Influence of the Preparation Method of Ag-K/CeO2-ZrO2-Al2O3 Catalysts on Their Structure and Activity for the Simultaneous Removal of Soot and NOx
Abstract
:1. Introduction
| Soot Combustion | (1) | |
| Ammonia Generation | (2) | |
| Selective Catalytic Reduction | (3) |
| Non-Selective Catalytic Reduction | (4) | |
| Soot Oxidation by N2O | (5) | |
| (6) |
2. Results and Discussion
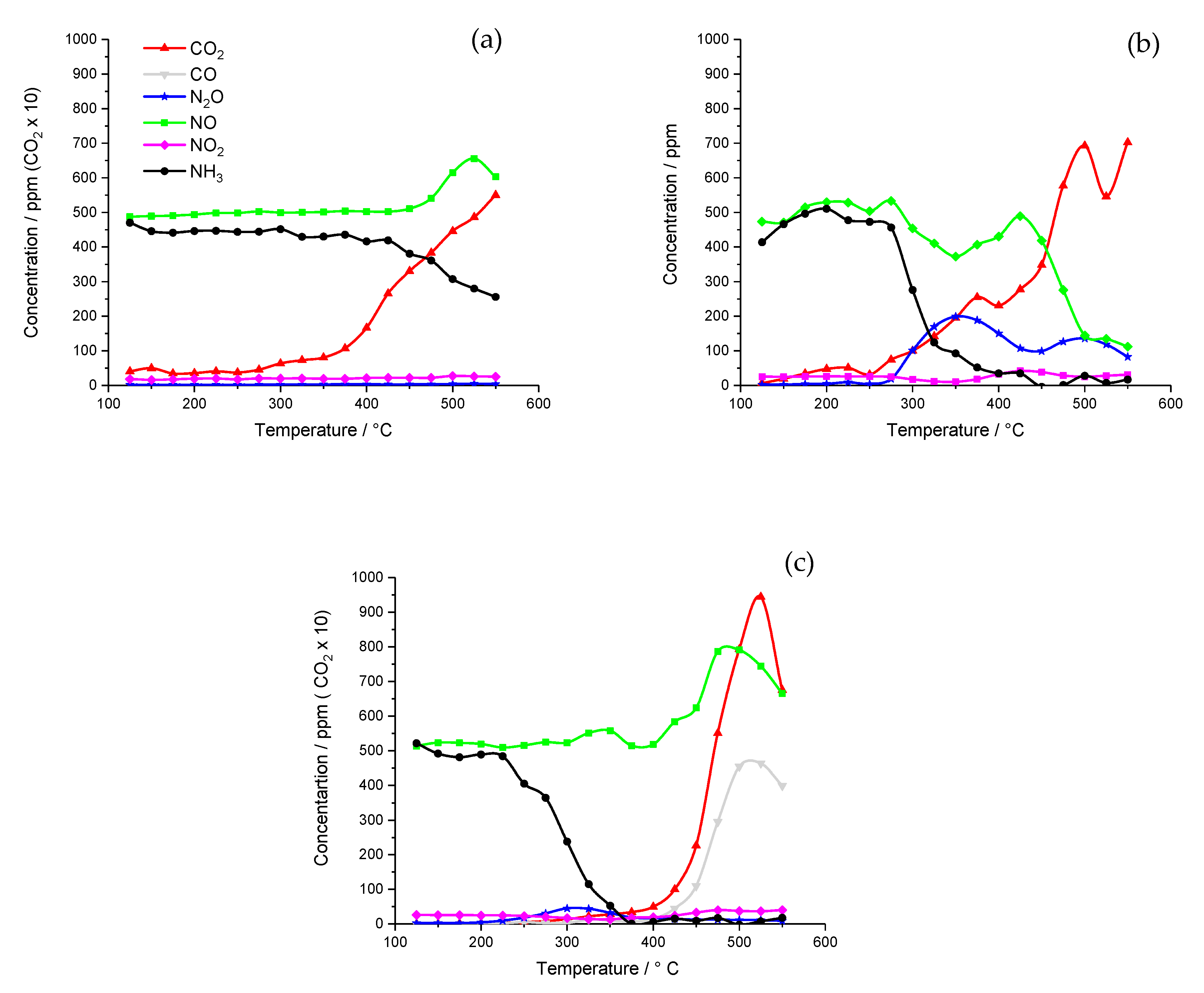
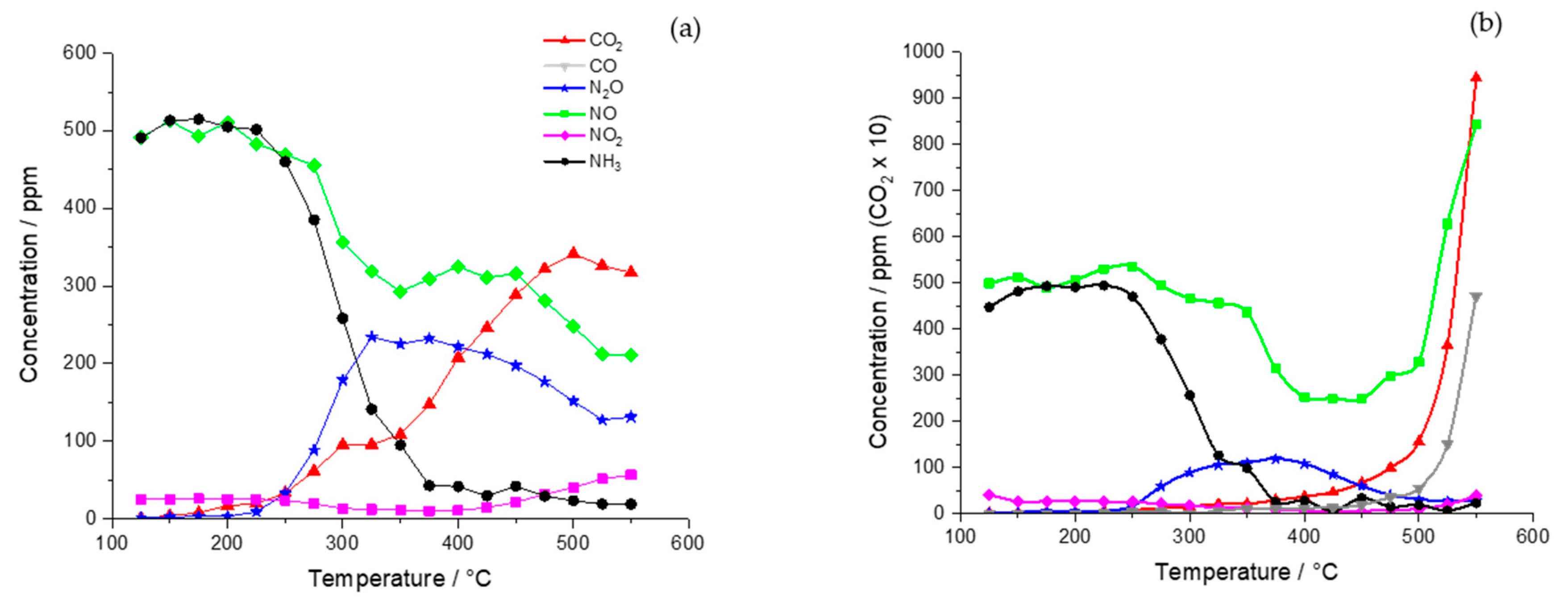
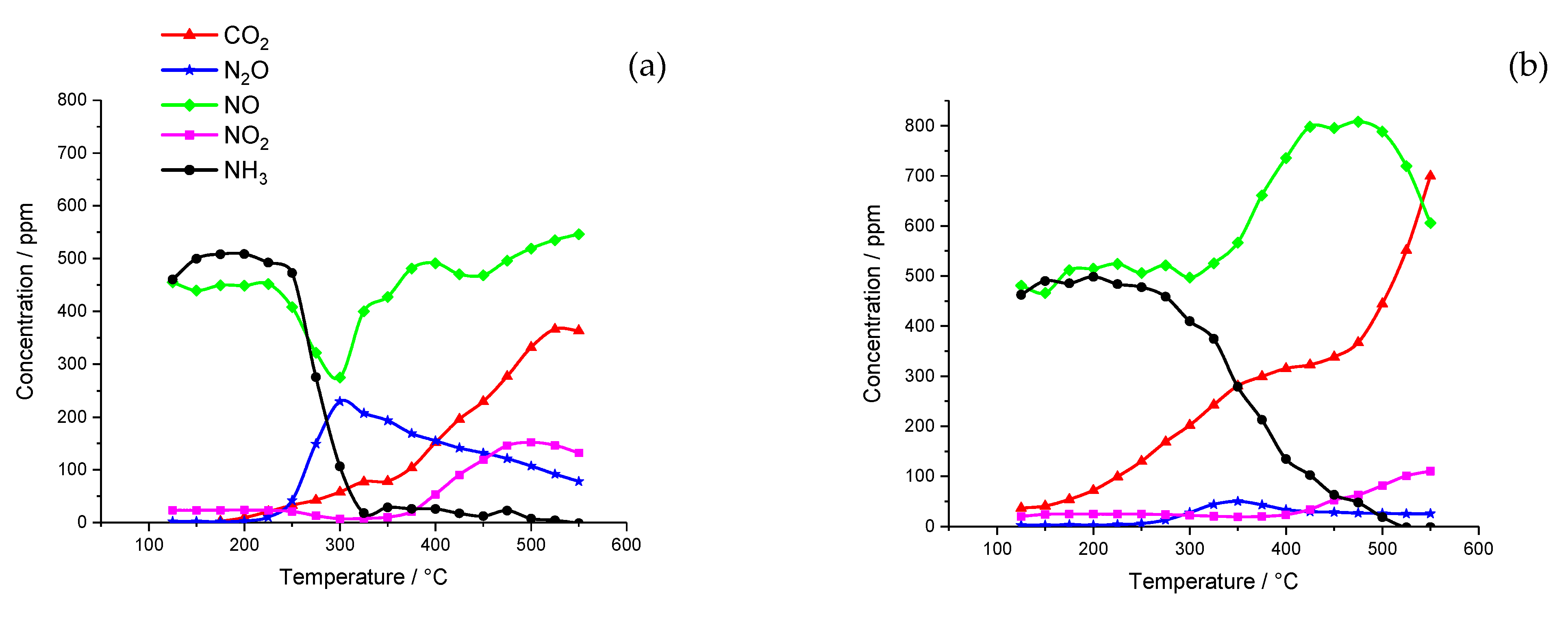
2.1. Catalyst Activity
2.1.1. Chemical Vapor Impregnation (CVI) Catalysts
2.1.2. Incipient Wetness Catalyst
2.1.3. Wet Impregnation Catalyst
2.2. Catalyst Characterization
3. Materials and Methods
3.1. Catalyst Preparation
3.1.1. Ceria Zirconia Alumina (CZA) Support
3.1.2. Wet Impregnation (IMP)
3.1.3. Incipient Wetness (IW)
3.1.4. Chemical Vapour Impregnation (CVI)
3.2. Characterization
3.3. Catalyst Performance Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization Regional Office for Europe. Health Effects of Particulate Matter; World Health Organization Regional Office for Europe: Copenhagen Ø, Denmark, 2013. [Google Scholar]
- Cohen, A.J.; Anderson, R.; Ostra, B.; Dev Pandey, K.; Krzyzanowski, M.; Künzli, N.; Gutschmidt, K.; Pope, A.; Romieu, I.; Samet, V.; et al. The global burden of disease due to outdoor air pollution. J. Toxicol. Environ. Health Part A 2005, 68, 1301–1307. [Google Scholar] [CrossRef]
- Health Assessment Document for Diesel Engine Exhaust; United States Environemntal Protection Agency: Washington, DC, USA, 2002; Volume 67.
- Union, T.E. Commission Regulation (EU) 2016/427-of 10 March 2016-amending Regulation (EC) No 692/2008 as regards emissions from light passenger and commercial vehicles (Euro 6). Off. J. Eur. 2016/427. Available online: https://eur-lex.europa.eu/eli/reg/2016/427/oj (accessed on 3 March 2016).
- Dieselnet. Available online: https://www.dieselnet.com/standards/eu/ld.php (accessed on 28 August 2019).
- Twigg, M.V. Catalytic control of emissions from cars. Catal. Today 2011, 163, 33–41. [Google Scholar] [CrossRef]
- Twigg, M.V. An essay book review of ‘Urea-SCR technology for deNOx after treatment of diesel exhausts’. Johns. Matthey Technol. Rev. 2015, 59, 221–232. [Google Scholar] [CrossRef]
- Grabchenko, M.V.; Mamontov, G.V.; Zaikovskii, V.I.; La Parola, V.; Liotta, L.F.; Vodyankina, O.V. Design of Ag-CeO2/SiO2 catalyst for oxidative dehydrogenation of ethanol: Control of Ag–CeO2 interfacial interaction. Catal. Today 2019, 333, 2–9. [Google Scholar] [CrossRef]
- Hu, S.; Wang, W.; Wang, Y.; Xu, Q.; Zhu, J. Interaction of Zr with CeO2 (111) Thin Film and Its Influence on Supported Ag Nanoparticles. J. Phys. Chem. C 2015, 119, 18257–18266. [Google Scholar] [CrossRef]
- Liu, S.; Wu, X.; Liu, W.; Chen, W.; Ran, R.; Li, M.; Weng, D. Soot oxidation over CeO2 and Ag/CeO2: Factors determining the catalyst activity and stability during reaction. J. Catal. 2016, 337, 188–198. [Google Scholar] [CrossRef]
- Gao, Y.; Duan, A.; Liu, S.; Wu, X.; Liu, W.; Li, M.; Chen, S.; Wang, X.; Weng, D. Study of Ag/CexNd1-xO2 nanocubes as soot oxidation catalysts for gasoline particulate filters: Balancing catalyst activity and stability by Nd doping. Appl. Catal. B Environ. 2017, 203, 116–126. [Google Scholar] [CrossRef]
- Kayama, T.; Yamazaki, K.; Shinjoh, H. Nanostructured Ceria−Silver Synthesized in a One-Pot Redox Reaction Catalyzes Carbon Oxidation. J. Am. Chem. Soc. 2010, 132, 13154–13155. [Google Scholar] [CrossRef]
- Chang, S.; Li, M.; Hua, Q.; Zhang, L.; Ma, Y.; Ye, B.; Huang, W. Shape-dependent interplay between oxygen vacancies and Ag–CeO2 interaction in Ag/CeO2 catalysts and their influence on the catalytic activity. J. Catal. 2012, 293, 195–204. [Google Scholar] [CrossRef]
- Sadlivskaya, M.V.; Mikheeva, N.N.; Zaikovskii, V.I.; Mamontov, G.V. Influence of Preparation Method of Ag–CeO2 Catalysts on Their Structure and Activity in Soot Combustion. Kinet. Catal. 2019, 60, 432–438. [Google Scholar] [CrossRef]
- Bueno-López, A. Diesel soot combustion ceria catalysts. Appl. Catal. B Environ. 2014, 146, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Peralta, M.A.; Zanuttini, M.S.; Querini, C.A. Activity and stability of BaKCo/CeO2 catalysts for diesel soot oxidation. Appl. Catal. B Environ. 2011, 110, 90–98. [Google Scholar] [CrossRef]
- Rao, K.N.; Venkataswamy, P.; Reddy, B.M. Structural Characterization and Catalytic Evaluation of Supported Copper-Ceria Catalysts for Soot Oxidation. Ind. Eng. Chem. Res. 2011, 50, 11960–11969. [Google Scholar] [CrossRef]
- Muroyama, H.; Hano, S.; Matsui, T.; Eguchi, K. Catalytic soot combustion over CeO2-based oxides. Catal. Today 2010, 153, 133–135. [Google Scholar] [CrossRef]
- Kašpar, J.; Fornasiero, P.; Graziani, M. Use of CeO2-based oxides in the three-way catalysis. Catal. Today 1999, 50, 285–298. [Google Scholar] [CrossRef]
- Trovarelli, A. Catalysis Reviews Catalytic Properties of Ceria and CeO2-Containing Materials Catalytic Properties of Ceria and CeO2 -Containing Materials. , Catal. Rev. Sci. Eng. 1996, 38, 439–520. [Google Scholar] [CrossRef]
- Aneggi, E.; de Leitenburg, C.; Dolcetti, G.; Trovarelli, A. Promotional effect of rare earths and transition metals in the combustion of diesel soot over CeO2 and CeO2–ZrO2. Catal. Today 2006, 114, 40–47. [Google Scholar] [CrossRef]
- Ramdas, R.; Nowicka, E.; Jenkins, R.; Sellick, D.; Davies, C.; Golunski, S. Using real particulate matter to evaluate combustion catalysts for direct regeneration of diesel soot filters. Appl. Catal. B Environ. 2015, 176, 436–443. [Google Scholar] [CrossRef] [Green Version]
- Davies, C.; Thompson, K.; Cooper, A.; Golunski, S.; Taylor, S.H.; Bogarra Macias, M.; Doustdar, O.; Tsolakis, A. Simultaneous removal of NOx and soot particulate from diesel exhaust by in-situ catalytic generation and utilisation of N2O. Appl. Catal. B Environ. 2018, 239, 10–15. [Google Scholar] [CrossRef]
- Rinkenburger, A.; Toriyama, T.; Yasuda, K.; Niessner, R. Catalytic Effect of Potassium Compounds in Soot Oxidation. ChemCatChem 2017, 9, 3513–3525. [Google Scholar] [CrossRef]
- Castoldi, L.; Matarrese, R.; Lietti, L.; Forzatti, P. Intrinsic reactivity of alkaline and alkaline-earth metal oxide catalysts for oxidation of soot. Appl. Catal. B Environ. 2009, 90, 278–285. [Google Scholar] [CrossRef]
- Xiong, S.; Liao, Y.; Dang, H.; Qia, F.; Yang, S. Promotion mechanism of CeO2 addition on the low temperature SCR reaction over MnOx/TiO2: A new insight from the kinetic study. RSC Adv. 2015, 5, 27785–27793. [Google Scholar] [CrossRef]
- Titov, A.; Klimenko, M.; Goryacheva, E.; Opolchenova, N.; Stepareva, N.; Sokolova, N. Preparation of Ceria and Yttria Nanopowders via Thermal Decomposition of Oxalates, Carbonates, and Hydroxides. Inorg. Mater. 2008, 44, 1101–1104. [Google Scholar] [CrossRef]
- Janoš, P.; Hladík, T.; Kormunda, M.; Ederer, J.; Šťastn Martin, M. Thermal Treatment of Cerium Oxide and Its Properties: Adsorption Ability versus Degradation Efficiency. Adv. Mater. Sci. Eng. 2014. [Google Scholar] [CrossRef] [Green Version]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef]
- Sartoretti, E.; Novara, C.; Giorgis, F.; Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D. In situ Raman analyses of the soot oxidation reaction over nanostructured ceria-based catalysts. Sci. Rep. 2009, 9. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.; Li, M.; Zhang, J.; Hu, X.; Zheng, J.; Zhang, N.; Chen, B.H. Constructing nano-structure on silver/ceria-zirconia towards highly active and stable catalyst for soot oxidation. Chem. Eng. J. 2017, 313, 544–555. [Google Scholar] [CrossRef]
- Martina, I.; Wiesinger, R.; Jembrih-Simburger, D.; Schreiner, M. Micro-Raman characterization of silver corrosion products: Instrumental set-up and reference database. E Preserv. Sci. 2012, 9, 1–6. [Google Scholar]
- Bosnick, K.A. Raman Studies of Mass-Selected Metal Clusters. Doctor of Philosophy. Ph.D. Thesis, University of Toronto, Toronto, ON, Canada, 2000. [Google Scholar]
- Bueno-Ferrer, C.; Parres-Esclapez, S.; Lozano-Castelló, D.; Bueno-López, A. Relationship between surface area and crystal size of pure and doped cerium oxides. J. Rare Earths 2010, 28, 647–653. [Google Scholar] [CrossRef]
- Aneggi, E.; Llorca, J.; de Leitenburg, C.; Dolcetti, G. Trovarelli, Soot combustion over silver-supported catalysts. Appl. Catal. B Environ. 2009, 91, 489–498. [Google Scholar] [CrossRef]
- Castoldi, L.; Aneggi, E.; Matarrese, R.; Bonzi, R.; Llorca, J.; Trovarelli, A.; Lietti, L. Silver-based catalytic materials for the simultaneous removal of soot and NOx. Catal. Today 2015, 258, 405–415. [Google Scholar] [CrossRef] [Green Version]
- Ferraria, A.M.; Carapeto, A.P.; Botelho Do Rego, A.M. X-ray photoelectron spectroscopy: Silver salts revisited. Vacuum 2012, 86, 1988–1991. [Google Scholar] [CrossRef]
- Neeft, J.P.A.; Van Pruissen, O.P.; Makkee, M.; Moulijn, J.A. Catalysts for the oxidation of soot from diesel exhaust gases. II. Contact between soot and catalyst under practical conditions. Appl. Catal. B Environ. 1997, 12, 21–31. [Google Scholar] [CrossRef]

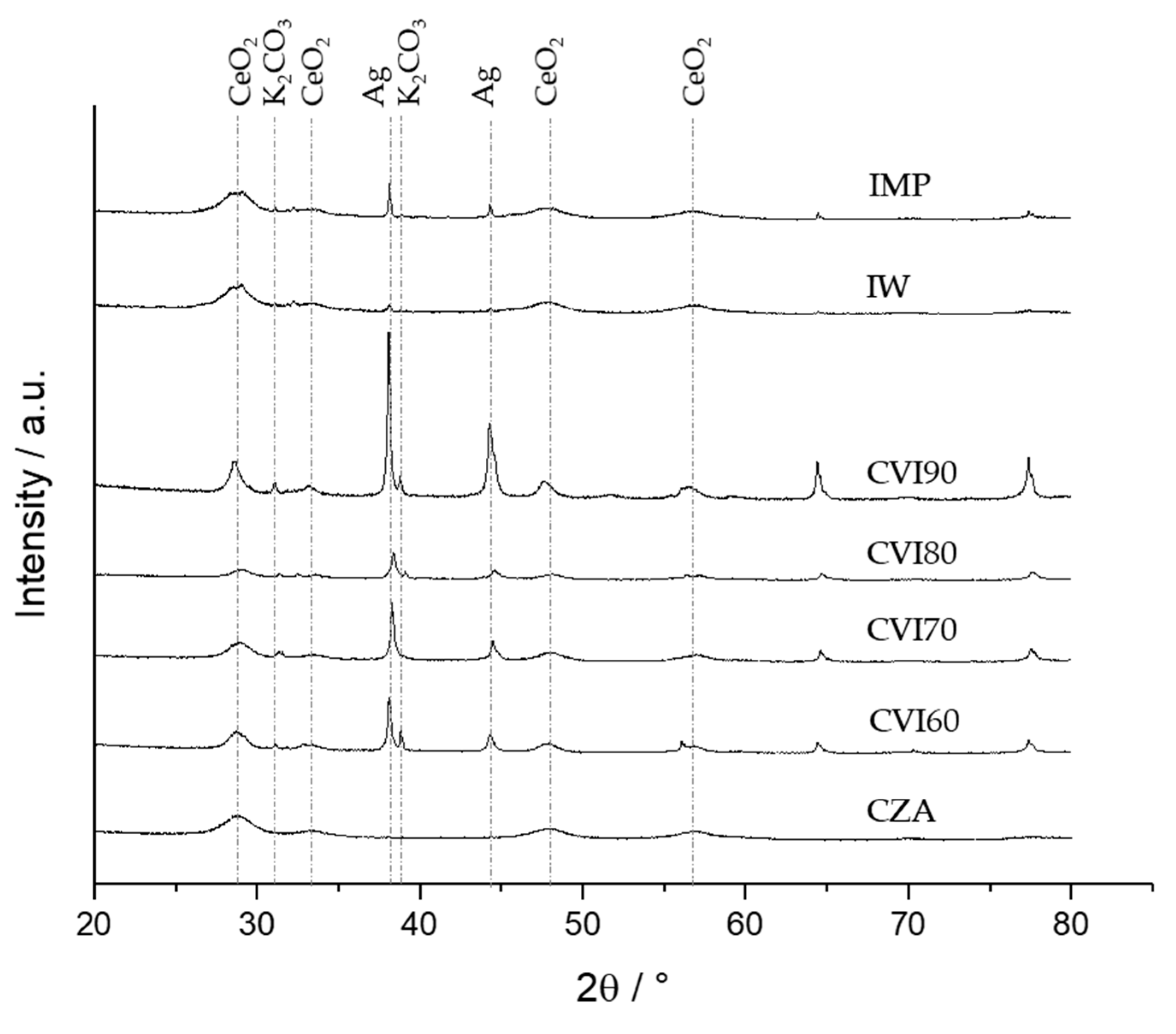
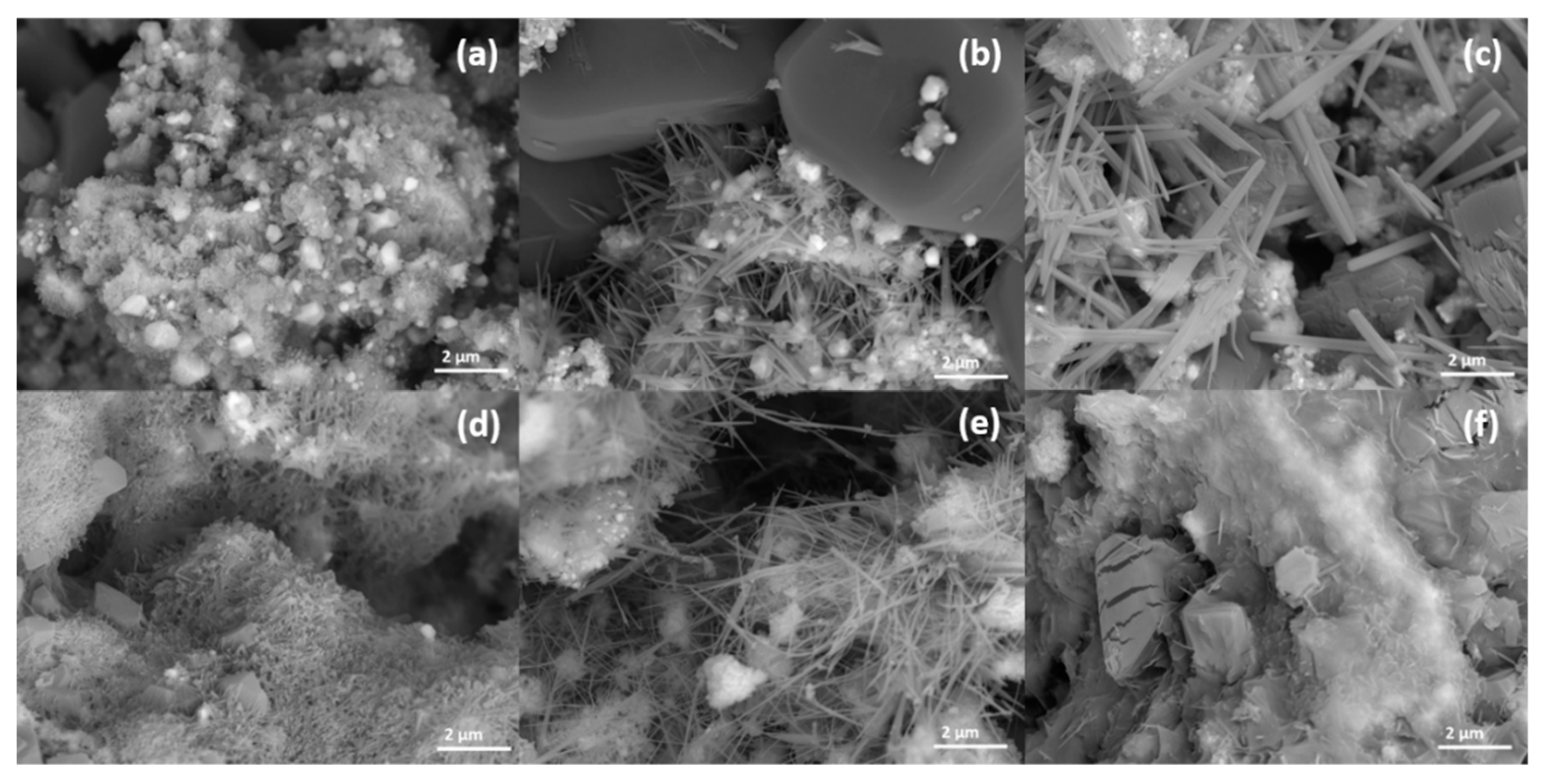
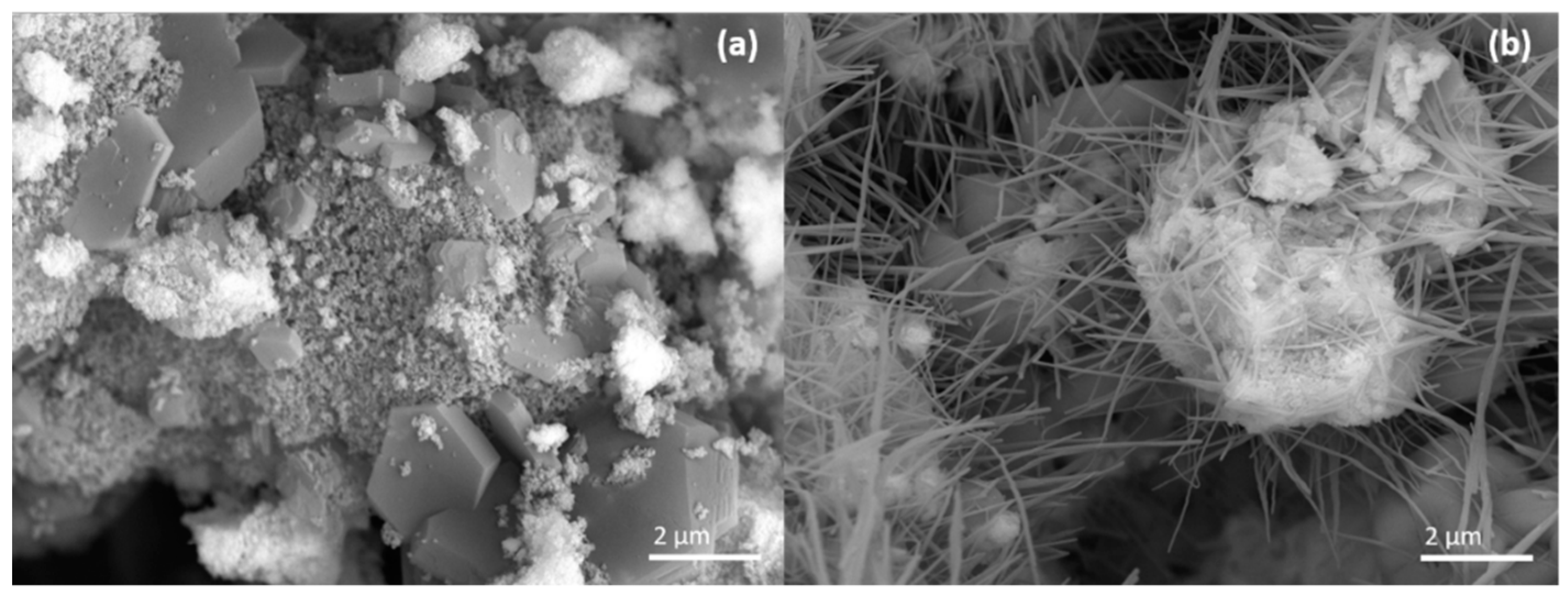
| Sample | Ag Crystallite Size [nm] | CeO2 Crystallite Size [nm] | CeO2 Defect Ratio | Catalyst Surface Area [m2 g−1] |
|---|---|---|---|---|
| CVI60 | 59 | 18 | - | 3.8 |
| CVI70 | 52 | 16 | 0.61 | 9.2 |
| CVI80 | 40 | 33 | 0.74 | 11.8 |
| CVI90 | <100 | >100 | 0.82 | 8.4 |
| IW | <100 | 18 | 0.0037 | 12.5 |
| IMP | 32 | 20 | - | 26.4 |
| Catalyst | Na | Ce | F | O | Ag | K | C | Zr | Al |
|---|---|---|---|---|---|---|---|---|---|
| CVI60 | 7.2 | 1.1 | 20.1 | 29.5 | 2.3 | 11.6 | 24.6 | 0.4 | 3.4 |
| CVI70 | 5.9 | 1.1 | 17.0 | 31.8 | 2.4 | 14.2 | 22.4 | 0.3 | 5.0 |
| CVI80 | 3.8 | 0.8 | 9.1 | 37.6 | 0.7 | 11.5 | 29.5 | 0.2 | 6.7 |
| CVI90 | 3.4 | 0.6 | 5.1 | 32.0 | 0.3 | 7.2 | 47.4 | 0.1 | 4.6 |
| IW | 1.3 | 2.3 | - | 53.6 | 0.1 | 11.6 | 21.7 | 0.6 | 8.9 |
| IMP | 0.9 | 1.7 | - | 45.9 | 0.1 | 10.5 | 33.1 | 0.4 | 7.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cooper, A.; Davies, T.E.; Morgan, D.J.; Golunski, S.; Taylor, S.H. Influence of the Preparation Method of Ag-K/CeO2-ZrO2-Al2O3 Catalysts on Their Structure and Activity for the Simultaneous Removal of Soot and NOx. Catalysts 2020, 10, 294. https://doi.org/10.3390/catal10030294
Cooper A, Davies TE, Morgan DJ, Golunski S, Taylor SH. Influence of the Preparation Method of Ag-K/CeO2-ZrO2-Al2O3 Catalysts on Their Structure and Activity for the Simultaneous Removal of Soot and NOx. Catalysts. 2020; 10(3):294. https://doi.org/10.3390/catal10030294
Chicago/Turabian StyleCooper, Anna, Thomas E. Davies, David J. Morgan, Stan Golunski, and Stuart H. Taylor. 2020. "Influence of the Preparation Method of Ag-K/CeO2-ZrO2-Al2O3 Catalysts on Their Structure and Activity for the Simultaneous Removal of Soot and NOx" Catalysts 10, no. 3: 294. https://doi.org/10.3390/catal10030294
APA StyleCooper, A., Davies, T. E., Morgan, D. J., Golunski, S., & Taylor, S. H. (2020). Influence of the Preparation Method of Ag-K/CeO2-ZrO2-Al2O3 Catalysts on Their Structure and Activity for the Simultaneous Removal of Soot and NOx. Catalysts, 10(3), 294. https://doi.org/10.3390/catal10030294






