Figure 1.
Catalytic activity results obtained for monometallic Ni/ZrO2 catalysts in the oxy-steam reforming of methane expressed as methane conversion, and the selectivity of the CO and CO2 and hydrogen yield formed during the process. (A) Methane conversion. (B) The selectivity towards CO and CO2 and hydrogen yield.
Figure 1.
Catalytic activity results obtained for monometallic Ni/ZrO2 catalysts in the oxy-steam reforming of methane expressed as methane conversion, and the selectivity of the CO and CO2 and hydrogen yield formed during the process. (A) Methane conversion. (B) The selectivity towards CO and CO2 and hydrogen yield.
Figure 2.
Catalytic activity results obtained in the oxy-steam reforming of methane expressed as methane conversion, and the selectivity of the CO and CO2 formed during the process and hydrogen yield. (A,B)—20%Ni/ZrO2, (C,D)—20%Ni/5%La2O3-ZrO2, (E,F)—20%Ni/5%CeO2-ZrO2.
Figure 2.
Catalytic activity results obtained in the oxy-steam reforming of methane expressed as methane conversion, and the selectivity of the CO and CO2 formed during the process and hydrogen yield. (A,B)—20%Ni/ZrO2, (C,D)—20%Ni/5%La2O3-ZrO2, (E,F)—20%Ni/5%CeO2-ZrO2.
Figure 3.
Results of the catalytic activity measurements performed in the temperature range 400–900 °C in the OSRM of a liquified natural gas (LNG) reaction on a 20%Ni/ZrO2 catalyst. (A) Hydrocarbon conversion. (B) The selectivity of CO and CO2 and hydrogen yield.
Figure 3.
Results of the catalytic activity measurements performed in the temperature range 400–900 °C in the OSRM of a liquified natural gas (LNG) reaction on a 20%Ni/ZrO2 catalyst. (A) Hydrocarbon conversion. (B) The selectivity of CO and CO2 and hydrogen yield.
Figure 4.
Results of the catalytic activity measurements performed in the temperature range 400–900 °C in the OSRM of the LNG reaction on a 20%Ni/5%La2O3-ZrO2 catalyst. (A) Hydrocarbon conversion. (B) The selectivity of CO and CO2 and hydrogen yield.
Figure 4.
Results of the catalytic activity measurements performed in the temperature range 400–900 °C in the OSRM of the LNG reaction on a 20%Ni/5%La2O3-ZrO2 catalyst. (A) Hydrocarbon conversion. (B) The selectivity of CO and CO2 and hydrogen yield.
Figure 5.
Results of the catalytic activity measurements performed in the temperature range 400–900 °C in the OSRM of the LNG reaction on a 20%Ni/5%CeO2-ZrO2 catalyst. (A) Hydrocarbon conversion. (B) The selectivity of CO and CO2 and hydrogen yield.
Figure 5.
Results of the catalytic activity measurements performed in the temperature range 400–900 °C in the OSRM of the LNG reaction on a 20%Ni/5%CeO2-ZrO2 catalyst. (A) Hydrocarbon conversion. (B) The selectivity of CO and CO2 and hydrogen yield.
Figure 6.
Results of the time-on-stream catalytic tests performed during 12 h of the OSRM of the LNG reaction on a 20%Ni/ZrO2 catalyst. (A) Hydrocarbon conversion. (B) The selectivity of CO and CO2 and hydrogen yield.
Figure 6.
Results of the time-on-stream catalytic tests performed during 12 h of the OSRM of the LNG reaction on a 20%Ni/ZrO2 catalyst. (A) Hydrocarbon conversion. (B) The selectivity of CO and CO2 and hydrogen yield.
Figure 7.
Results of the time-on-stream catalytic tests performed during 12 h of the OSRM of the LNG reaction on a 20%Ni/5%La2O3-ZrO2 catalyst. (A) Hydrocarbon conversion. (B) The selectivity of the CO and CO2 and hydrogen yield.
Figure 7.
Results of the time-on-stream catalytic tests performed during 12 h of the OSRM of the LNG reaction on a 20%Ni/5%La2O3-ZrO2 catalyst. (A) Hydrocarbon conversion. (B) The selectivity of the CO and CO2 and hydrogen yield.
Figure 8.
Results of the time-on-stream catalytic tests performed during 12 h of the OSRM of the LNG reaction on a 20%Ni/5%CeO2-ZrO2 catalyst. (A) Hydrocarbon conversion. (B) The selectivity of the CO and CO2 and hydrogen yield.
Figure 8.
Results of the time-on-stream catalytic tests performed during 12 h of the OSRM of the LNG reaction on a 20%Ni/5%CeO2-ZrO2 catalyst. (A) Hydrocarbon conversion. (B) The selectivity of the CO and CO2 and hydrogen yield.
Figure 9.
XRD curves of the ZrO2 and monometallic Ni/ZrO2 catalysts calcined in an air atmosphere for 4h at 400 °C.
Figure 9.
XRD curves of the ZrO2 and monometallic Ni/ZrO2 catalysts calcined in an air atmosphere for 4h at 400 °C.
Figure 10.
XRD curves of supports 5%La2O3-ZrO2, 5%CeO2-ZrO2, and monometallic Ni catalysts supported on ZrO2, 5%CeO2-ZrO2, and 5%La2O3-ZrO2 calcined in an air atmosphere for 4h at 400 °C.
Figure 10.
XRD curves of supports 5%La2O3-ZrO2, 5%CeO2-ZrO2, and monometallic Ni catalysts supported on ZrO2, 5%CeO2-ZrO2, and 5%La2O3-ZrO2 calcined in an air atmosphere for 4h at 400 °C.
Figure 11.
XRD curves of spent 20%Ni catalysts supported on ZrO2, 5%La2O3-ZrO2, and 5%CeO2-ZrO2 carriers.
Figure 11.
XRD curves of spent 20%Ni catalysts supported on ZrO2, 5%La2O3-ZrO2, and 5%CeO2-ZrO2 carriers.
Figure 12.
Temperature programmed reduction profiles of ZrO2 and Ni catalysts supported on ZrO2 oxide calcined in an air atmosphere for 4h at 400 °C.
Figure 12.
Temperature programmed reduction profiles of ZrO2 and Ni catalysts supported on ZrO2 oxide calcined in an air atmosphere for 4h at 400 °C.
Figure 13.
Temperature programmed reduction profiles of 20%Ni catalysts supported on 5%La2O3-ZrO2, 5%CeO2-ZrO2, and ZrO2 calcined in an air atmosphere for 4h at 400 °C.
Figure 13.
Temperature programmed reduction profiles of 20%Ni catalysts supported on 5%La2O3-ZrO2, 5%CeO2-ZrO2, and ZrO2 calcined in an air atmosphere for 4h at 400 °C.
Figure 14.
BET adsorption–desorption isotherms for supports and monometallic Ni catalysts calcined in an air atmosphere at 400 °C for 4 h.
Figure 14.
BET adsorption–desorption isotherms for supports and monometallic Ni catalysts calcined in an air atmosphere at 400 °C for 4 h.
Figure 15.
BET adsorption–desorption isotherms for monometallic Ni catalysts calcined in an air atmosphere at 400 °C for 4 h.
Figure 15.
BET adsorption–desorption isotherms for monometallic Ni catalysts calcined in an air atmosphere at 400 °C for 4 h.
Figure 16.
TPD-NH3 profile of ZrO2, 5%CeO2-ZrO2, and 5%La2O3-ZrO2 carriers calcined in an air atmosphere for 4 h at 400 °C.
Figure 16.
TPD-NH3 profile of ZrO2, 5%CeO2-ZrO2, and 5%La2O3-ZrO2 carriers calcined in an air atmosphere for 4 h at 400 °C.
Figure 17.
TPD-NH3 profile of Ni/ZrO2 catalysts reduced in hydrogen for 1 h at 500 °C.
Figure 17.
TPD-NH3 profile of Ni/ZrO2 catalysts reduced in hydrogen for 1 h at 500 °C.
Figure 18.
TPD-NH3 profile of 20%Ni/ZrO2, 20%Ni/5%CeO2-ZrO2, and 20%Ni/5%La2O3-ZrO2 catalysts reduced in hydrogen for 1 h at 500 °C.
Figure 18.
TPD-NH3 profile of 20%Ni/ZrO2, 20%Ni/5%CeO2-ZrO2, and 20%Ni/5%La2O3-ZrO2 catalysts reduced in hydrogen for 1 h at 500 °C.
Figure 19.
Measurements for monometallic Ni catalysts supported on ZrO2 oxide calcined at 400 °C in an air atmosphere for 4 h.
Figure 19.
Measurements for monometallic Ni catalysts supported on ZrO2 oxide calcined at 400 °C in an air atmosphere for 4 h.
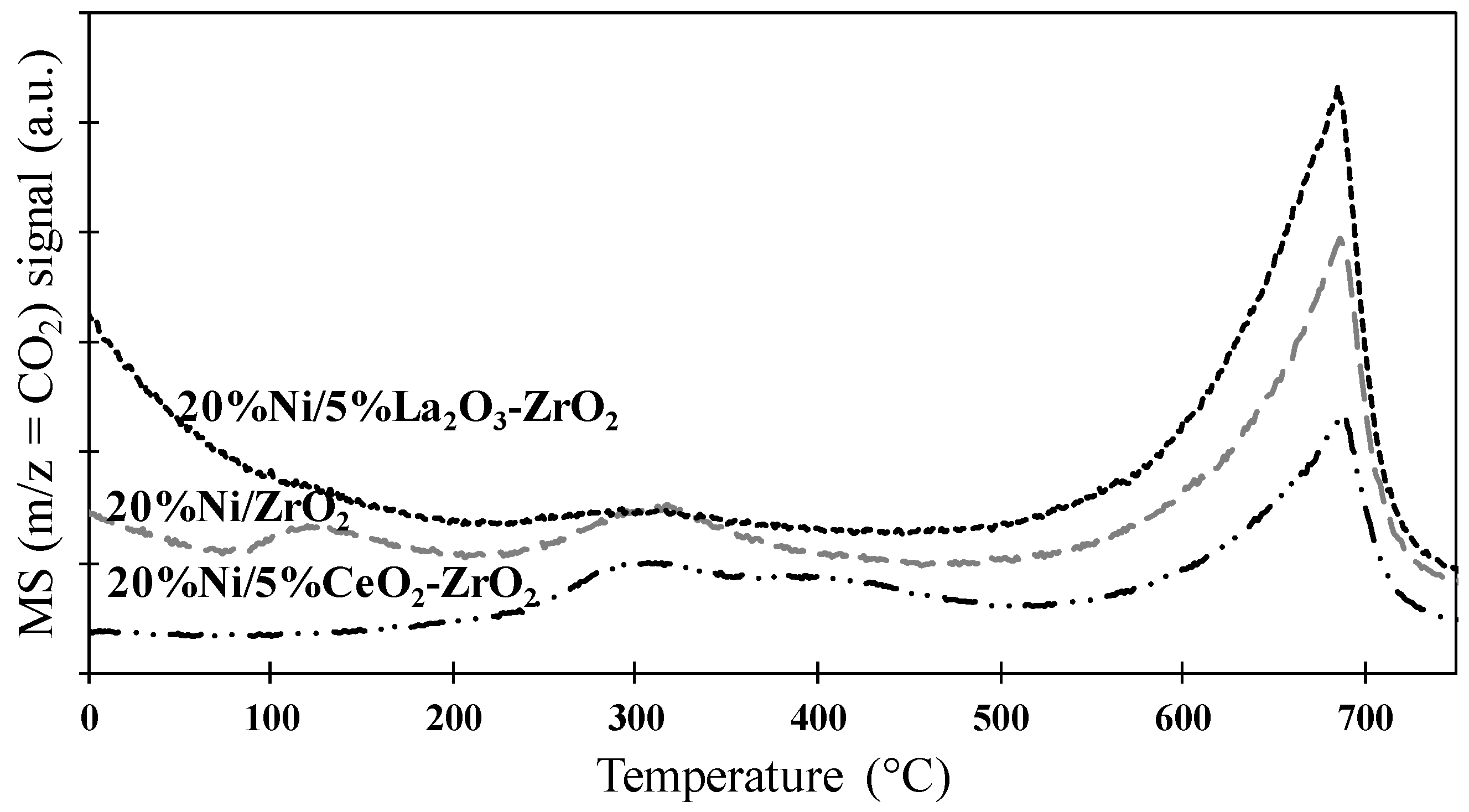
Figure 20.
MS profiles for m/z = 44 (CO2) obtained during the thermal analysis performed in an air atmosphere of the spent (after twelve hours of the oxy-steam reforming of LNG process at 700 °C, molar ratio between reagents was used: C:H2O:O2 = 1:2.7:0.35, total gas flow 51 cm3/min) monometallic Ni catalysts supported on ZrO2, 5%CeO2-ZrO2, or 5%La2O3-ZrO2 oxides supports.
Figure 20.
MS profiles for m/z = 44 (CO2) obtained during the thermal analysis performed in an air atmosphere of the spent (after twelve hours of the oxy-steam reforming of LNG process at 700 °C, molar ratio between reagents was used: C:H2O:O2 = 1:2.7:0.35, total gas flow 51 cm3/min) monometallic Ni catalysts supported on ZrO2, 5%CeO2-ZrO2, or 5%La2O3-ZrO2 oxides supports.
Table 1.
Size of NiO crystallites estimated by the Scherrer method for a NiO peak (200) for an angle of 2theta = 43.36° obtained from XRD measurements performed for Ni catalysts calcined in an air atmosphere at 400 °C for 4 h.
Table 1.
Size of NiO crystallites estimated by the Scherrer method for a NiO peak (200) for an angle of 2theta = 43.36° obtained from XRD measurements performed for Ni catalysts calcined in an air atmosphere at 400 °C for 4 h.
| No. | Catalyst | The Size of NiO Crystallites [nm] |
|---|
| 1 | 20Ni%ZrO2 | 32 |
| 2 | 20%Ni-5%CeO2-ZrO2 | 24 |
| 3 | 20%Ni-5%La2O3-ZrO2 | 20 |
Table 2.
Crystallite size of metallic nickel calculated from XRD measurements performed for spent Ni catalysts (after catalytic tests performed in the oxy-steam reforming of LNG process in the temperature range 400–700 °C, a molar ratio between reagents was used: C:H2O:O2 = 1:2.7:0.35, the total gas flow was 51 cm3/min).
Table 2.
Crystallite size of metallic nickel calculated from XRD measurements performed for spent Ni catalysts (after catalytic tests performed in the oxy-steam reforming of LNG process in the temperature range 400–700 °C, a molar ratio between reagents was used: C:H2O:O2 = 1:2.7:0.35, the total gas flow was 51 cm3/min).
| No. | Catalyst | The Size of Ni° Crystallites [nm] |
|---|
| 1 | 20%Ni/ZrO2 | 35 |
| 2 | 20%Ni/5%CeO2-ZrO2 | 22 |
| 3 | 20%Ni/5%La2O3-ZrO2 | 41 |
Table 3.
Amount of hydrogen consumed during the TPR run and the estimated reduction degree for the nickel catalysts.
Table 3.
Amount of hydrogen consumed during the TPR run and the estimated reduction degree for the nickel catalysts.
| Catalysts | H2 Consumed (mol/gcat) × 10+4 | Reduction of NiO (%) |
|---|
| 5%Ni/ZrO2 | 0.84 | 99 |
| 10%Ni/ZrO2 | 1.69 | 99 |
| 20%Ni/ZrO2 | 2.70 | 80 |
| 30%Ni/ZrO2 | 3.60 | 71 |
| 20%Ni/5%La2O3-ZrO2 | 3.36 | 99 |
| 20%Ni/5%CeO2-ZrO2 | 2.01 | 60 |
Table 4.
Specific surface area and pore size distributions of support and monometallic Ni-supported catalysts calcined in an air atmosphere at 400 °C for 4 h.
Table 4.
Specific surface area and pore size distributions of support and monometallic Ni-supported catalysts calcined in an air atmosphere at 400 °C for 4 h.
| Materials | BET Surface Area (m2/g) | Monolayer Capacity (cm3/g) | Average Pore Radius (nm) |
|---|
| ZrO2 | 109.6 | 0.26 | 3.4 |
| 5%CeO2-ZrO2 | 98.5 | 0.22 | 3.2 |
| 5%La2O3-ZrO2 | 92.7 | 0.21 | 2.9 |
| 5%Ni/ZrO2 | 94.4 | 0.23 | 3.5 |
| 10%Ni/ZrO2 | 85.0 | 0.18 | 3.1 |
| 20%Ni/ZrO2 | 76.8 | 0.18 | 3.4 |
| 20%Ni/5%CeO2-ZrO2 | 79.4 | 0.17 | 3.2 |
| 20%Ni/5%La2O3-ZrO2 | 73.6 | 0.17 | 3.4 |
| 30%Ni/ZrO2 | 63.4 | 0.17 | 3.2 |
Table 5.
Amount of NH3 adsorbed on the surface of ZrO2 and the reduced monometallic Ni-supported catalysts (reduction for 1 h in pure hydrogen at 500 °C) calculated from the surface under the peaks recorded during temperature-programmed desorption measurements.
Table 5.
Amount of NH3 adsorbed on the surface of ZrO2 and the reduced monometallic Ni-supported catalysts (reduction for 1 h in pure hydrogen at 500 °C) calculated from the surface under the peaks recorded during temperature-programmed desorption measurements.
| Catalytic Systems | Total Acidity (mmol/g) | Weak Centers (mmol/g) | Medium Centers (mmol/g) | Strong Centers (mmol/g) |
|---|
| 100–600 °C | 100–300 °C | 300–450 °C | >450 °C |
|---|
| ZrO2 | 0.101 | 0.054 | 0.040 | 0.007 |
| 5%CeO2-ZrO2 | 1.192 | 0.638 | 0.470 | 0.084 |
| 5%La2O3-ZrO2 | 1.384 | 0.688 | 0.608 | 0.088 |
| 5% Ni/ZrO2 | 0.142 | 0.078 | 0.060 | 0.004 |
| 10% Ni/ZrO2 | 0.179 | 0.120 | 0.540 | 0.005 |
| 20% Ni/ZrO2 | 0.217 | 0.125 | 0.086 | 0.006 |
| 20% Ni/5%CeO2-ZrO2 | 0.620 | 0.328 | 0.234 | 0.057 |
| 20% Ni/5%La2O3-ZrO2 | 0.734 | 0.332 | 0.320 | 0.081 |
| 30% Ni/ZrO2 | 0.360 | 0.170 | 0.170 | 0.020 |
Table 6.
TOF-SIMS results obtained for spent Ni catalysts supported on ZrO2 oxide.
Table 6.
TOF-SIMS results obtained for spent Ni catalysts supported on ZrO2 oxide.
| | Number of Counts × 103 |
|---|
| Ion | 5%Ni/ZrO2 | 10%Ni/ZrO2 | 20%Ni/ZrO2 | 30%Ni/ZrO2 |
|---|
| Ni+ | 45.0 | 67.1 | 180.3 | 163.9 |
| NiOH+ | 3.0 | 5.1 | 12.0 | 9.0 |
| Zr+ | 16.2 | 17.4 | 14.5 | 7.1 |
| ZrO+ | 56.5 | 60.3 | 46.6 | 18.4 |
| ZrOH+ | 26.5 | 29.0 | 21.6 | 9.0 |
| NiZrO+ | - | 0.4 | 0.5 | - |
Table 7.
The relation between the intensity of selected ions and the nickel content estimated by TOF-SIMS measurements.
Table 7.
The relation between the intensity of selected ions and the nickel content estimated by TOF-SIMS measurements.
| Catalyst | Ion Ratio Intensities of Ni+/ZrO+ |
|---|
| 5%Ni/ZrO2 | 0.78 |
| 10%Ni/ZrO2 | 1.11 |
| 20%Ni/ZrO2 | 3.87 |
| 30%Ni/ZrO2 | 8.90 |