The Effects of the Crystalline Phase of Zirconia on C–O Activation and C–C Coupling in Converting Syngas into Aromatics
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of ZrO2 Samples
2.1.1. Texture Properties of the Crystalline Phase of ZrO2 Samples
2.1.2. Interactions of CO with the ZrO2 Samples
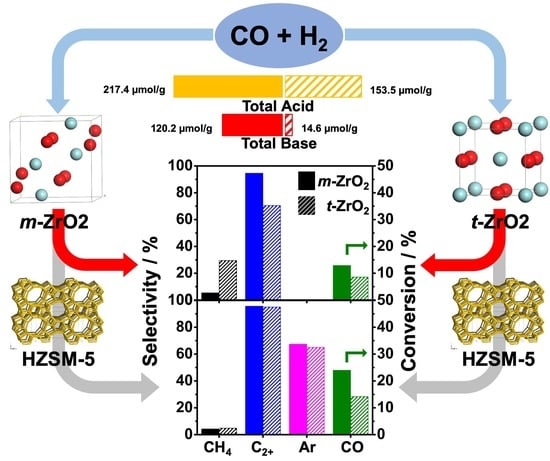
2.1.3. Acidity and Basicity Analysis of the ZrO2 Samples
2.2. Catalytic Performance
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Niziolek, A.M.; Onel, O.; Guzman, Y.A.; Floudas, C.A. Biomass-Based production of benzene, toluene, and xylenes via methanol: Process synthesis and deterministic global optimization. Energy Fuels 2016, 30, 4970–4998. [Google Scholar] [CrossRef]
- Torres Galvis, H.M.; de Jong, K.P. Catalysts for production of lower olefins from synthesis gas: A review. ACS Catal. 2013, 3, 2130–2149. [Google Scholar] [CrossRef]
- Olsbye, U.; Svelle, S.; Bjorgen, M.; Beato, P.; Janssens, T.V.; Joensen, F.; Bordiga, S.; Lillerud, K.P. Conversion of methanol to hydrocarbons: How zeolite cavity and pore size controls product selectivity. Angew. Chem. Int. Ed. 2012, 51, 5810–5831. [Google Scholar] [CrossRef] [PubMed]
- Kasipandi, S.; Bae, J.W. Recent advances in direct synthesis of value-added aromatic chemicals from syngas by cascade reactions over bifunctional catalysts. Adv. Mater. 2019, 31, 1803390. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Hu, J.; Han, J.; Yu, F. Synthesis of gasoline-Range hydrocarbons from nitrogen-Rich syngas over a Mo/HZSM-5 bi-functional catalyst. J. Energy Inst. 2016, 89, 782–792. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Wang, X.; Peng, X.; Yang, Y.; Cheng, K.; Zhang, Q.; Wang, Y. Mesoporous zeolite Y-Supported Co nanoparticles as efficient Fischer−Tropsch catalysts for selective synthesis of diesel fuel. Ind. Eng. Chem. Res. 2016, 55, 13008–13019. [Google Scholar] [CrossRef]
- Wang, C.; Xu, L.; Wang, Q. Review of directly producing light olefins via CO hydrogenation. J. Nat. Gas Chem. 2003, 12, 10–16. [Google Scholar]
- Jiao, F.; Li, J.; Pan, X.; Xiao, J.; Li, H.; Ma, H.; Wei, M.; Pan, Y.; Zhou, Z.; Li, M.; et al. Selective conversion of syngas to light olefins. Science 2016, 351, 1065–1068. [Google Scholar] [CrossRef]
- Cheng, K.; Gu, B.; Liu, X.; Kang, J.; Zhang, Q.; Wang, Y. Direct and highly selective conversion of synthesis gas into lower olefins: Design of a bifunctional catalyst combining methanol synthesis and carbon–Carbon coupling. Angew. Chem. Int. Ed. 2016, 55, 4725–4728. [Google Scholar] [CrossRef]
- Yang, J.; Pan, X.; Jiao, F.; Li, J.; Bao, X. Direct conversion of syngas to aromatics. Chem. Commun. 2017, 53, 11146–11149. [Google Scholar] [CrossRef]
- Cheng, K.; Zhou, W.; Kang, J.; He, S.; Shi, S.; Zhang, Q.; Pan, Y.; Wen, W.; Wang, Y. Bifunctional catalysts for one-step conversion of syngas into aromatics with excellent selectivity and stability. Chem 2017, 3, 334–347. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, W.; Yang, Y.; Cheng, K.; Kang, J.; Zhang, L.; Zhang, G.; Min, X.; Zhang, Q.; Wang, Y. Design of efficient bifunctional catalysts for direct conversion of syngas into lower olefins via methanol/dimethyl ether intermediates. Chem. Sci. 2018, 9, 4708–4718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Wang, S.; Qin, F.; Huang, L.; Yue, Y.; Hua, W.; Qiao, M.; He, H.; Shen, W.; Xu, H. Ceria-Zirconia/zeolite bifunctional catalyst for highly selective conversion of syngas into aromatics. ChemCatChem 2018, 10, 4519–4524. [Google Scholar] [CrossRef]
- Zhou, W.; Shi, S.; Wang, Y.; Zhang, L.; Wang, Y.; Zhang, G.; Min, X.; Cheng, K.; Zhang, Q.; Kang, J.; et al. Selective conversion of syngas to aromatics over a Mo-ZrO2/H-ZSM-5 bifunctional catalyst. ChemCatChem 2019, 11, 1681–1688. [Google Scholar] [CrossRef]
- Liu, J.; He, Y.; Yan, L.; Li, K.; Zhang, C.; Xiang, H.; Wen, X.; Li, Y. Nano-Sized ZrO2 derived from metal–Organic frameworks and their catalytic performance for aromatic synthesis from syngas. Catal. Sci. Technol. 2019, 9, 2982–2992. [Google Scholar] [CrossRef]
- Xu, H.; Li, M.; Nawaz, M.A.; Liu, D. Doping of K and Zn elements in FeZr-Ni/ZSM-5: Highly selective catalyst for syngas to aromatics. Catal. Commun. 2019, 121, 95–99. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, J.; Wang, J.; Ma, G.; Lin, J.; Yang, Y.; Li, Y.; Zhang, C.; Ding, M. Selective conversion of syngas to aromatics over Fe3O4@MnO2 and hollow HZSM-5 bifunctional catalysts. ACS Catal. 2019, 9, 5147–5156. [Google Scholar] [CrossRef]
- Pokrovski, K.; Jung, K.T.; Bell, A.T. Investigation of CO and CO2 adsorption on tetragonal and monoclinic zirconia. Langmuir 2001, 17, 4297–4303. [Google Scholar] [CrossRef]
- Fan, Y.; Cheng, S.; Wang, H.; Tian, J.; Xie, S.; Pei, Y.; Qiao, M.; Zong, B. Pt–WOx on monoclinic or tetrahedral ZrO2: Crystal phase effect of zirconia on glycerol hydrogenolysis to 1,3-Propanediol. Appl. Catal. B Environ. 2017, 217, 331–341. [Google Scholar] [CrossRef]
- Liu, S.; Wang, H.; Wei, Y.; Zhang, R.; Royer, S. Morphology-Oriented ZrO2-supported vanadium oxide for the NH3-SCR process: Importance of structural and textural properties. ACS Appl. Mater. Interfaces 2019, 11, 22240–22254. [Google Scholar] [CrossRef]
- Foraita, S.; Fulton, J.L.; Chase, Z.A.; Vjunov, A.; Xu, P.; Barath, E.; Camaioni, D.M.; Zhao, C.; Lercher, J.A. Impact of the oxygen defects and the hydrogen concentration on the surface of tetragonal and monoclinic ZrO2 on the reduction rates of stearic acid on Ni/ZrO2. Chem. Eur. J. 2015, 21, 2423–2434. [Google Scholar] [CrossRef] [PubMed]
- De Souza, P.M.; Rabelo-Neto, R.C.; Borges, L.E.P.; Jacobs, G.; Davis, B.H.; Graham, U.M.; Resasco, D.E.; Noronha, F.B. Effect of zirconia morphology on hydrodeoxygenation of phenol over Pd/ZrO2. ACS Catal. 2015, 5, 7385–7398. [Google Scholar] [CrossRef]
- Gu, H.; Ding, J.; Zhong, Q.; Zeng, Y.; Song, F. Promotion of surface oxygen vacancies on the light olefins synthesis from catalytic CO2 hydrogenation over Fe-K/ZrO2 catalysts. Int. J. Hydrog. Energ. 2019, 44, 11808–11816. [Google Scholar] [CrossRef]
- Maruya, K.-I.; Komiya, T.; Hayakawa, T.; Lu, L.; Yashima, M. Active sites on ZrO2 for the formation of isobutene from CO and H2. J. Mol. Catal. A Chem. 2000, 159, 97–102. [Google Scholar] [CrossRef]
- Witoon, T.; Chalorngtham, J.; Dumrongbunditkul, P.; Chareonpanich, M.; Limtrakul, J. CO2 hydrogenation to methanol over Cu/ZrO2 catalysts: Effects of zirconia phases. Chem. Eng. J. 2016, 293, 327–336. [Google Scholar] [CrossRef]
- Ma, Z.-Y.; Yang, C.; Wei, W.; Li, W.-H.; Sun, Y.-H. Catalytic performance of copper supported on zirconia polymorphs for CO hydrogenation. J. Mol. Catal. A Chem. 2005, 231, 75–81. [Google Scholar] [CrossRef]
- He, D.; Ding, Y.; Luo, H.; Li, C. Effects of zirconia phase on the synthesis of higher alcohols over zirconia and modified zirconia. J. Mol. Catal. A Chem. 2004, 208, 267–271. [Google Scholar] [CrossRef]
- Bachiller-Baeza, B.; Rodriguez-Ramos, I.; Guerrero-Ruiz, A. Interaction of carbon dioxide with the surface of zirconia polymorphs. Langmuir 1998, 14, 3556–3564. [Google Scholar] [CrossRef]
- Wu, X.; Tan, M.; Tian, S.; Song, F.; Ma, Q.; He, Y.; Yang, G.; Tsubaki, N.; Tan, Y. Designing ZrO2-Based catalysts for the direct synthesis of isobutene from syngas: The studies on Zn promoter role. Fuel 2019, 243, 34–40. [Google Scholar] [CrossRef]
- Li, Y.; He, D.; Zhu, Q.; Zhang, X.; Xu, B. Effects of redox properties and acid–Base properties on isosynthesis over ZrO2-Based catalysts. J. Catal. 2004, 221, 584–593. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, L.; Yue, B.; Chen, X.; He, H. Simultaneous characterization of solid acidity and basicity of metal oxide catalysts via the solid-state NMR technique. J. Phys. Chem. C 2018, 122, 24094–24102. [Google Scholar] [CrossRef]
- Zheng, A.; Huang, S.-J.; Liu, S.-B.; Deng, F. Acid properties of solid acid catalysts characterized by solid-state 31P NMR of adsorbed phosphorous probe molecules. Phys. Chem. Chem. Phys. 2011, 13, 14889–14901. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Venkatraman, T.; Song, W. NMR study of tungstated zirconia catalyst: Acidic properties of tungstated zirconia and influence of tungsten loading. Appl. Catal. A Gen. 2002, 224, 77–87. [Google Scholar] [CrossRef]
- Li, Y.; He, D.; Yuan, Y.; Cheng, Z.; Zhu, Q. Selective formation of isobutene from CO hydrogenation over zirconium dioxide based catalysts. Energy Fuels 2001, 15, 1434–1440. [Google Scholar] [CrossRef]
- Stöcker, M. Methanol-To-Hydrocarbons: Catalytic materials and their behavior. Micropor. Mesopor. Mater. 1999, 29, 3–48. [Google Scholar] [CrossRef]
- Li, W.; Huang, H.; Li, H.; Zhang, W.; Liu, H. Facile synthesis of pure monoclinic and tetragonal zirconia nanoparticles and their phase effects on the behavior of supported molybdena catalysts for methanol-Selective oxidation. Langmuir 2008, 24, 8358–8366. [Google Scholar] [CrossRef]





| Sample | SBET (m2/g) | Vpore (cm3/g) a | Dpore (nm) | Crystallite Size (nm) b |
|---|---|---|---|---|
| m-ZrO2 | 60 | 0.23 | 15.8 | 10.2 |
| t-ZrO2 | 67 | 0.33 | 19.5 | 11.7 |
| Sample | Acid Density (µmol/g) | Base Density (µmol/g) | |||||
|---|---|---|---|---|---|---|---|
| Lewis Acid | Brønsted Acid | Total | Weak | Moderate | Strong | Total | |
| m-ZrO2 | 217.4 | - | 217.4 | 49.0 | 68.1 | 3.1 | 120.2 |
| t-ZrO2 | 145.2 | 8.3 | 153.5 | 9.1 | 5.5 | - | 14.6 |
| Catalyst | CO Conv. (%) | CO2 sel. (%) | Product Selectivity (%) b | ||||
|---|---|---|---|---|---|---|---|
| CH4 | C2–4= | C2–40 | C5+ | Ar | |||
| m-ZrO2 | 12.9 | 38.3 | 5.4 | 57.8 | 6.7 | 30.1 | - |
| t-ZrO2 | 8.6 | 36.9 | 29.5 | 26.1 | 19.3 | 25.1 | - |
| m-ZrO2/HZSM-5-mix | 24.0 | 36.4 | 4.3 | 2.7 | 21.4 | 4.2 | 67.4 |
| t-ZrO2/HZSM-5-mix | 14.2 | 33.5 | 4.9 | 2.6 | 20.6 | 6.9 | 65.0 |
| m-ZrO2/HZSM-5-db | 14.7 | 41.7 | 5.8 | 1.0 | 66.2 | 22.1 | 4.9 |
| t-ZrO2/HZSM-5-db | 8.7 | 38.5 | 21.0 | 1.5 | 49.5 | 14.2 | 13.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Fang, Y.; Huang, Z.; Xu, H.; Shen, W. The Effects of the Crystalline Phase of Zirconia on C–O Activation and C–C Coupling in Converting Syngas into Aromatics. Catalysts 2020, 10, 262. https://doi.org/10.3390/catal10020262
Wang S, Fang Y, Huang Z, Xu H, Shen W. The Effects of the Crystalline Phase of Zirconia on C–O Activation and C–C Coupling in Converting Syngas into Aromatics. Catalysts. 2020; 10(2):262. https://doi.org/10.3390/catal10020262
Chicago/Turabian StyleWang, Sheng, Yue Fang, Zhen Huang, Hualong Xu, and Wei Shen. 2020. "The Effects of the Crystalline Phase of Zirconia on C–O Activation and C–C Coupling in Converting Syngas into Aromatics" Catalysts 10, no. 2: 262. https://doi.org/10.3390/catal10020262