Virgin Heavy Gas Oil from Oil Sands Bitumen as FCC Feed
Abstract
:1. Introduction
2. Results and Discussion
2.1. Material Properties
2.2. Conversion and Yield
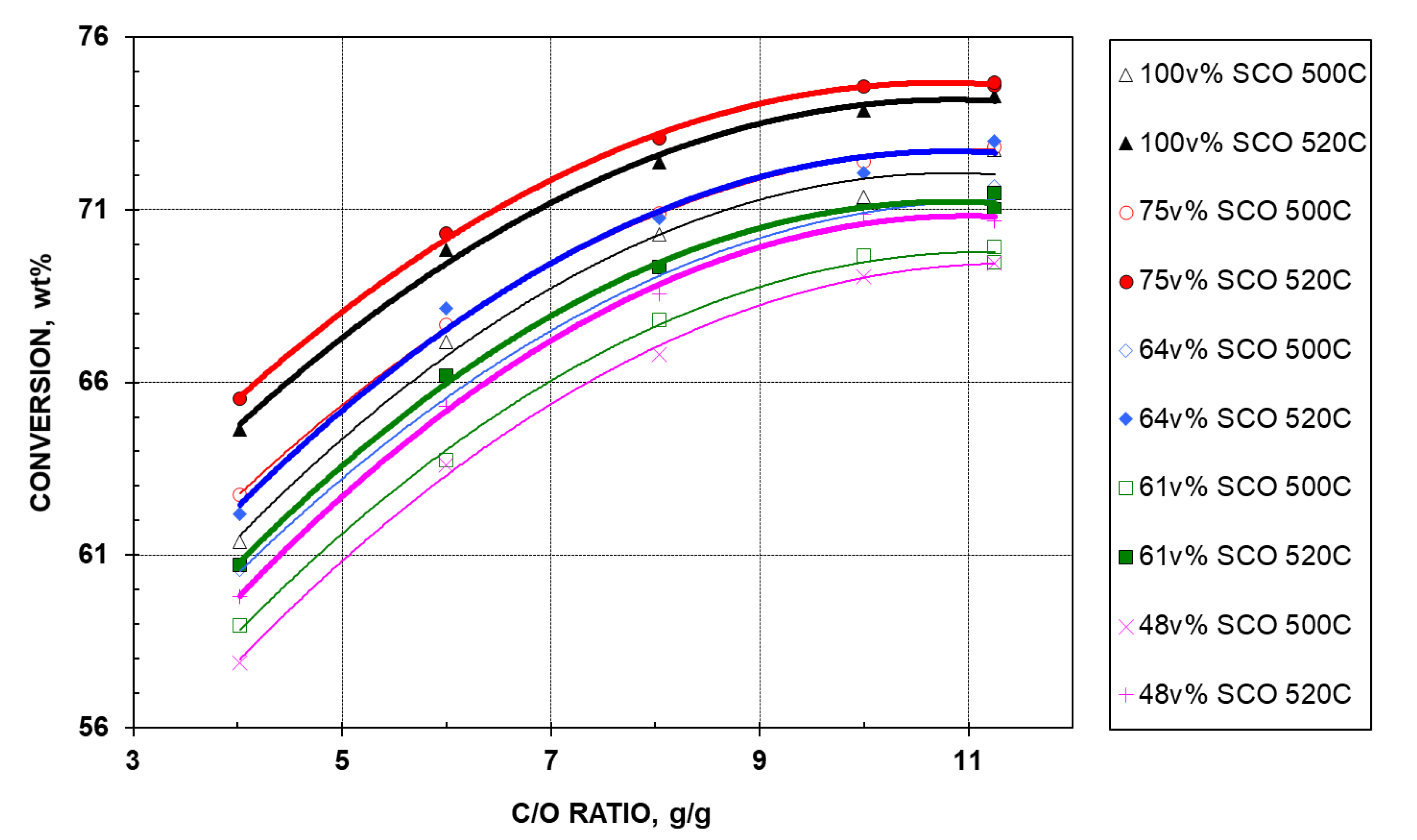
2.2.1. Conversion
- At a certain temperature, conversion of a feed increases with CTO, and levels off at high CTOs. This behavior is not unexpected, as higher CTO ratios will give a greater number of active sites on the catalyst on which feed molecules can be cracked. At high CTOs, the gasoline precursors (crackable components) are gradually used up, and the cracking rate will be reduced.
- For a given feed, conversion is increased by an elevated temperature. This is explained easily, as an elevated temperature (1) promotes cracking, which produces more gases and more lighter liquid products, and (2) reduces the catalyst poisoning of basic nitrogen in the feed that is more prevalent at lower temperatures. At higher CTOs, the nitrogen effect is also reduced, as there is less nitrogen in the system, resulting from having less oil in contact with the same amount of catalyst. This explains the observed narrower conversion gap between two temperatures for the same feed as CTO increases (Figure 1). However, careful examination reveals that not all feeds increase in conversion at higher temperature to the same degree. In general, the better the quality of the feed (or the richer the SCO HGO in the feed), the larger the resulting conversion gap at the same CTO. This is because (1) the better feed with more gasoline precursors can be cracked better at a higher temperature (520 °C) to produce more light products, resulting in higher conversions across the whole CTO range, and (2) the better feed also contains more nitrogen, leading to lower conversions at a lower temperature (500 °C). The combined effect is responsible for the observed larger conversion gap between the two temperatures for the feed with better quality.
- Perhaps the most surprising observation is that, at a given temperature (500 °C or 520 °C) and the same CTO, the order of conversion does not follow the order of feed quality-the blend containing 75 v% SCO HGO (called “75% feed” for simplicity) shows the highest conversion, followed by 100%, 64%, 61%, and 48% feed, in sequence. Possible explanations are as follows.
- ✓
- Nitrogen content is lower in bitumen HGO at 1355 wppm as compared to 1584 wppm N in SCO HGO, and lower N feeds should crack better.
- ✓
- In a comprehensive study involving ~30 different nitrogen compounds, Fu and Schaffer [11] reported that the poisoning effect of a nitrogen compound on cracking yields depended strongly on its molecular structure with respect to type of heterocyclic, molecular size, the numbers of nitrogen contained in rings, the degree of saturation, and the presence of alkyl substitutes. On the issue of degree of saturation, hydrogenation of heterocyclic rings can dramatically change the distribution of nitrogen types, e.g., conversion of a neutral compound such as pyrrole (C4H5N) to a base such as pyrrolidine (C4H9N).
- ✓
- Bitumen HGO has a higher monoaromatics content than SCO HGO (28.9 vs. 23.0 wt%). This boosts the gasoline precursors (and FCC performance) of some of their blends, although bitumen HGO contains less saturates (Table 1).
- ✓
- Being virgin in nature (not having been exposed to any cracking or reaction environment), bitumen HGO is believed to be more reactive than its counterpart from SCO in terms of having larger or bulkier molecules and longer aromatic side chains, which are easier to crack. The 75% SCO-HGO feed has more of these longer/bulkier molecules than the 100% SCO-HGO feed, and should behave better upon catalytic cracking.
2.2.2. Dry Gas Yield
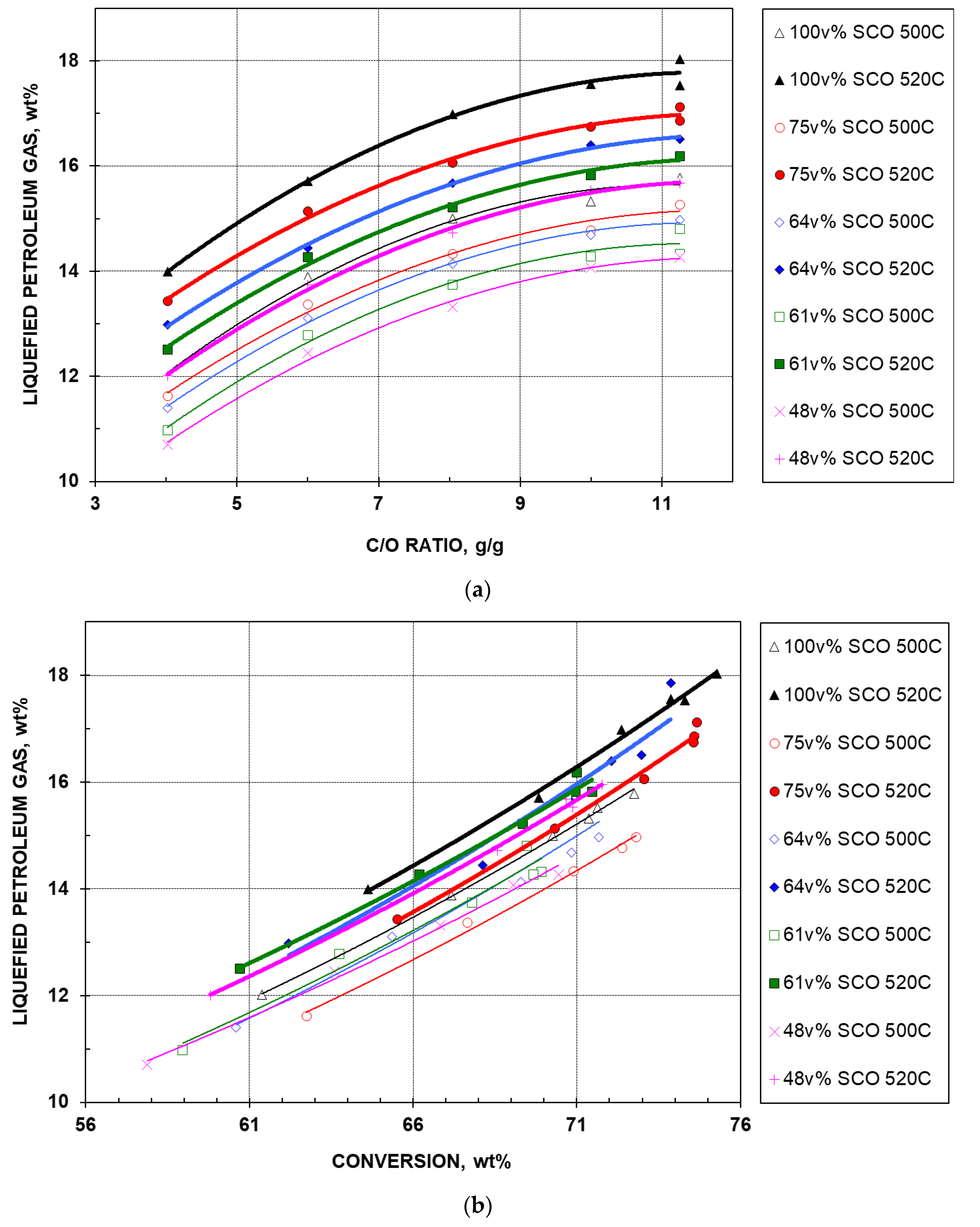
2.2.3. Liquefied Petroleum Gas Yield
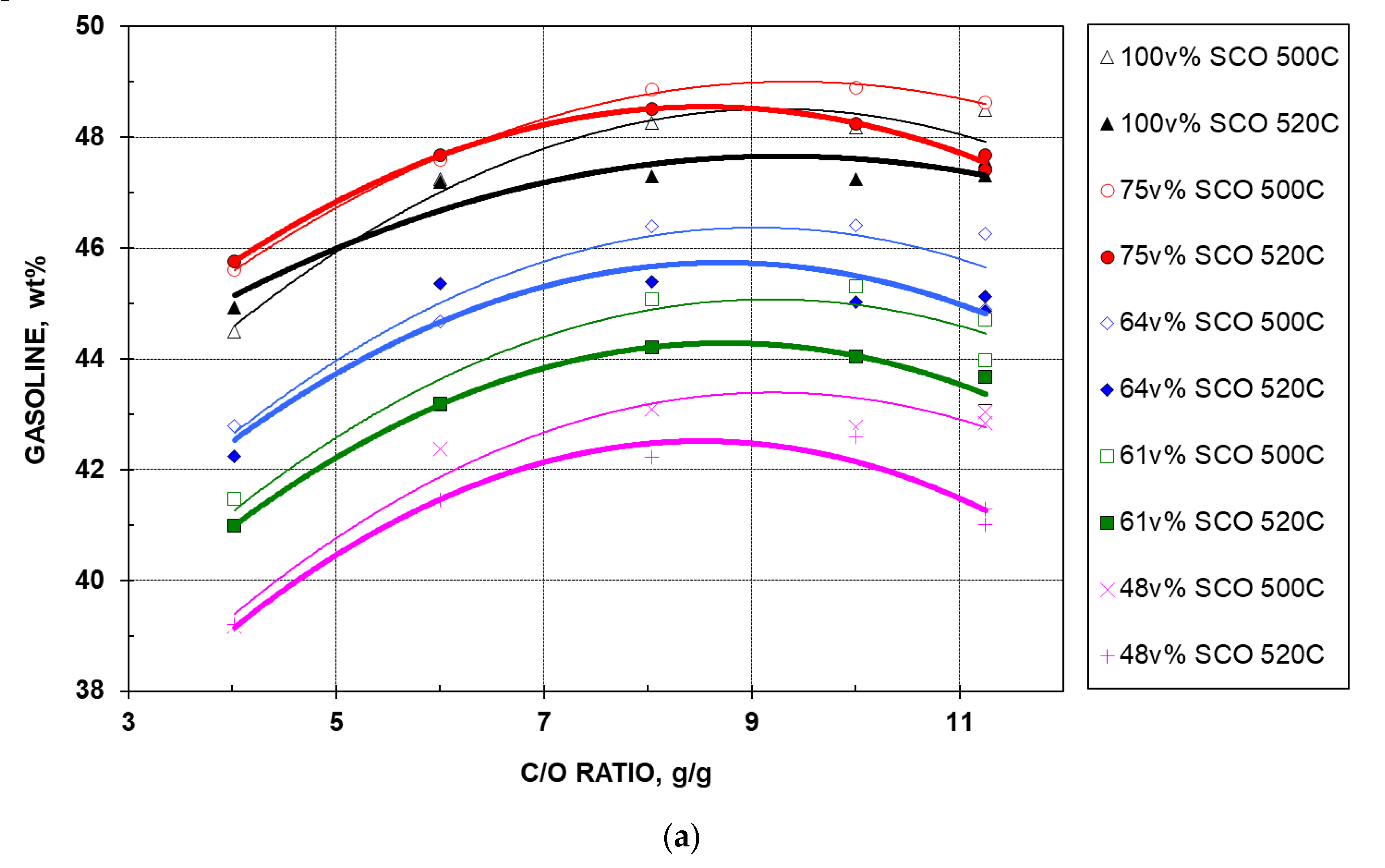
2.2.4. Gasoline Yield
- For the five feeds at a given temperature, gasoline yield increases with CTO, but only up to a certain point (at a CTO of ~9 at 500 °C), after which the yield begins to decline at higher CTOs. A maximum signifies that the rate of formation of gasoline from cracking of its precursors (i.e., feed, HCO, and/or LCO) is exceeded by the consumption of gasoline to produce LPG, dry gas, and coke. This behavior is usually termed “over-cracking”. For the same feed, the maximum is reached at a lower CTO when the temperature is higher. This is understandable, as higher temperature favors production of gases and coke at the expense of gasoline.
- For the five feeds at a given CTO, gasoline yield decreases when the temperature is increased from 500 to 520 °C, except for the 100 and 75% feeds, which show an opposite trend at very low CTOs. The latter observation can be explained by the nitrogen poisoning effect (of the two nitrogen-rich feeds), which is reduced at higher temperature (520 °C). The gap between the two yield curves at different temperatures widens as CTO increases, which is attributable to the earlier over-cracking (at a lower CTO) of the feed cracked at a higher temperature (520 °C), as mentioned above.
- As in the case of conversion, among the five feeds at a given temperature and CTO, the 75% feed gives the highest gasoline yield, followed by the 100%, 64%, 61%, and 48% feeds, in sequence. Again, the lower nitrogen content and the virgin nature of bitumen HGO in the blend is believed the key factor contributing to the high gasoline yield of the 75% feed. However, the poisoning effect of polynuclear aromatics comes into play, resulting in low gasoline yields at elevated additions of bitumen HGO.
- Figure 4a was converted into Figure 4b through the conversion–CTO relationship that is shown in Figure 1. In Figure 4b, over-cracking phenomena are observed at 520 °C with an overall general trend, indicating that the occurrence of over-cracking depends on the aromatic content of the feed—when aromatics content is higher, the maximum appears at a lower conversion. Higher temperature can shift over-cracking to a lower conversion. The above observation agrees well with our previous finding [14]. Figure 4b also confirms the superior performance of the 75% feed, which gives the highest gasoline yield, as well as the order of performance of all five feeds at a given temperature.
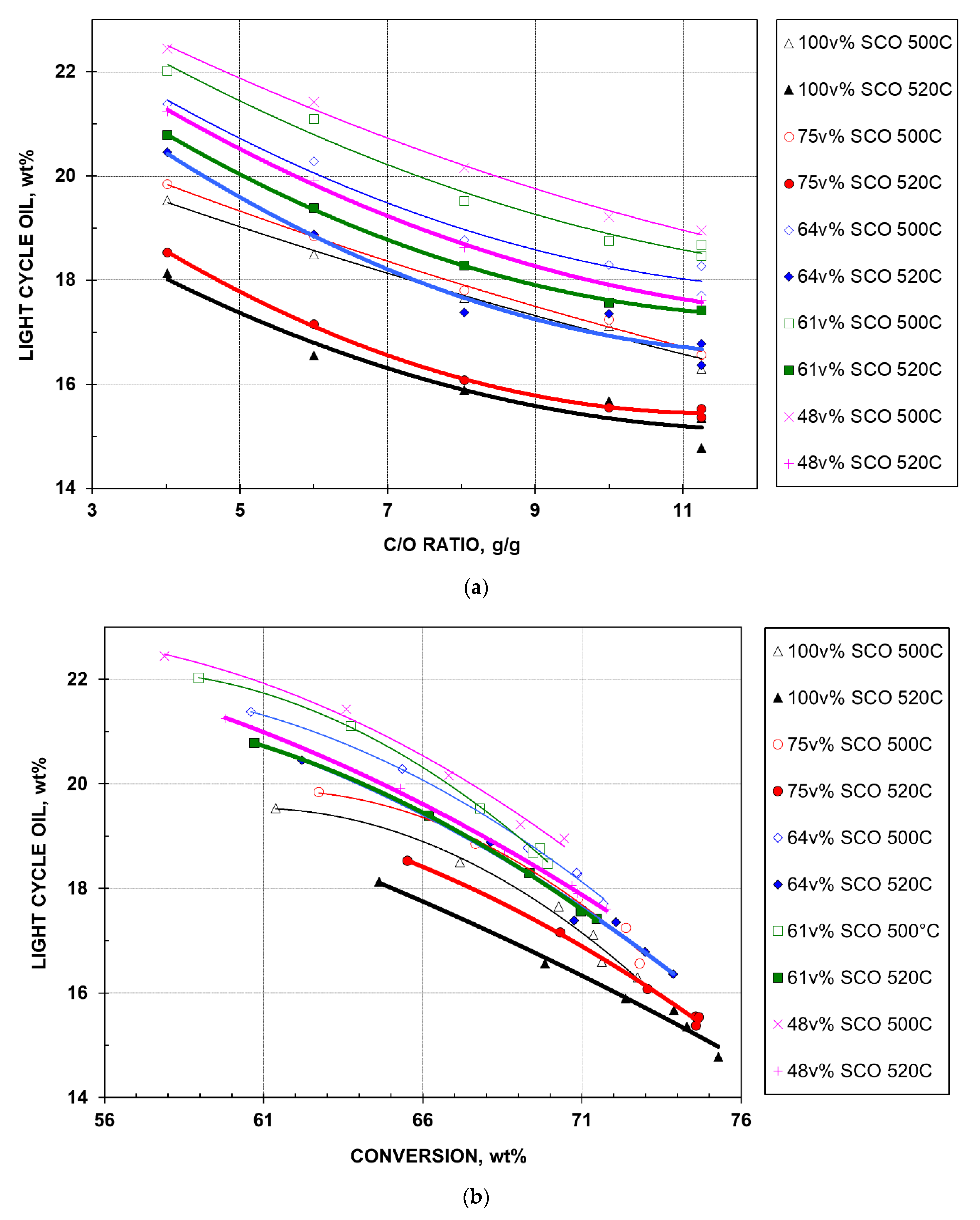
2.2.5. Light Cycle Oil Yield
- For a given temperature and feed, LCO yield decreases monotonously and in parallel with CTO. At high CTOs, the concave yield curves level off, approaching the limit of LCO precursors, which is defined as the sum of diaromatics, 2-ring aromatic sulfur, and half of the 3-ring aromatic sulfur [6]. This definition assumes that the remaining core compounds will boil in the LCO range (221–343 °C) after the precursor side chains are removed.
- At a given temperature and the same CTO, among the five feeds, the 100% SCO HGO feed exhibits the lowest LCO yield, followed by the 75, 64, 61, and 48% feeds, sequentially. This is in agreement with their respective LCO precursor contents in Table 1. However, Figure 5a seems to reveal that the 75% feed is cracked excessively, with LCO yield close to that of the 100% feed. Again, this is credited by the lower nitrogen content and the virgin nature of bitumen HGO in blend.
- For the same feed at a given CTO, LCO yield is observed to decrease at higher temperatures. This helps drive the cracking of large molecules (e.g., those found in LCO) into smaller molecules (gases and gasoline). However, the yield gap between the two temperatures seems to depend on the feed quality, with the 100% SCO HGO feed showing the largest gap.
- The LCO selectivity (yield over conversion) plot is shown in Figure 5b, which combines Figure 1 and Figure 5a. Nearly all yield curves exhibit convex decreases. In theory, they should merge (by definition of conversion, see Section 2.2.1) at 100 wt% conversion with 0 wt% LCO. Note that the five feeds contain 1.9 to 4.8 wt% material in the LCO boiling range (Table 1). Thus, when catalytic cracking occurs, the LCO yield of each feed should initially increase, and then decrease after reaching a maximum (as conversion increases). This behavior is not observed in Figure 5b. In Figure 5b, the maxima occur at lower conversions, which are not attained in this study.
2.2.6. Heavy Cycle Oil Yield
2.2.7. Coke Yield
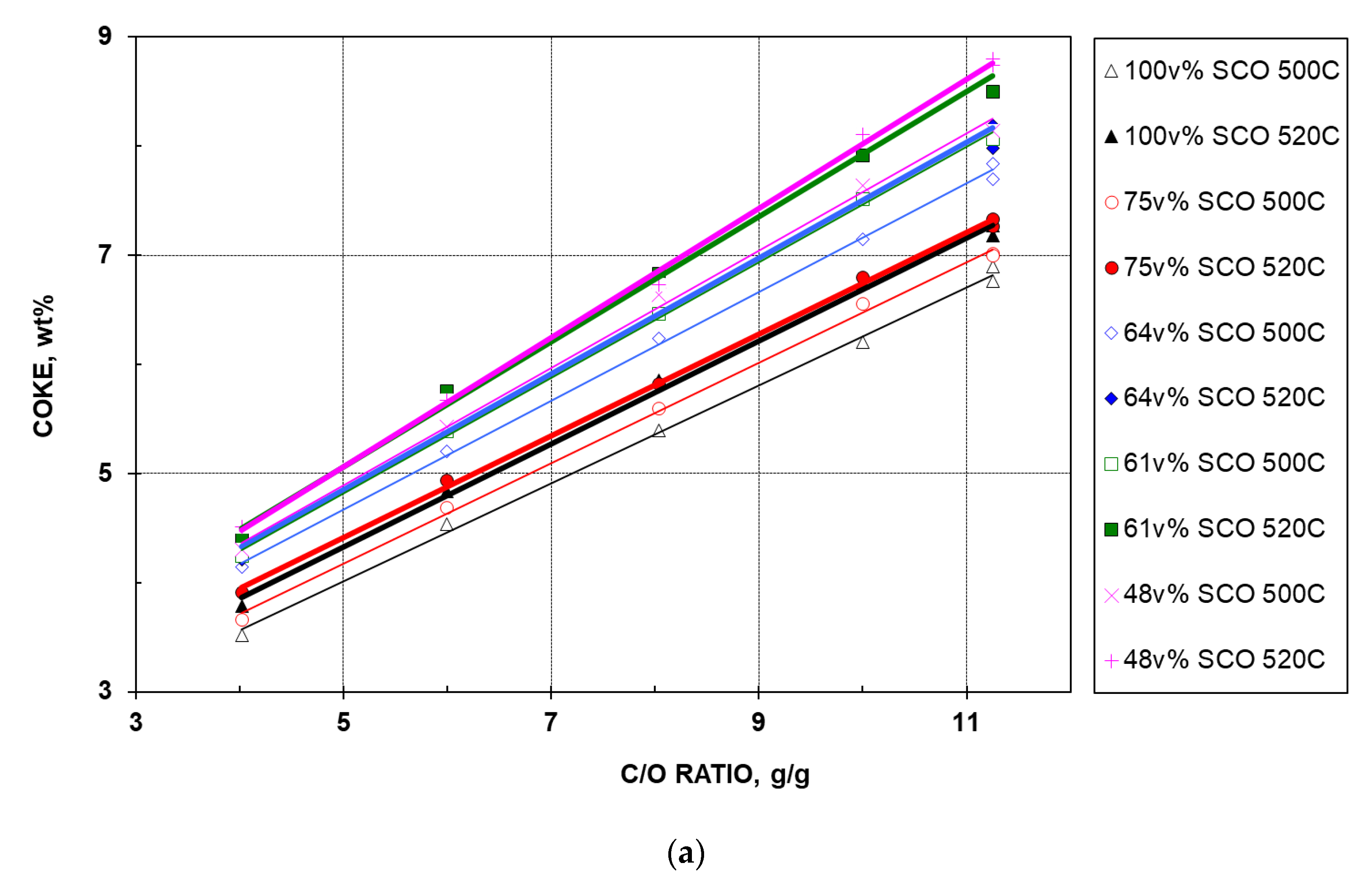
- For the five feeds at a given temperature, coke yield increases linearly with CTO.
- For a given feed and CTO, coke yield increases with elevated temperature. This increase is understandable as a higher severity (either CTO or temperature) promotes cracking with coke as a product.
- At a given temperature and the same CTO, coke yield depends on the feed quality. In line with theory, the 100% SCO HGO feed, containing the least aromatics (Table 1), gives the lowest coke yield, followed by the 75, 64, 61, and 48% feeds, sequentially. Note that the difference between coke yields of the two best feeds at 520 °C is minimal.
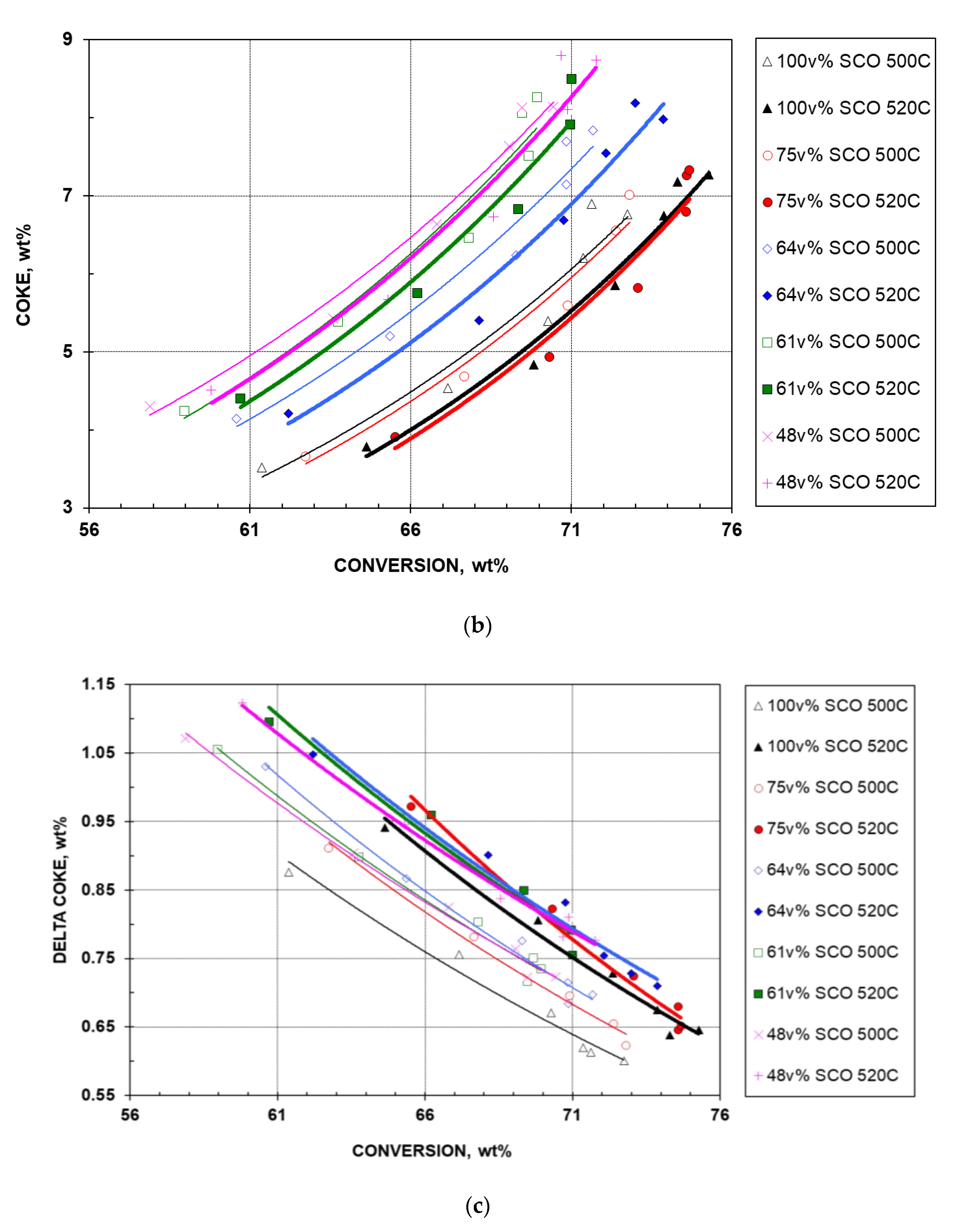
- The corresponding coke selectivity plot derived from Figure 1 and Figure 7a is shown in Figure 7b. For the five feeds at a given temperature, coke exhibits an exponential increase in yield at higher conversions. For the same feed at a given conversion, a decreased coke yield is observed as temperature increases. At a given temperature and the same conversion, coke yield decreases with improved feed quality except for the 75% feed, which exhibits slightly lower coke yield than the best feed, the pure SCO HGO. These observations can be explained by the data in Figure 1 and Figure 7a.
2.3. Assessment of Blends as FCC Feeds
- At a given temperature, as feedstock quality decreases with lower SCO HGO addition, the gasoline first increases then decreases; the LPG and coke first decrease (based on data point, not trend line, for coke) then increase; dry gas, LCO, and delta coke increase at all times (very minor increase for delta coke); and HCO decreases at all times.
- Among the blends at a given temperature, the 75% feed is no doubt the best FCC feed which outperforms the 100% SCO-HGO feed with higher gasoline and diesel and lower HCO and coke, although there is a mild increase in dry gas and a drop in LPG.
- HGO from Synsynbit with 64 v% SCO addition also behaves acceptably with a small decrease in gasoline and a slight increase in diesel.
- HGO from Synbit containing more than 50 v% bitumen is, of course, expected to perform relatively poorly. Given the much lower market price of synbit, this option may still be worth considering.
- Increasing the temperature from 500 to 520 °C reduces gasoline, LCO, and coke, but increases all gaseous products, HCO, and delta coke (very little increase for delta coke).
3. Experimental
3.1. Materials
3.2. Apparatus
3.3. Experimental Conditions
3.4. Error Analysis of ACE Data
4. Conclusions
- Virgin HGO from raw bitumen contains less nitrogen than its counterpart from SCO, and has relatively low Ni + V and MCR. These poisons impose no serious threat to the cracking catalyst. As a result, in the presence of a suitable amount of SCO HGO, the cracking performance of the blend, in terms of conversion and yields of gasoline, diesel, and coke, improves. It can be accepted as FCC feed.
- The major challenge is the mild increase in the dry gas yield, notably due to the augmentation of H2S, and higher sulfur levels in gasoline and diesel, which may need desulfurization prior to being sent to gasoline and diesel pools.
- For a given HGO blend at a constant conversion, higher cracking temperature produces more dry gas and delta coke and less gasoline. Thus, it is advantageous to crack the blend at a lower temperature, thereby reducing secondary reactions.
- In this study, overcracking, where gasoline started to decline at a given conversion, is observed in many occasions. This is governed by the aromatics in the feed―the higher the aromatics, the lower the conversion at which overcracking is observed. Higher temperature can shift overcracking to a lower conversion.
- Gasoline yield is strongly affected by gasoline precursors, defined as feed saturates plus monoaromatics, whereas LCO yield is by LCO precursors, defined as the summation of diaromatics, 2-ring aromatic sulfur, and 1/2 of the 3-ring aromatic sulfur.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yui, S. Producing quality synthetic crude oil from Canadian oil sands bitumen. J. Jpn. Petro. Inst. 2008, 51, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Yui, S.; Dodge, T. Producing quality diesel fuel and FCC feedstock from bitumen-derived heavy gas oils by hydrocracking. The Commemorative International Symposium on the 50th Anniversary of JPI/38th Petroleum-Petrochemical Symposium of JPI. Jpn. Petro. Inst. 2008, 121. [Google Scholar] [CrossRef]
- Ng, S.H.; Wang, J.; Fairbridge, C.; Zhu, Y.; Yang, L.; Ding, F.; Yui, S. Study of Canadian FCC feeds from various origins and treatments. 1. Ranking of feedstocks based on feed quality and product distribution. Energy Fuels 2004, 18, 160–171. [Google Scholar] [CrossRef]
- Yui, S.; Matsumoto, N.; Sasaki, Y. Athabasca oil sands produce quality FCC feeds. Oil Gas. J. 1998, 96, 43–51. [Google Scholar]
- Ng, S.H.; Yang, H.; Wang, J.; Zhu, Y.; Fairbridge, C.; Yui, S. Comparison of catalytic cracking performance between riser reactor and microactivity test (MAT) unit. Energy Fuels 2001, 15, 783–785. [Google Scholar] [CrossRef]
- Ng, S.H.; Wang, J.; Fairbridge, C.; Zhu, Y.X.; Zhu, Y.J.; Yang, L.; Ding, F.; Yui, S. Study of Canadian FCC feeds from various origins and treatments. 2. Some specific cracking characteristics and comparisons of product yields and qualities between a riser reactor and a MAT unit. Energy Fuels 2004, 18, 172–187. [Google Scholar] [CrossRef]
- Ng, S.H.; Humphries, A.; Fairbridge, C.; Zhu, Y.; Khulbe, C.; Tsai, T.Y.R.; Ding, F.; Charland, J.P.; Yui, S. Comparisons of FCC product yields and qualities between reactors using Canadian heavy feeds. Fuel Process. Technol. 2005, 86, 1335–1350. [Google Scholar] [CrossRef]
- Fisher, I.P. Effect of feedstock variability on catalytic cracking yields. Appl. Catal. 1990, 65, 189–210. [Google Scholar] [CrossRef]
- Scherzer, J. Correlation between catalyst formulation and catalytic properties. In Fluid Catalytic Cracking: Science and Technology; Magee, J.S., Mitchell, M.M., Jr., Eds.; Studies in Surface Science and Catalysis 76; Elsevier Science Publishers B. V.: Amsterdam, The Netherlands, 1993; pp. 145–182. [Google Scholar]
- Ng, S.H.; Zhu, Y.; Humphries, A.; Zheng, L.; Ding, F.; Gentzis, T.; Charland, J.-P.; Yui, S. FCC study of Canadian oil-sands derived vacuum gas oils. 1. Feed and catalyst effects on yield structure. Energy Fuels 2002, 16, 1196–1208. [Google Scholar] [CrossRef]
- Fu, C.M.; Schaffer, A.M. Effect of nitrogen compounds on cracking catalysts. Ind. Eng. Chem. Prod. Res. Dev. 1985, 24, 68–75. [Google Scholar] [CrossRef]
- John, T.M.; Wojciechowski, B.W. Effect of reaction temperature on product distribution in the catalytic cracking of a neutral distillate. J. Catal. 1975, 37, 348–357. [Google Scholar] [CrossRef]
- Keyworth, D.A.; Reid, T.A.; Asim, M.Y.; Gilman, R.H. Offsetting the cost of lower sulfur in gasoline. In Proceeding of the 1992 NPRA Annual Meeting, New Orleans, LA, USA, 22–24 March 1992. AM-92-17. [Google Scholar]
- Ng, S.H.; Al-Sabawi, M.; Wang, J.; Ling, H.; Zheng, Y.; Wei, Q.; Ding, F.C.; Little, E. FCC coprocessing oil sands heavy gas oil and canola oil. 1. Yield structure. Fuel 2015, 156, 163–176. [Google Scholar] [CrossRef]
- ASTM D5236-13. Standard Test Method for Distillation of Heavy Hydrocarbon Mixtures (Vacuum Potstill Method); ASTM International: West Conshohocken, PA, USA, 2010. [Google Scholar] [CrossRef]
- Purvin & Gertz, Inc. Oil Sands Products Analysis for Asian Markets. 2005. Available online: https://open.alberta.ca/publications/oil-sands-products-analysis-for-asian-markets (accessed on 15 April 2005).
- Kayser, J.C. Versatile fluidized bed reactor. U.S. Patent 6069012 30 May 2000. [Google Scholar]
- ASTM D7964/D7964M-14. Standard Test Method for Determining Activity of Fluid Catalytic Cracking (FCC) Catalysts in a Fluidized Bed; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar] [CrossRef]
- Ng, S.H.; Shi, Y.; Heshka, N.E.; Zhang, Y.; Little, E. Laboratory production of biofuels and biochemicals from a rapeseed oil through catalytic cracking conversion. J. Vis. Exp. 2016, 115, e54390. [Google Scholar] [CrossRef] [PubMed]




| HGO (343 °C–FBP) | ||||||
|---|---|---|---|---|---|---|
| SCO in (SCO + bitumen) blend, v% | 100.00 | 75.37 | 64.00 | 61.09 | 48.00 | 0.00 |
| SCO in (SCO + bitumen) blend, wt% | 100.00 | 74.55 | 62.98 | 60.04 | 46.91 | 0.00 |
| density at 15.6 °C, g/cm3 | 0.9336 | 0.9429 | 0.9480 | 0.9489 | 0.9545 | 0.9755 |
| °API gravity | 20.1 | 18.6 | 17.8 | 17.6 | 16.7 | 13.6 |
| carbon, wt% | 87.5 | 86.8 | 86.5 | 86.9 | 86.1 | 84.8 |
| hydrogen, wt% | 11.9 | 11.0 | 11.3 | 11.0 | 11.0 | 10.7 |
| H/C atomic ratio, mol/mol | 1.62 | 1.51 | 1.56 | 1.51 | 1.53 | 1.51 |
| sulfur, wt% | 0.44 | 1.24 | 1.60 | 1.66 | 2.12 | 3.69 |
| nitrogen, wppm | 1584 | 1526 | 1502 | 1495 | 1465 | 1355 |
| MCR, wt% | 0.00 | 0.10 | 0.13 | 0.03 | 0.07 | 0.26 |
| Ni, wppm | <0.1 | 0.1 | 0.3 | 0.1 | <0.1 | <0.1 |
| V,c wppm | 0.10 | 0.15 | 0.17 | 0.18 | 0.20 | 0.30 |
| simulated distillation, wt% | - | - | - | - | - | - |
| 343 °C− | 1.9 | 3.8 | 4.0 | 4.0 | 4.8 | 7.2 |
| 525 °C+ | 6.5 | 6.8 | 8.3 | 7.9 | 8.9 | 11.8 |
| saturates, wt% | 46.5 | 47.3 | 43.5 | 44.6 | 40.5 | 32.5 |
| paraffins | 6.0 | 4.4 | 3.4 | 3.8 | 3.2 | 1.9 |
| cycloparaffins | 40.5 | 42.9 | 40.0 | 40.8 | 37.3 | 30.6 |
| aromatics, wt% | 48.6 | 47.0 | 50.0 | 49.4 | 52.6 | 58.5 |
| monoaromatics | 23.0 | 23.7 | 24.4 | 25.9 | 27.9 | 28.9 |
| diaromatics | 15.9 | 16.7 | 17.5 | 17.9 | 18.4 | 22.2 |
| triaromatics | 8.0 | 5.1 | 5.1 | 4.4 | 4.8 | 5.0 |
| tetraaromatics and up | 1.3 | 1.0 | 2.2 | 1.0 | 1.0 | 1.1 |
| aromatic sulfur | 0.4 | 0.4 | 0.7 | 0.1 | 0.5 | 1.3 |
| 2-ring compounds | 0.4 | 0.4 | 0.7 | 0.1 | 0.5 | 1.3 |
| 3-ring compounds | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| polars, wt% | 4.9 | 5.3 | 6.0 | 6.0 | 6.9 | 9.1 |
| asphaltenes, wt% | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| gasoline precursors,a wt% | 69.5 | 71.0 | 67.9 | 70.5 | 68.4 | 61.4 |
| light cycle oil precursors,b wt% | 16.3 | 17.1 | 18.2 | 18.0 | 18.9 | 23.5 |
| Catalyst | Albemarle E-CAT |
|---|---|
| total surface area, m2/g | 141 |
| zeolite surface area, m2/g | 54.0 |
| matrix surface area, m2/g | 87.0 |
| zeolite/matrix (Z/M) | 0.62 |
| micropore volume, cm3/g | 0.03 |
| Al2O3, wt% | 51.0 |
| RE2O3, wt% (on catalyst) * | 0.80 |
| Na2O, wt% | 0.38 |
| Fe2O3, wt% | 0.63 |
| Ni, wppm | 50 |
| V, wppm | 60 |
| apparent bulk density, g/cm3 | 0.83 |
| average particle size, µm | 75 |
| particle size distribution, % | - |
| −105 µm | 79 |
| −80 µm | 57 |
| −60 µm | 30 |
| −40 µm | 7 |
| −20 µm | 0 |
| v% SSB in Blend | 100.00 | 75.37 | 64.00 | 61.09 | 48.00 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Run No. | 791 | 796 | 797 | 802 | 945 | 950 | 951 | 956 | 1246 | 1251 | 1252 | 1257 | 963 | 968 | 969 | 974 | 1264 | 1269 | 1270 | 1275 |
| Temp., °C | 500 | 520 | 500 | 520 | 500 | 520 | 500 | 520 | 500 | 520 | ||||||||||
| Conv., wt% | 71.63 | 72.75 | 74.30 | 75.28 | 72.81 | 67.79 | 74.59 | 74.67 | 70.85 | 71.68 | 72.98 | 73.87 | 69.93 | 69.47 | 71.47 | 71.00 | 69.46 | 70.44 | 70.68 | 71.77 |
| Diff, % | 1.55 | 1.32 | 7.15 | 0.12 | 1.16 | 1.20 | 0.66 | 0.66 | 1.40 | 1.52 | ||||||||||
| Yield, wt% | ||||||||||||||||||||
| Dry Gas | 1.71 | 1.71 | 2.27 | 2.39 | 2.21 | 2.19 | 2.78 | 2.81 | 2.64 | 2.60 | 3.15 | 3.16 | 2.63 | 2.63 | 3.23 | 3.25 | 2.74 | 2.78 | 3.43 | 3.43 |
| Diff, % | 0.28 | 5.21 | 0.62 | 1.06 | 1.80 | 0.20 | 0.05 | 0.75 | 1.47 | 0.06 | ||||||||||
| LPG | 15.52 | 15.78 | 17.54 | 18.03 | 14.97 | 15.26 | 16.86 | 17.12 | 15.62 | 14.97 | 16.51 | 17.86 | 14.32 | 14.80 | 15.82 | 16.19 | 13.69 | 14.26 | 15.68 | 15.95 |
| Diff, % | 1.64 | 2.80 | 1.96 | 1.52 | 4.23 | 7.89 | 3.29 | 2.28 | 4.08 | 1.74 | ||||||||||
| Gasoline | 47.50 | 48.50 | 47.31 | 47.59 | 48.63 | 43.33 | 47.68 | 47.42 | 44.89 | 46.27 | 45.13 | 44.86 | 44.70 | 43.97 | 43.67 | 43.06 | 43.04 | 42.84 | 41.30 | 41.00 |
| Diff, % | 2.08 | 0.58 | 11.52 | 0.55 | 0.01 | 0.61 | 1.66 | 1.41 | 0.48 | 0.71 | ||||||||||
| LCO | 16.59 | 16.30 | 15.36 | 14.78 | 16.56 | 19.89 | 15.37 | 15.53 | 18.27 | 17.70 | 16.79 | 16.36 | 18.47 | 18.68 | 17.42 | 16.97 | 18.27 | 18.96 | 18.06 | 17.60 |
| Diff, % | 1.80 | 3.86 | 18.25 | 1.05 | 3.17 | 2.55 | 1.15 | 2.63 | 3.72 | 2.57 | ||||||||||
| HCO | 11.78 | 10.96 | 10.35 | 9.94 | 10.62 | 12.32 | 10.04 | 9.79 | 10.87 | 10.62 | 10.23 | 9.77 | 11.60 | 11.85 | 11.11 | 12.04 | 12.27 | 10.60 | 11.26 | 10.63 |
| Diff, % | 7.25 | 3.99 | 14.83 | 2.51 | 2.38 | 4.61 | 2.09 | 7.98 | 14.62 | 5.74 | ||||||||||
| Coke | 6.89 | 6.76 | 7.18 | 7.27 | 7.01 | 7.00 | 7.27 | 7.33 | 7.70 | 7.84 | 8.19 | 7.99 | 8.27 | 8.07 | 8.75 | 8.50 | 8.13 | 8.14 | 8.80 | 8.74 |
| Diff, % | 1.94 | 1.24 | 0.21 | 0.84 | 1.91 | 2.55 | 2.49 | 2.90 | 0.05 | 0.66 | ||||||||||
| Delta Coke | 0.61 | 0.60 | 0.64 | 0.65 | 0.62 | 0.62 | 0.65 | 0.65 | 0.68 | 0.70 | 0.73 | 0.71 | 0.73 | 0.72 | 0.78 | 0.76 | 0.72 | 0.72 | 0.78 | 0.78 |
| Diff, % | 1.94 | 1.24 | 0.21 | 0.84 | 1.91 | 2.55 | 2.49 | 2.90 | 0.05 | 0.66 | ||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, S.H.; Heshka, N.E.; Zheng, Y.; Ling, H.; Wang, J.; Liu, Q.; Little, E.; Ding, F.; Wang, H. Virgin Heavy Gas Oil from Oil Sands Bitumen as FCC Feed. Catalysts 2020, 10, 277. https://doi.org/10.3390/catal10030277
Ng SH, Heshka NE, Zheng Y, Ling H, Wang J, Liu Q, Little E, Ding F, Wang H. Virgin Heavy Gas Oil from Oil Sands Bitumen as FCC Feed. Catalysts. 2020; 10(3):277. https://doi.org/10.3390/catal10030277
Chicago/Turabian StyleNg, Siauw H., Nicole E. Heshka, Ying Zheng, Hao Ling, Jinsheng Wang, Qianqian Liu, Edward Little, Fuchen Ding, and Hui Wang. 2020. "Virgin Heavy Gas Oil from Oil Sands Bitumen as FCC Feed" Catalysts 10, no. 3: 277. https://doi.org/10.3390/catal10030277






