F.; Cheng, H.-M.; Yang, Q.-H. Ultra-thick graphene bulk supercapacitor electrodes for compact energy storage. Energy Environ. Sci. 2016, 9, 3135–3142. [Google Scholar] [CrossRef]
- Misnon, I.I.; Zain, N.K.M.; Jose, R. Conversion of oil palm kernel shell biomass to activated carbon for supercapacitor electrode application. Waste Biomass Valorization 2019, 10, 1731–1740. [Google Scholar] [CrossRef]
- Prataap, R.V.; Arunachalam, R.; Raj, R.P.; Mohan, S.; Peter, L. Effect of electrodeposition modes on ruthenium oxide electrodes for supercapacitors. Curr. Appl. Phys. 2018, 18, 1143–1148. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Alinajafi, H.A.; Rezaei, B. Adenine decorated@ reduced graphene oxide, a new environmental friendly material for supercapacitor application. J. Alloy. Compd. 2018, 735, 1010–1016. [Google Scholar] [CrossRef]
- Yumak, T. Electrochemical Performance of Fabricated Supercapacitors Using MnO2/Activated Carbon Electrodes. Hacet. J. Biol. Chem. 2019, 47, 115–122. [Google Scholar]
- Williams, P.T.; Reed, A.R. Development of activated carbon pore structure via physical and chemical activation of biomass fibre waste. Biomass Bioenergy 2006, 30, 144–152. [Google Scholar] [CrossRef]
- Prauchner, M.J.; Rodríguez-Reinoso, F. Chemical versus physical activation of coconut shell: A comparative study. Microporous Mesoporous Mater. 2012, 152, 163–171. [Google Scholar] [CrossRef]
- Ho, M.; Khiew, P.; Isa, D.; Tan, T.; Chiu, W.; Chia, C.H. A review of metal oxide composite electrode materials for electrochemical capacitors. Nano 2014, 9, 1430002. [Google Scholar] [CrossRef]
- Cao, J.-P.; He, S.; Wu, Y.; Zhao, X.-Y.; Wei, X.-Y.; Takarada, T. Synthesis of NiO/Activated Carbon Composites and Their Application as Electrode Materials for Capacitors. Int. J. Electrochem. Sci 2017, 12, 2704–2718. [Google Scholar] [CrossRef]
- Kim, D.-W.; Rhee, K.-Y.; Park, S.-J. Synthesis of activated carbon nanotube/copper oxide composites and their electrochemical performance. J. Alloy. Compd. 2012, 530, 6–10. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Dunn, B.; Gogotsi, Y. Multidimensional materials and device architectures for future hybrid energy storage. Nat. Commun. 2016, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 1–17. [Google Scholar] [CrossRef]
- Zhang, C.; Kremer, M.P.; Seral-Ascaso, A.; Park, S.H.; McEvoy, N.; Anasori, B.; Gogotsi, Y.; Nicolosi, V. Stamping of flexible, coplanar micro-supercapacitors using MXene inks. Adv. Funct. Mater. 2018, 28, 1705506. [Google Scholar] [CrossRef]
- Patil, A.; Lokhande, A.; Chodankar, N.; Kumbhar, V.; Lokhande, C. Engineered morphologies of β-NiS thin films via anionic exchange process and their supercapacitive performance. Mater. Des. 2016, 97, 407–416. [Google Scholar] [CrossRef]
- Yang, G.; Park, S.-J. MnO2 and biomass-derived 3D porous carbon composites electrodes for high performance supercapacitor applications. J. Alloy. Compd. 2018, 741, 360–367. [Google Scholar] [CrossRef]
- Tseng, L.-H.; Hsiao, C.-H.; Nguyen, D.D.; Hsieh, P.-Y.; Lee, C.-Y.; Tai, N.-H. Activated carbon sandwiched manganese dioxide/graphene ternary composites for supercapacitor electrodes. Electrochim. Acta 2018, 266, 284–292. [Google Scholar] [CrossRef]
- Babkova, T.; Fei, H.; Kazantseva, N.E.; Sapurina, I.Y.; Sáha, P. Enhancing the supercapacitor performance of flexible MnOxCarbon cloth electrodes by Pd-decoration. Electrochim. Acta 2018, 272, 1–10. [Google Scholar] [CrossRef]
- Han, G.; Liu, Y.; Zhang, L.; Kan, E.; Zhang, S.; Tang, J.; Tang, W. MnO 2 nanorods intercalating graphene oxide/polyaniline ternary composites for robust high-performance supercapacitors. Sci. Rep. 2014, 4, 4824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidheswaran, M.A.; Destaillats, H.; Sullivan, D.P.; Larsen, J.; Fisk, W.J. Quantitative room-temperature mineralization of airborne formaldehyde using manganese oxide catalysts. Appl. Catal. B Environ. 2011, 107, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Ali, G.A.; Yusoff, M.M.; Ng, Y.H.; Lim, H.N.; Chong, K.F. Potentiostatic and galvanostatic electrodeposition of manganese oxide for supercapacitor application: A comparison study. Curr. Appl. Phys. 2015, 15, 1143–1147. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.I.; Park, S.-J. One-pot synthesis of reduced graphene oxide/anatase titanium dioxide composites for photocatalytic degradation of methylene blue. J. Nanosci. Nanotechnol. 2018, 18, 6173–6179. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Manivannan, S.; Kim, K.; Kwon, H.-I.; Jin, S.H. Petal-like MoS2 nanostructures with metallic 1 T phase for high performance supercapacitors. Curr. Appl. Phys. 2018, 18, 345–352. [Google Scholar] [CrossRef]
- Yang, C.-S.; Jang, Y.S.; Jeong, H.K. Bamboo-based activated carbon for supercapacitor applications. Curr. Appl. Phys. 2014, 14, 1616–1620. [Google Scholar] [CrossRef]
- Yuan, A.; Zhang, Q. A novel hybrid manganese dioxide/activated carbon supercapacitor using lithium hydroxide electrolyte. Electrochem. Commun. 2006, 8, 1173–1178. [Google Scholar] [CrossRef]
- Malak-Polaczyk, A.; Matei-Ghimbeu, C.; Vix-Guterl, C.; Frackowiak, E. Carbon/λ-MnO2 composites for supercapacitor electrodes. J. Solid State Chem. 2010, 183, 969–974. [Google Scholar] [CrossRef]
- Subramanian, V.; Luo, C.; Stephan, A.M.; Nahm, K.S.; Thomas, S.; Wei, B. Supercapacitors from Activated Carbon Derived from Banana Fibers. J. Phys. Chem. C 2007, 111, 7527–7531. [Google Scholar] [CrossRef]
- Kim, T.-W.; Park, S.-J. Synthesis of reduced graphene oxide/thorn-like titanium dioxide nanofiber aerogels with enhanced electrochemical performance for supercapacitor. J. Colloid Interface Sci. 2017, 486, 287–295. [Google Scholar] [CrossRef] [PubMed]



| Sample | X-ray Photoelectron Spectroscopy (at. %) | ICP-OES (at. %) | ||
|---|---|---|---|---|
| C | O | Mn | Mn | |
| AC | 81.34 | 18.66 | - | - |
| MO | - | 74.85 | 25.10 | 28.61 |
| AM 0.1 | 85.34 | 13.45 | 1.15 | 2.17 |
| AM 0.5 | 82.45 | 15.26 | 3.00 | 4.69 |
| AM 1 | 71.73 | 24.53 | 3.75 | 7.96 |
| AM 2 | 71.19 | 23.40 | 5.41 | 9.13 |
| AM 3 | 63.22 | 28.85 | 7.93 | 12.37 |
| Samples | a SBET (m2 g−1) | b Vtotal (cm3 g−1) | c Vmeso (cm3 g−1) | d Vmicro (cm3 g−1) | e Dp (nm) |
|---|---|---|---|---|---|
| AC | 1844 | 1.316 | 0.526 | 0.790 | 3.8 |
| AM 0.1 | 1805 | 1.047 | 0.419 | 0.628 | 3.7 |
| AM 0.5 | 1569 | 1.099 | 0.427 | 0.672 | 3.6 |
| AM 1 | 1479 | 1.047 | 0.419 | 0.628 | 3.6 |
| AM 2 | 1172 | 0.817 | 0.276 | 0.541 | 3.5 |
| AM 3 | 1017 | 0.708 | 0.239 | 0.469 | 3.4 |
| MO | 4 | 0.002 | 0.002 | 0.000 | 28.1 |
| Sample | Specific Capacitance (F g−1) | Ref. |
|---|---|---|
| MnO2/Activated carbon | 62.4 (0.1 A g−1) | [38] |
| LiMn2O4/Activated carbon | 48 (1 A g−1) | [39] |
| ZnCl2 treated activated carbon | 74 (0.5 A g−1) | [40] |
| Activated carbon/MnO2 | 60.3 (1 A g−1) | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.R.; Lee, J.W.; Yang, G.; Heo, Y.-J.; Park, S.-J. Activated Carbon/MnO2 Composites as Electrode for High Performance Supercapacitors. Catalysts 2020, 10, 256. https://doi.org/10.3390/catal10020256
Choi JR, Lee JW, Yang G, Heo Y-J, Park S-J. Activated Carbon/MnO2 Composites as Electrode for High Performance Supercapacitors. Catalysts. 2020; 10(2):256. https://doi.org/10.3390/catal10020256
Chicago/Turabian StyleChoi, Jang Rak, Ji Won Lee, Guijun Yang, Young-Jung Heo, and Soo-Jin Park. 2020. "Activated Carbon/MnO2 Composites as Electrode for High Performance Supercapacitors" Catalysts 10, no. 2: 256. https://doi.org/10.3390/catal10020256
APA StyleChoi, J. R., Lee, J. W., Yang, G., Heo, Y. -J., & Park, S. -J. (2020). Activated Carbon/MnO2 Composites as Electrode for High Performance Supercapacitors. Catalysts, 10(2), 256. https://doi.org/10.3390/catal10020256





