Synthesis of Pd-M@HCS(M = Co, Ni, Cu) Bimetallic Catalysts and Their Catalytic Performance for Direct Synthesis of H2O2
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Pd@HCS and Pd-M@HCS
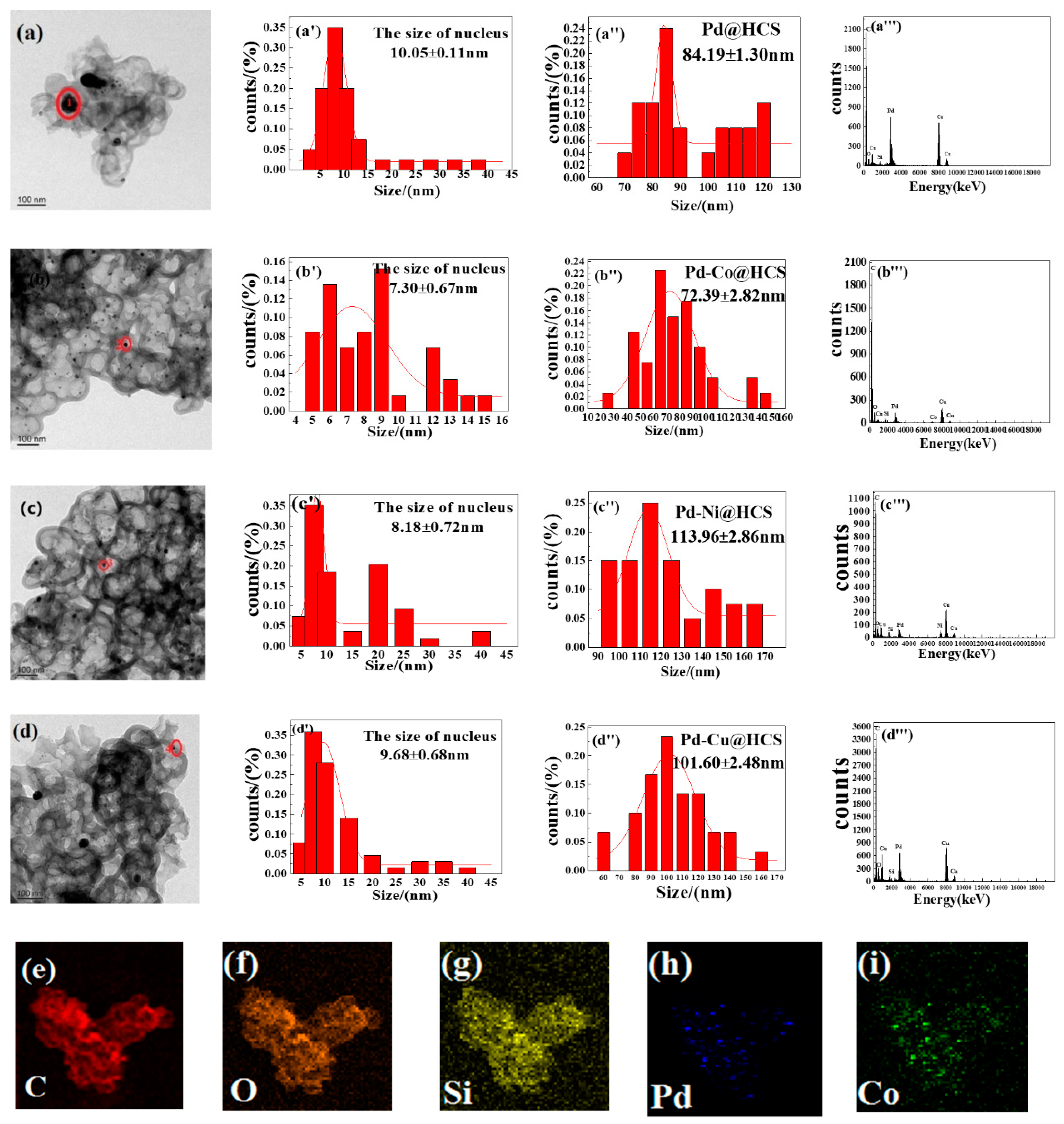
2.1.1. Morphology of Pd@HCS and Pd-M@HCS
2.1.2. XRD Pattern of Pd@HCS and Pd-M@HCS
2.1.3. N2 Adsorption–Desorption of Pd@HCS and Pd-M@HCS
2.1.4. Activity Test of Pd@HCS and Pd-M@HCS for DSHP
2.2. Characterization of Pd-Co@HCS-(X)
2.2.1. XRD pattern of Pd-Co@HCS-(X)
2.2.2. N2 Adsorption–Desorption of Pd-Co@HCS-(X)
2.2.3. Activity Test of Pd-Co@HCS-(X) for DSHP
3. Experimental
3.1. Materials
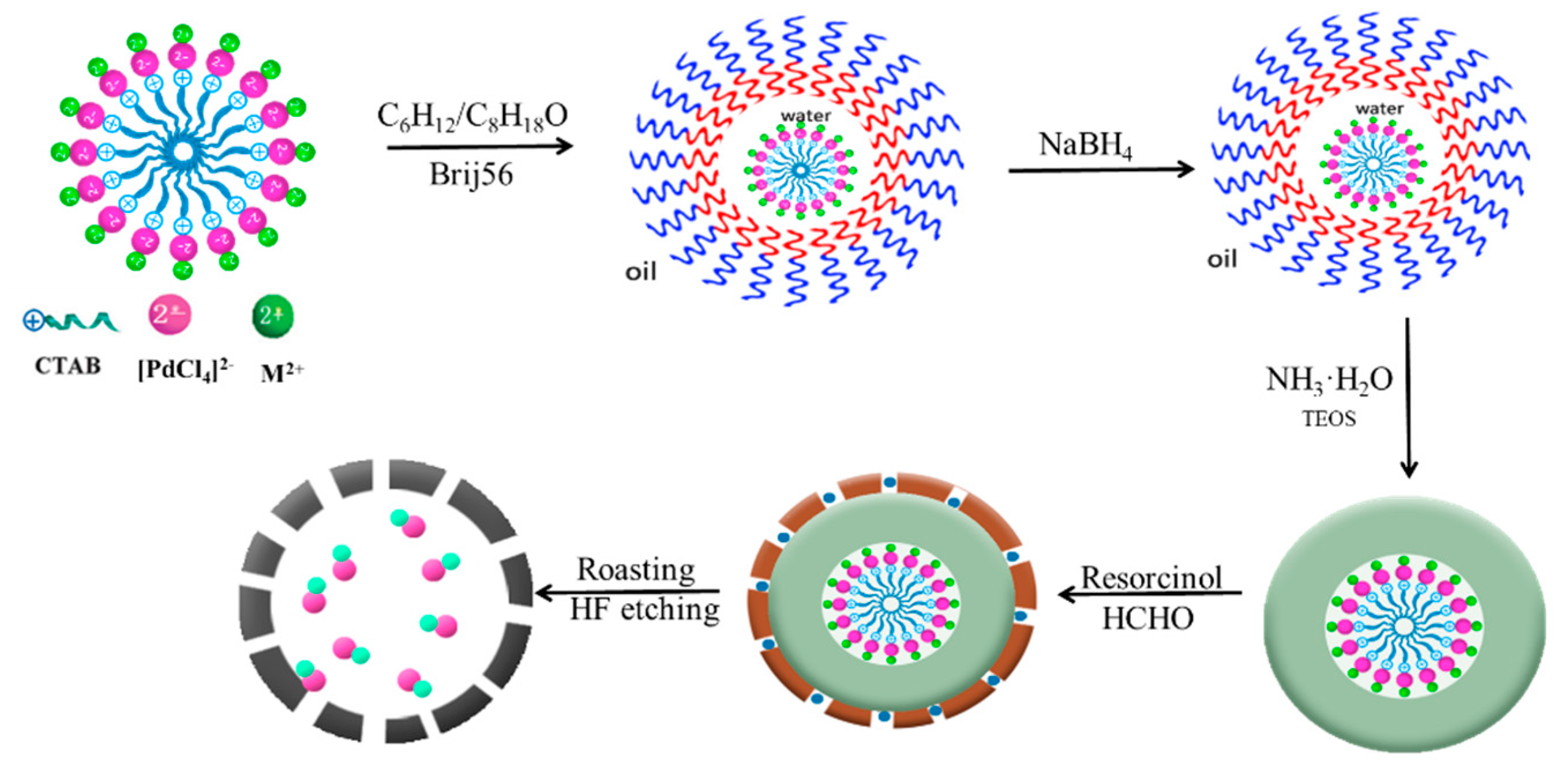
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Activity Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Edwards, J.K.; Freakley, S.J.; Carley, A.F.; Kiely, C.J.; Hutchings, G.J. Strategies for designing supported gold-palladium bimetallic catalysts for the direct synthesis of hydrogen peroxide. Acc. Chem. Res. 2014, 47, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Hage, R.; Lienke, A. Applications of transition-metal catalysts to textile and wood-pulp bleaching. Angew. Chem. Int. Ed. 2005, 45, 206–222. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.P.; Lee, I.H. Decomposition of acetic acid by advanced oxidation processes. Korean J. Chem. Eng. 2009, 26, 387–391. [Google Scholar]
- Kim, H.; Lee, W.; Ahn, C.; Kim, B.; Kim, J.; Oh, H. Kinetic correlation between degradation and dechlorination of perchloroethylene in the Fenton reaction. Korean J. Chem. Eng. 2010, 27, 1750–1754. [Google Scholar] [CrossRef]
- Choi, S.S.; Sang, H.S.; Kang, D.G.; Ha, J.H.; Cha, H.J. Removal of neurotoxic ethyl parathion pesticide by two-stage chemical/enzymatic treatment system using Fenton’s reagent and organophosphorous hydrolase. Korean J. Chem. Eng. 2010, 27, 900–904. [Google Scholar] [CrossRef]
- Campos-Martin, J.M.; Blanco-Brieva, G.; Fierro, J.L.G. Hydrogen peroxide synthesis:an outlook beyond the anthraquinone process. Angew. Chem. Int. Ed. 2006, 45, 6962–6984. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, S.; Sieber, V. Recent Advances in the Direct Synthesis of Hydrogen Peroxide Using Chemical Catalysis—A Review. Catalysts 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Samanta, C. Direct synthesis of hydrogen peroxide from hydrogen and oxygen: An overview of recent developments in the process. Appl. Catal. A Gen. 2008, 350, 133–149. [Google Scholar] [CrossRef]
- Lewis, R.J.; Hutchings, G.J. Recent Advances in the Direct Synthesis of H2O2. ChemCatChem 2019, 11, 298–308. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, D.; Zhang, X.; Li, L.; Hou, H.; Niwa, O.; You, T. Pd−Ni Alloy Nanoparticle/Carbon Nanofiber Composites: Preparation, Structure, and Superior Electrocatalytic Properties for Sugar Analysis. Anal. Chem. 2014, 86, 5898–5905. [Google Scholar] [CrossRef]
- Feng, L.; Chong, H.; Li, P.; Xiang, J.; Fu, F.; Yang, S.; Yu, H.; Sheng, H.; Zhu, M. Pd−Ni Alloy Nanoparticles as Effective Catalysts for Miyaura−Heck Coupling Reactions. J. Phys. Chem. C 2015, 119, 11511–11515. [Google Scholar] [CrossRef]
- Solsona, B.E.; Edwards, J.K.; Landon, P.; Carley, A.F.; Herzing, A.; Kiely, C.J.; Hutchings, G.J. Direct Synthesis of Hydrogen Peroxide from H2 and O2 Using Al2O3 Supported Au-Pd Catalysts. Chem. Mater. 2006, 18, 2689–2695. [Google Scholar] [CrossRef]
- Freakley Simon, J.; He, Q.; Harrhy Jonathan, H.; Lu, L.; Crole David, A.; Morgan David, J.; Ntainjua Edwin, N.; Edwards Jennifer, K.; Carley Albert, F.; Borisevich Albina, Y.; et al. Palladium-tin catalysts for the direct synthesis of H2O2 with high selectivity. Science 2016, 351, 965–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paunovic, V.; Ordomsky, V.; D’Angelo, M.F.N.; Schouten, J.C.; Nijhuis, T.A. Direct synthesis of hydrogen peroxide over Au–Pd catalyst in a wall-coated microchannel. J. Catal. 2014, 309, 325–332. [Google Scholar] [CrossRef]
- Lu, A.H.; Spliethoff, B.; Schüth, F. Aqueous Synthesis of Ordered Mesoporous Carbon via Self-Assembly Catalyzed by Amino Acid. Chem. Mater. 2008, 20, 5314–5319. [Google Scholar] [CrossRef]
- Yokoi, T.; Sakamoto, Y.; Terasaki, O.; Kubota, Y.; Okubo, T.; Tatsumi, T. Periodic Arrangement of Silica Nanospheres Assisted by Amino Acids. J. Am. Chem. Soc. 2006, 128, 13664–13665. [Google Scholar] [CrossRef]
- Liu, J.; Qiao, S.Z.; Liu, H.; Chen, J.; Orpe, A.; Zhao, D.; Lu, G.Q. Extension of The Stöber Method to the Preparation of Monodisperse Resorcinol–Formaldehyde Resin Polymer and Carbon Spheres. Angew. Chem. 2011, 50, 5947–5951. [Google Scholar] [CrossRef]
- Liu, P.; Lin, Q.; Pan, H.; Zhao, J.; Zhao, C.; Wang, Y. Direct synthesis of hydrogen peroxide from hydrogen and oxygen over yolk–shell nanocatalyst Pd@HCS with controlled Pd nanoparticle size. J. Catal. 2019, 377, 511–523. [Google Scholar] [CrossRef]
- Liu, Z.; Qi, J.; Liu, M.; Zhang, S.; Fan, Q.; Liu, H.; Liu, H.; Liu, K.; Zheng, H.; Yin, Y.; et al. Aqueous Synthesis of Ultrathin Platinum/Non-Noble Metal Alloy Nanowires for Enhanced Hydrogen Evolution Activity. Angew. Chem. 2018. [Google Scholar] [CrossRef]
- Vondrova, M.; Klimczuk, T.; Miller, V.L.; Kirby, B.W.; Yao, N.; Cava, R.J.; Bocarsly, A.B. Supported Superparamagnetic Pd/Co Alloy Nanoparticles Prepared from a Silica/Cyanogel Co-gel. Chem. Mater. 2005, 17, 6216–6218. [Google Scholar] [CrossRef]
- Ma, J.; Xu, L.; Xu, L.; Wang, H.; Xu, S.; Li, H.; Xie, S.; Li, H. Highly Dispersed Pd on Co−B Amorphous Alloy: Facile Synthesis via Galvanic Replacement Reaction and Synergetic Effect between Pd and Co. Am. Chem. Soc. 2013, 3, 985–992. [Google Scholar] [CrossRef]
- Liu, D.; Guo, Q.; Hou, H.; Niwa, O.; You, T. PdxCoy Nanoparticle/Carbon Nanofiber Composites with Enhanced Electrocatalytic Properties. ACS Catal. 2014, 4, 1825–1829. [Google Scholar] [CrossRef]
- Bai, Z.; Xu, P.; Chao, S.; Yan, H.; Cui, Q.; Niu, L.; Yang, L.; Qian, J. A facile one-step preparation of a Pd–Co bimetallic hollow nanosphere electrocatalyst for ethanol oxidation. Catal. Sci. Technol. 2013, 3, 2843–2848. [Google Scholar] [CrossRef]






| Catalysts | SBET a (m2/g) | Vmic b (cm3/g) | Dpore c (nm) | Pd Loading d (wt%) |
|---|---|---|---|---|
| Pd@HCS | 379.01 | 0.09 | 15.83 | 4.05 |
| Pd-Co@HCS | 683.36 | 0.16 | 8.78 | 4.05 |
| Pd-Ni@HCS | 434.52 | 0.15 | 13.81 | 4.03 |
| Pd-Cu@HCS | 523.38 | 0.19 | 11.46 | 4.02 |
| Catalysts | SBET (m2/g) | Vmic (cm3/g) | Dpore (nm) | Pd Loading (wt%) |
|---|---|---|---|---|
| Pd-Co@HCS-(1) | 487.96 | 0.17 | 12.29 | 4.05 |
| Pd-Co@HCS-(2.8) | 601.61 | 0.19 | 9.97 | 4.05 |
| Pd-Co@HCS-(4.4) | 683.36 | 0.16 | 8.78 | 4.05 |
| Pd-Co@HCS-(6.6) | 400.03 | 0.13 | 14.99 | 4.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Pan, H.; Lin, Q.; Shi, Y.; Zhang, J. Synthesis of Pd-M@HCS(M = Co, Ni, Cu) Bimetallic Catalysts and Their Catalytic Performance for Direct Synthesis of H2O2. Catalysts 2020, 10, 303. https://doi.org/10.3390/catal10030303
Wang Y, Pan H, Lin Q, Shi Y, Zhang J. Synthesis of Pd-M@HCS(M = Co, Ni, Cu) Bimetallic Catalysts and Their Catalytic Performance for Direct Synthesis of H2O2. Catalysts. 2020; 10(3):303. https://doi.org/10.3390/catal10030303
Chicago/Turabian StyleWang, Yaodan, Hongyan Pan, Qian Lin, Yongyong Shi, and Jiesong Zhang. 2020. "Synthesis of Pd-M@HCS(M = Co, Ni, Cu) Bimetallic Catalysts and Their Catalytic Performance for Direct Synthesis of H2O2" Catalysts 10, no. 3: 303. https://doi.org/10.3390/catal10030303
APA StyleWang, Y., Pan, H., Lin, Q., Shi, Y., & Zhang, J. (2020). Synthesis of Pd-M@HCS(M = Co, Ni, Cu) Bimetallic Catalysts and Their Catalytic Performance for Direct Synthesis of H2O2. Catalysts, 10(3), 303. https://doi.org/10.3390/catal10030303




