Abstract
A ZSM-5 zeolite with a hierarchical pore structure was synthesized by the desilication-recrystallization method using tetraethyl ammonium hydroxide (TEAOH) and cetyltrimethylammonium bromide (CTAB) as the desilication and structure-directing agents, respectively. The MnOx/ZSM-5 catalyst was synthesized by the ethanol dispersion method and applied for the low-temperature selective catalytic reduction of NOx with NH3. The results showed that NOx conversion of the hierarchical MnOx/ZSM-5 catalyst could reach 100% at about 120 °C and could be maintained in the temperature range of 120–240 °C with N2 selectivity over 90%. Furthermore, the hierarchical MnOx/ZSM-5catalyst presented better SO2 resistance performance than the traditional catalyst in the presence of 100 ppm SO2 at 120 °C. XRD, SEM, TEM, XPS, BET, NH3-TPD, and TG were applied to characterize the structural properties of the MnOx/ZSM-5 catalysts. These results showed that the MnOx/ZSM-5 catalyst had micropores (0.78 nm) and mesopores (3.2 nm) leading to a larger specific surface area, which improved the mass transfer of reactants and products while reducing the formation of sulfates. The better catalytic performance over hierarchical MnOx/ZSM-5 catalyst could be attributed to the higher concentration of Mn4+ and chemisorbed oxygen species and higher surface acidity. The improved SO2 resistance was related to the catalyst’s hierarchical pore structure.
1. Introduction
Selective catalytic reduction of NOx by NH3 (NH3-SCR) is the most widely applied technology to remove NOx from stationary sources. The catalyst is the key factor that can determine the efficiency of the selective catalytic reduction system [1]. The temperature window of traditional commercial catalyst (V2O5-WO3(MoO3)/TiO2) is 300–400 °C, and low-temperature NH3-SCR catalysts, which can be placed downstream of the desulfurizer and electrostatic precipitator, have attracted increasing attention in recent years [2]. Research on the low-temperature NH3-SCR reaction using transition metal oxides (MnOx [3], FeOx [4], CuOx [5], CeOx [6]) as the active components has become widespread. Among these metal oxides, MnOx exhibits excellent low-temperature catalytic activity, which can be attributed to the variable valence states of manganese and oxygen species [7]. However, there still remains a challenge of SO2 poisoning for low-temperature NH3-SCR catalysts. The deposition of sulfite and sulfate species and sulfate of manganese oxide on the catalysts’ surface lead to the inhibition of the catalysts. Therefore, improving the SO2 tolerance of catalysts is required. Many efforts have been made to promote SO2 tolerance of NH3-SCR catalysts. Yu et al. [8] demonstrated that Pr modification could enhance the sulfur dioxide resistance of MnOx/SAPO-34 catalyst. Compared with manganese oxide, SO2 preferentially combines with PrOx to form praseodymium sulfate and protecting the active sites of Mn. Fan et al. [9] demonstrated that when the cerium oxides were incorporated in the framework and the surface of the Mn/SAPO-34 catalyst, the sulfate of the manganese oxide and the formation of NH4HSO4 could be inhibited. In recent years, catalysts with hierarchical pore structures have attracted much attention for environmental catalytic applications. Due to their adjustable pore sizes and unique structural properties, Gao et al. [10] demonstrated manganese oxide nanoparticles with a hierarchical mesopore structure in the walls of the macroporous skeleton supported by three-dimensionally ordered macroporous carbon, which exhibited better low-temperature NH3-SCR reaction activity, stability, as well as H2O and SO2 resistance. Fang et al. [11] prepared foam-like Fe2O3@CuOx monolith catalysts with a three-dimensional hierarchical structure and displayed a higher activity, stability, as well as SO2 and H2O resistance than the catalyst without Fe2O3. In addition, some research studies have also focused on the hierarchical zeolite supported metal oxides. Pt nanoparticles were encapsulated in a hollow ZSM-5 through a “dissolution-recrystallization” process [12]. The Pt/hollow ZSM-5 catalyst showed excellent SO2 and H2O tolerance, but the active temperature range of the catalyst was very narrow. When the catalyst showed a maximum NO conversion at 90 °C, the activity then decreased with increasing temperature. Other catalysts like hierarchical Fe/ZSM-5 [13], hierarchical Fe-Beta [14], and hierarchical Fe-ZSM-5@CeO2 [15] were synthesized and applied in the SCR reaction.
The ZSM-5 zeolite has adjustable surface acidity, shape selectivity, and a stable framework structure, which has been widely applied as a carrier in the SCR reaction. In this work, a hierarchical ZSM-5 zeolite was successfully prepared via a desilication-recrystallization method, and cetyltrimethylammonium bromide (CTAB) was used as the structure-directing agent. The hierarchical MnOx/ZSM-5 catalyst was synthesized by the ethanol dispersion method and applied in the NH3-SCR reaction. We studied the effect of the hierarchical pore structure on the catalytic performance through comparison with a traditional MnOx/ZSM-5 catalyst. The hierarchical MnOx/ZSM-5 catalyst showed better low-temperature NH3-SCR catalytic activity and higher SO2 tolerance. Furthermore, XRD, SEM, TEM, XPS, BET, NH3-TPD, and TG were employed to explore the structural properties of the MnOx/ZSM-5 catalyst. The results of comparable MnOx/ZSM-5 catalysts demonstrated that a larger specific surface area and pore size, higher surface acidity, and higher concentration of Mn4+ and chemisorbed oxygen species could be provided by the hierarchical pore structure, thus promoting the low-temperature NH3-SCR catalytic performance.
2. Results and Discussion
2.1. XRD Results
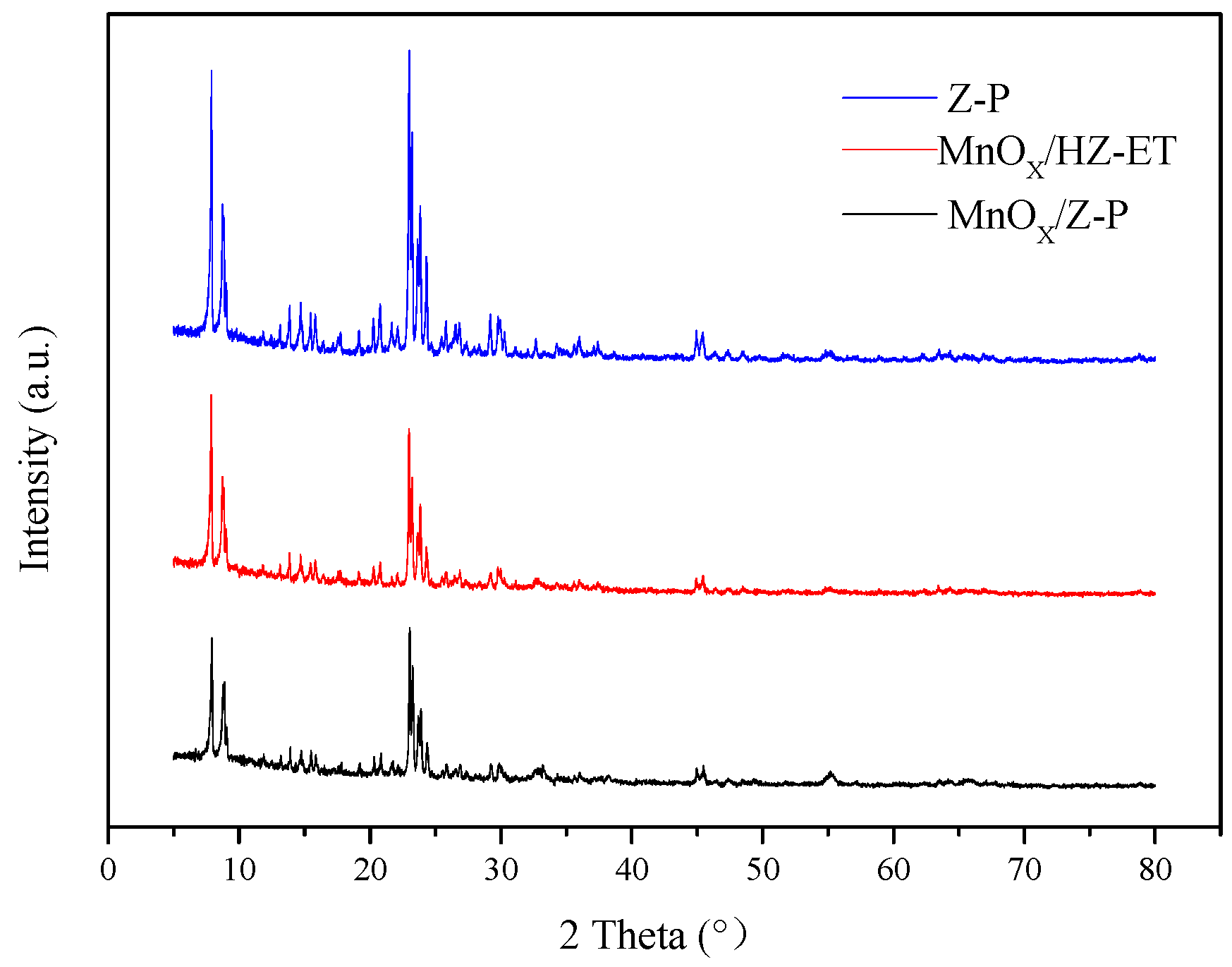
The XRD patterns of MnOx/HZ-ET (hierarchical MnOx/ZSM-5, the treated ZSM-5 was denoted as HZ-ET) and MnOx/Z-P (conventional MnOx/ZSM-5, ZSM-5 zeolite without treatment was denoted as Z-P) are shown in Figure 1. Two diffraction peaks between 2θ = 7 and 10° and three diffraction peaks between 2θ = 22 and 25° were observed, and these peaks were attributed to the characteristic diffraction peaks of the MFI zeolite, indicating that the MFI characteristic structure of ZSM-5 was not destroyed after desilication-recrystallization. No peaks of manganese oxide could be seen in the XRD spectra, indicating that manganese oxide in the catalyst existed either in an amorphous or highly dispersed state. TEM images would be used to further elucidate the structure of the MnOx. The relative crystallinity results of MnOx/HZ-ET and MnOx/Z-P are shown in Table 1. Compared with the parent ZSM-5 (Z-P), the lower relative crystallinity (RC) of the MnOx/HZ-ET was attributed to the desilication, which destroyed the crystal structure [16] and the interaction between the MnOx and the zeolite [17]. The RC of MnOx/HZ-ET was higher than MnOx/Z-P because of recrystallization, which indicated that the framework structure of MnOx/HZ-ET was more complete.
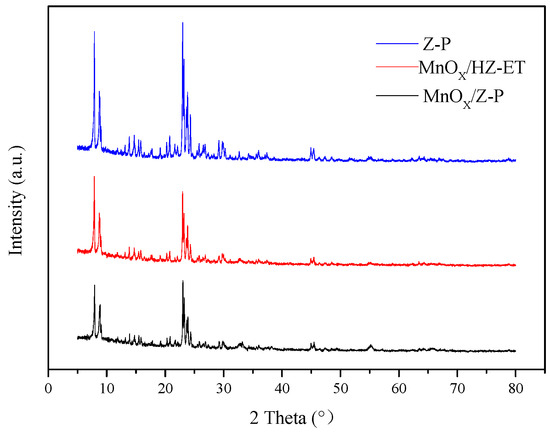
Figure 1.
XRD patterns of the catalysts.

Table 1.
Relative crystallinity (RC) of the catalysts.
2.2. SEM and TEM Results
Figure 2 shows the SEM images of MnOx/Z-P and MnOx/HZ-ET catalysts. The two catalysts showed a hexagonal morphology of the characteristic MFI structure. Compared with the smooth surface of the MnOx/Z-P catalyst (Figure 2a), the surface of the MnOx/HZ-ET catalyst (Figure 2b) was rougher, and the pore structure could be seen on the surface.

Figure 2.
SEM images of the catalysts. (a) MnOx/Z-P, (b) MnOx/HZ-ET.
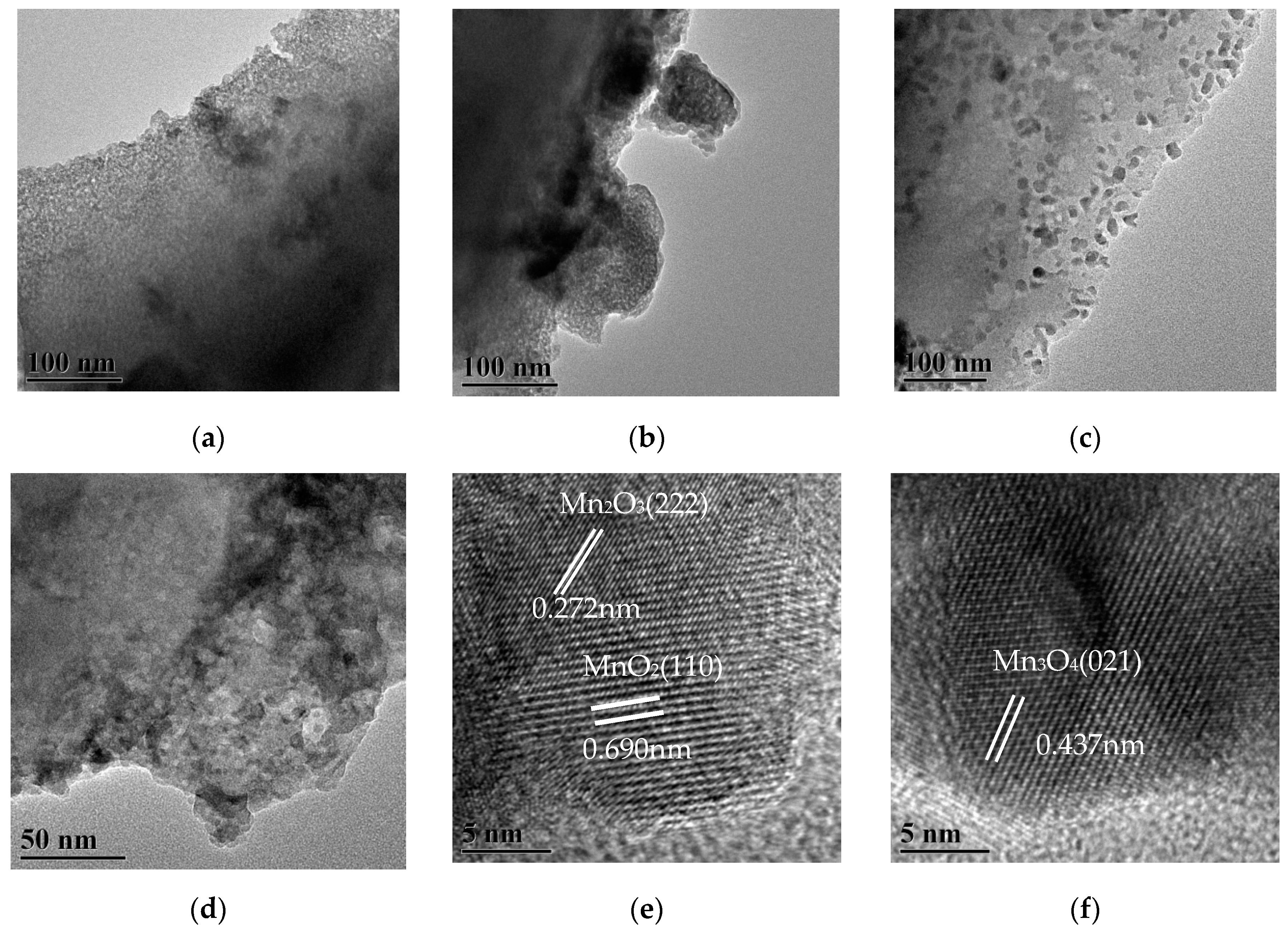
Figure 3 shows TEM images of MnOx/HZ-ET catalysts. Regular mesopores are observed in Figure 3a,b. At the same time, some irregular, larger pore size mesopores compared with the above regular mesopores can be observed in Figure 3c,d. The micropores and regular/irregular mesopores were exhibited in MnOx/HZ-ET with different size ranges. HRTEM images of MnOx/HZ-ET catalyst are shown in Figure 3e,f. The lattice fringe spacing of 0.437 nm in Figure 3f corresponds to the (021) plane of Mn3O4, while the lattice fringe spacings of 0.690 nm and 0.272 nm in Figure 3e correspond to the (110) plane of MnO2 and the (222) plane of Mn2O3, respectively. Combined with the XRD analysis, the results of TEM images showed that manganese oxide was not amorphous, but existed in various forms of crystallization in highly dispersed states. The (110) planes of MnO2 in Figure 3e was considered as the most active crystal plane in the NH3-SCR reaction [18] and provided a large number of active sites and oxygen vacancies. This was one of the reasons why the MnOx/HZ-ET catalyst showed better low-temperature SCR activity than MnOx/Z-P.
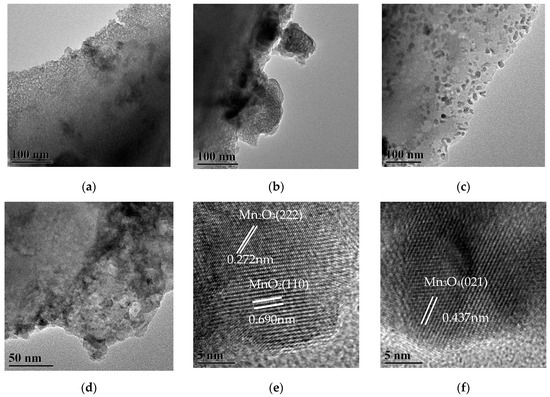
Figure 3.
(a–d) TEM images of the MnOx/HZ-ET, (e–f) HR-TEM images of the MnOx/HZ-ET.
2.3. BET Results
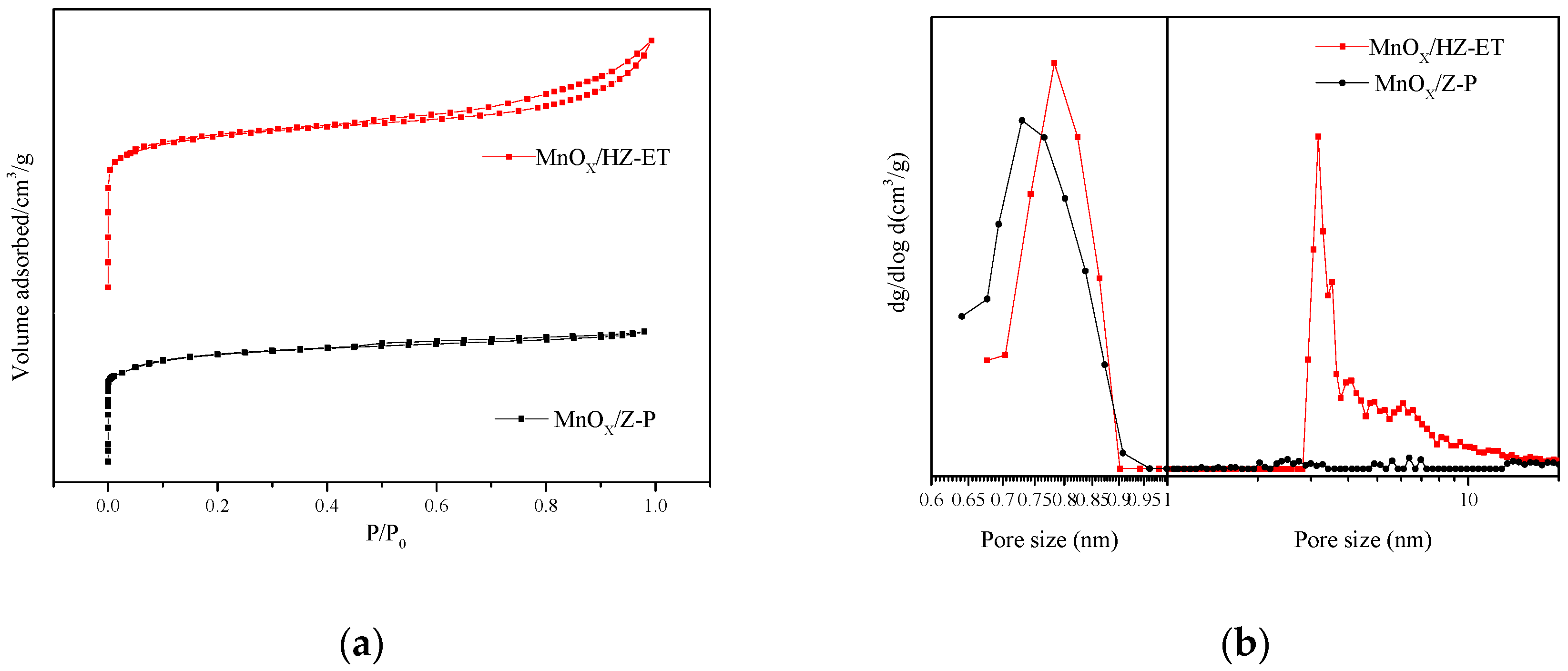
Figure 4 shows the nitrogen adsorption-desorption isotherms and the pore size distribution curves of MnOx/Z-P and MnOx/HZ-ET catalysts. Figure 4a shows that MnOx/Z-P exhibited type I isotherms with no distinct hysteresis loop, which indicated that MnOx/Z-P was a typical microporous material, where MnOx/HZ-ET displayed typical type I isotherms at low relative pressure (P/P0 < 0.01) and type IV isotherms with a clear hysteresis loop at higher relative pressure (P/P0 > 0.4), demonstrating the co-existence of micropores and mesopores created through the desilication-recrystallization processes. Figure 4b shows that the micropore size distributions of MnOx/HZ-ET catalyst centered around 0.78 nm, which was larger than that of MnOx/Z-P (around 0.73 nm). The enlargement of the micropores in MnOx/HZ-ET may be due to the destruction of pore walls by treatment with TEAOH [19]. The pore size distribution of MnOx/Z-P (Figure 4b) showed no obvious peaks in the range of 2–20 nm, and MnOx/HZ-ET showed a narrow and intense peak at 3.2 nm. In addition, MnOx/HZ-ET showed broad peaks between 4 and 15 nm, which possibly indicated that the intracrystalline mesopores were caused by desilication and existed in an irregular state [16]; this result was consistent with the TEM images.
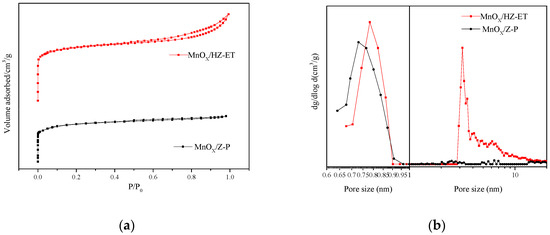
Figure 4.
(a) N2 adsorption-desorption isotherms and (b) pore size distribution of the catalysts.
Table 2 shows the corresponding results of structural properties of MnOx/Z-P and MnOx/HZ-ET catalysts. The specific surface areas and the external surface areas of MnOx/HZ-ET were 328 m2/g and 124 m2/g, which was higher than MnOx/Z-P (247 m2/g and 12 m2/g, respectively). MnOx/HZ-ET contained more mesopores and less micropores than MnOx/Z-P, which was attributed to the transformation of part of the microporous phase into the mesoporous phase. The larger specific surface area and pore size within hierarchical MnOx/HZ-ET improved the mass transfer of reactants and products during the NH3-SCR reaction, thus improving its catalytic performance compared to MnOx/Z-P, which only had microporous structures.

Table 2.
Structural properties of the catalysts.
2.4. XPS Results
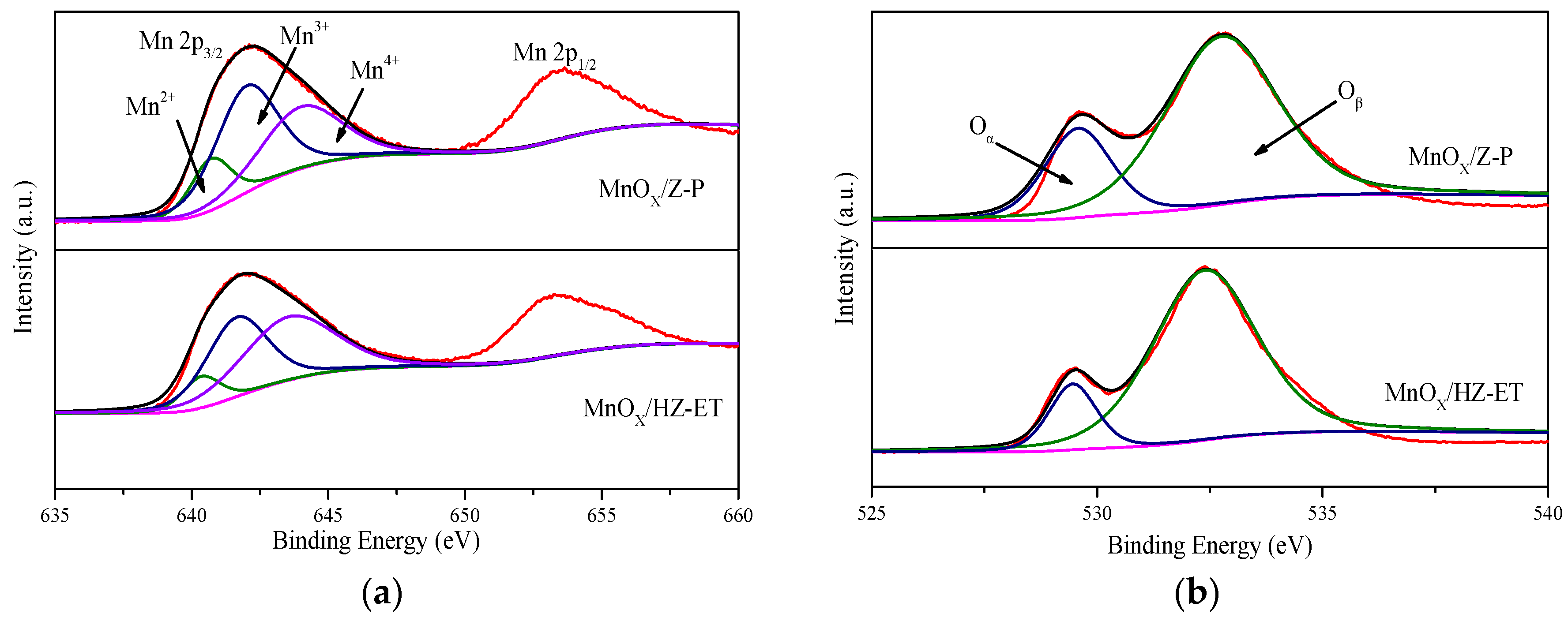
The surface atomic composition was analyzed by XPS. Figure 5 shows the XPS spectra of Mn 2p and O 1s for the MnOx/Z-P and MnOx/HZ-ET catalysts. The XPS spectra of Mn 2p3/2 could be fit into three characteristic peaks ascribed to Mn2+ at 640.4 eV, Mn3+ at 642.0 eV, and Mn4+ at 644.1 eV. The XPS spectra of O 1s could be divided into two characteristic peaks ascribed to lattice oxygen species (Oα), around 529.7 eV, and chemisorbed oxygen species (Oβ), around 531.5 eV [20]. The corresponding results are summarized in Table 3. It was clear that the molar concentration of manganese on the surface of MnOx/HZ-ET (9.62%) was higher than MnOx/Z-P (8.47%), and the relative surface concentration ratios of Mn4+ (Mn4+/Mn) of MnOx/HZ-ET(49.09%) were much higher than MnOx/Z-P (36.36%). It was reported that Mn4+ could promote the oxidation of NO to NO2, and higher concentration ratios of Mn4+ species were beneficial for the improvement of their redox cycle, thus promoting the activity in the NH3-SCR reaction at low temperatures [21]. In addition, the percentage of chemisorbed oxygen species Oβ in MnOx/HZ-ET (87.09%) was much higher than MnOx/Z-P (75.41%). The chemisorbed oxygen species Oβ was reported to be more active than Oα because of their higher mobility. Higher percentage of Oβ was beneficial for the oxidation of NO to NO2, enhancing the low-temperature activity through the “fast SCR” reaction [22]. Therefore, the excellent low-temperature SCR activity of MnOx/HZ-ET was attributed to the higher percentage of active Mn4+ and chemisorbed oxygen species.
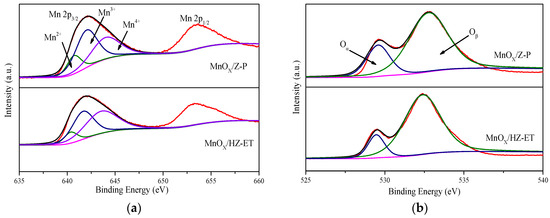
Figure 5.
XPS spectra of Mn 2p (a), O 1s (b) of the catalysts.

Table 3.
Surface atomic concentrations and relative concentration ratios of Mn and O.
2.5. NH3-TPD Results
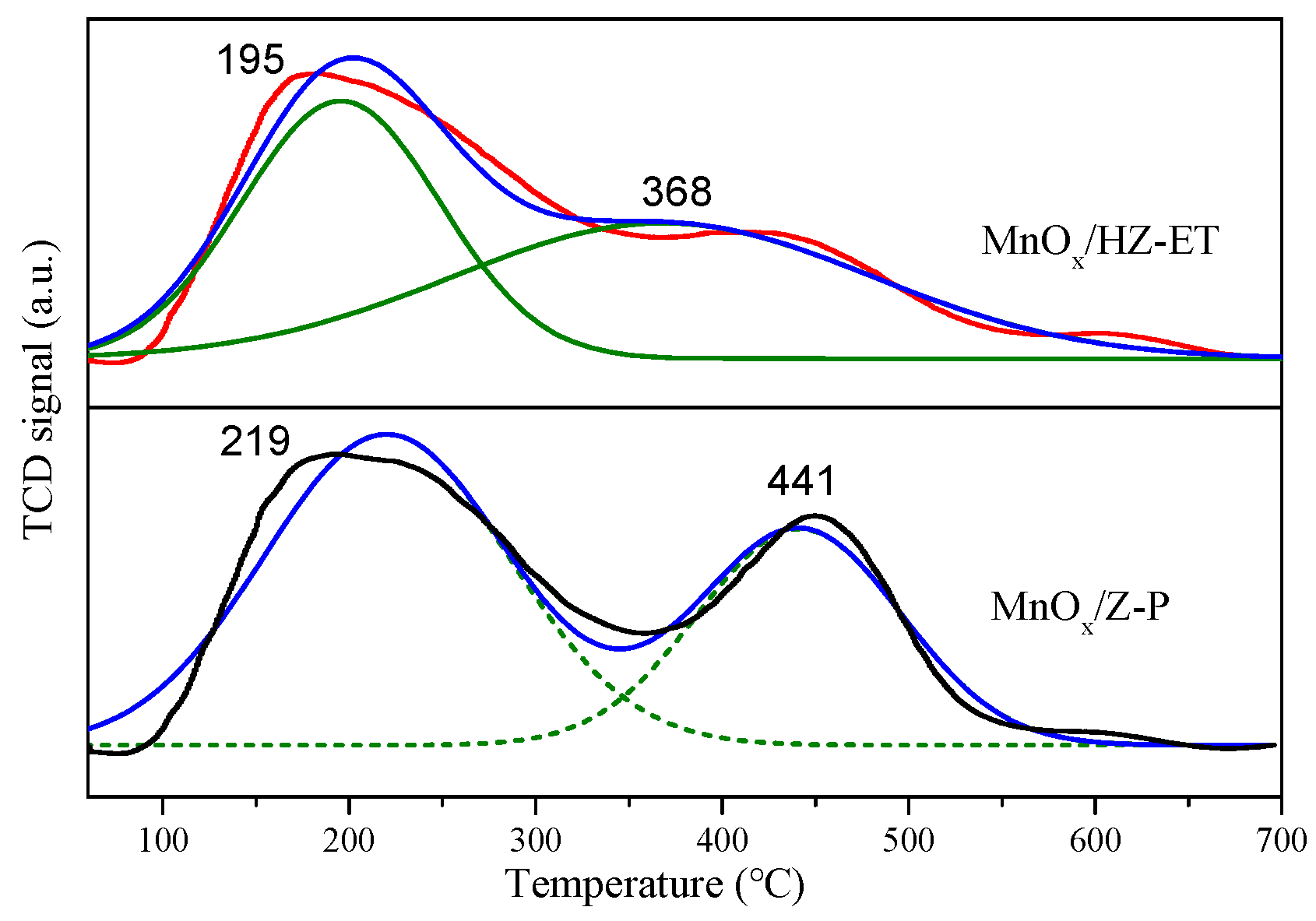
The surface acidity properties of the catalysts were analyzed by the NH3-TPD technique. Figure 6 shows that the NH3 desorption profiles of MnOx/HZ-ET and MnOx/Z-P exhibited two distinct desorption peaks. The desorption peaks below 350 °C were associated with the weakly acidic sites, while the desorption peaks ranging from 350 to 550 °C were associated with the strongly acidic sites [23]. The quantified results of NH3-TPD are summarized in Table 4, and the area of the peak was proportional to the amount of acid. It could be found that MnOx/HZ-ET showed more total acid amount than MnOx/Z-P. Although the weak acid amount of MnOx/Z-P was higher than MnOx/HZ-ET, the strong acid and total acid amount of MnOx/HZ-ET were both higher than MnOx/Z-P, which may be ascribed to the higher specific surface area of the MnOx/HZ-ET catalyst. It could be concluded that MnOx/HZ-ET with a hierarchical pore structure could provide more strong surface acidity sites, which would be beneficial for the adsorption and activation of NH3, resulting in increased low-temperature NH3-SCR performance.
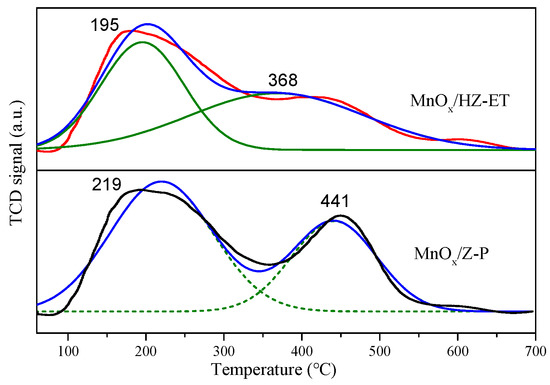
Figure 6.
NH3-TPD of the catalysts.

Table 4.
The NH3 desorption of the catalysts.
2.6. SCR Performance
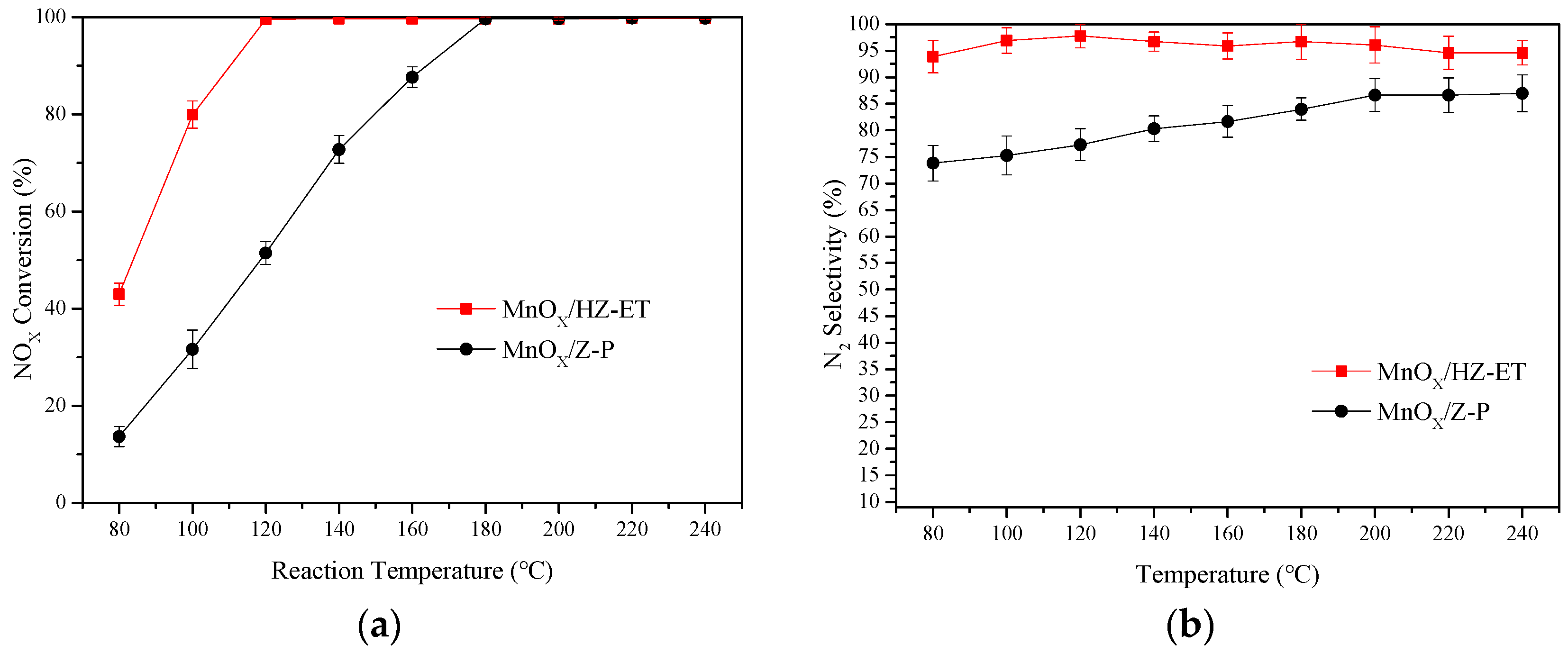
Low-temperature NH3-SCR activities and N2 selectivities of MnOx/HZ-ET and MnOx/Z-P were tested over the temperature range of 80–240 °C, and the results of the NOx conversion are shown in Figure 7. The MnOx/Z-P catalyst showed low NOx conversion in the temperature range of 80–180 °C, and 99% NOx conversion was obtained around 180 °C. Meanwhile, the N2 selectivity of MnOx/Z-P was lower than 90% over the whole temperature range. Compared with MnOx/Z-P, MnOx/HZ-ET showed significantly higher NOx conversion over the temperature range of 80–120 °C. When the temperature reached 120 °C, nearly 100% NOx conversion could be obtained with a broader operating temperature window (120–240 °C). Over 90% N2, selectivity could be maintained for the MnOx/HZ-ET catalyst throughout the entire temperature range. Conventional microporous catalysts were reported to have several drawbacks in the SCR reaction, such as diffusion limitations of the reactants and products [24]. Therefore, it was reasonable to deduce that the mass transfer of reactants and products could be enhanced with the existence of mesopores at low temperatures, and as a result of this, better low-temperature NH3-SCR performance could be obtained using the hierarchical MnOx/HZ-ET catalyst.
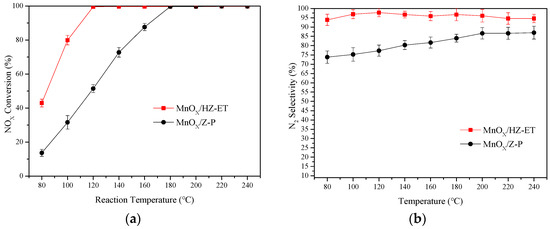
Figure 7.
SCR activity (a) and N2 selectivity (b) of MnOx/HZ-ET and MnOx/Z-P catalysts with a Mn loading of 15 wt.%.
2.7. Effect of SO2 on SCR Catalytic Activity
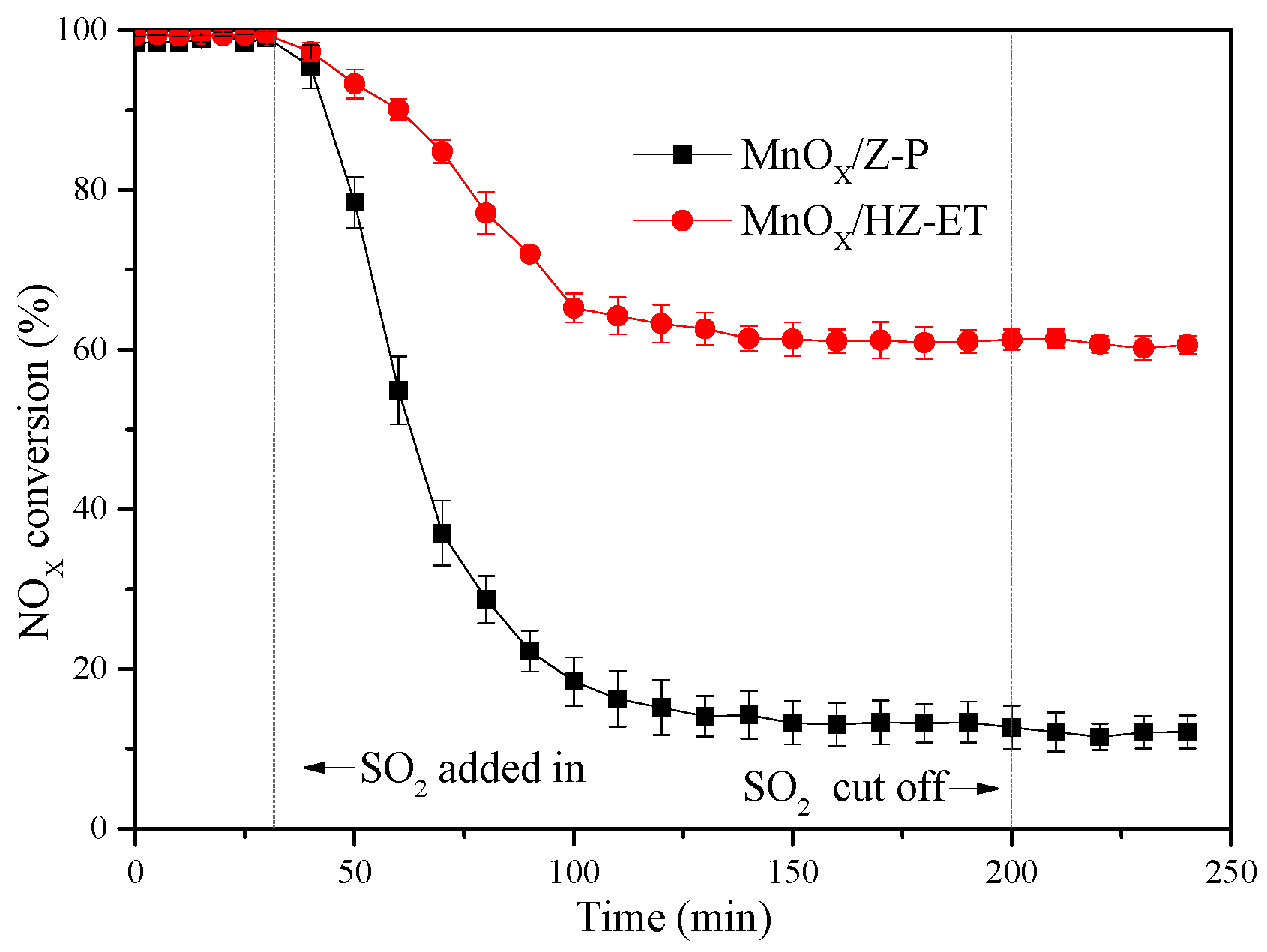
Figure 8 shows the NOx conversion for the MnOx/HZ-ET and MnOx/Z-P catalysts in the presence of 100 ppm SO2 at 120 °C and 180 °C, respectively. The results showed that NOx conversion noticeably decreased with the addition of SO2 into the feed gas. The SCR activity of the MnOx/Z-P catalyst decreased from 100% to 15% after 60 min of SO2 addition. The NOx conversion of MnOx/HZ-ET decreased from 100% to 60% after the SO2 was added for 1 h. The NOx conversion could not be recovered over the two catalysts when SO2 was absent from the reaction mixture, which indicated that the deactivation was irreversible. (NH4)2SO4 or NH4HSO4 may be formed during the reaction when SO2 was added into the reaction atmospheres. These species could only decompose over 300 °C and may block the zeolite channels, leading to reduced catalytic activities. At the same time, catalysts with a larger pore size could reduce the formation of sulfate species [25]. Comparing the SO2 tolerance of MnOx/HZ-ET and MnOx/Z-P, the hierarchical pore structure MnOx/HZ-ET catalyst could provide larger specific surface area and larger pore size, which was beneficial for its SO2 resistance.
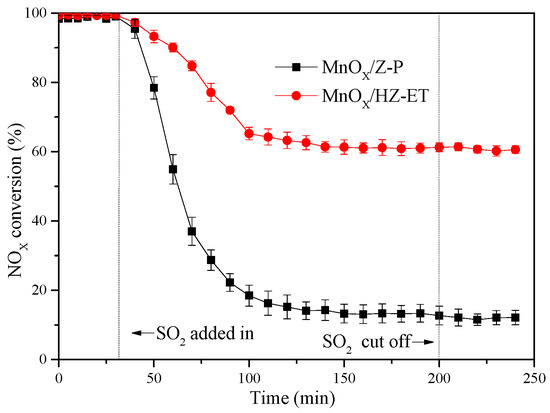
Figure 8.
The SO2 tolerance tests over the MnOx/HZ-ET and MnOx/Z-P; reaction conditions: 800 ppm NH3, 800 ppm NO, 100 ppm SO2, 5 vol. % O2, Ar to balance, gas hourly space velocity (GHSV) = 40,000 h−1.
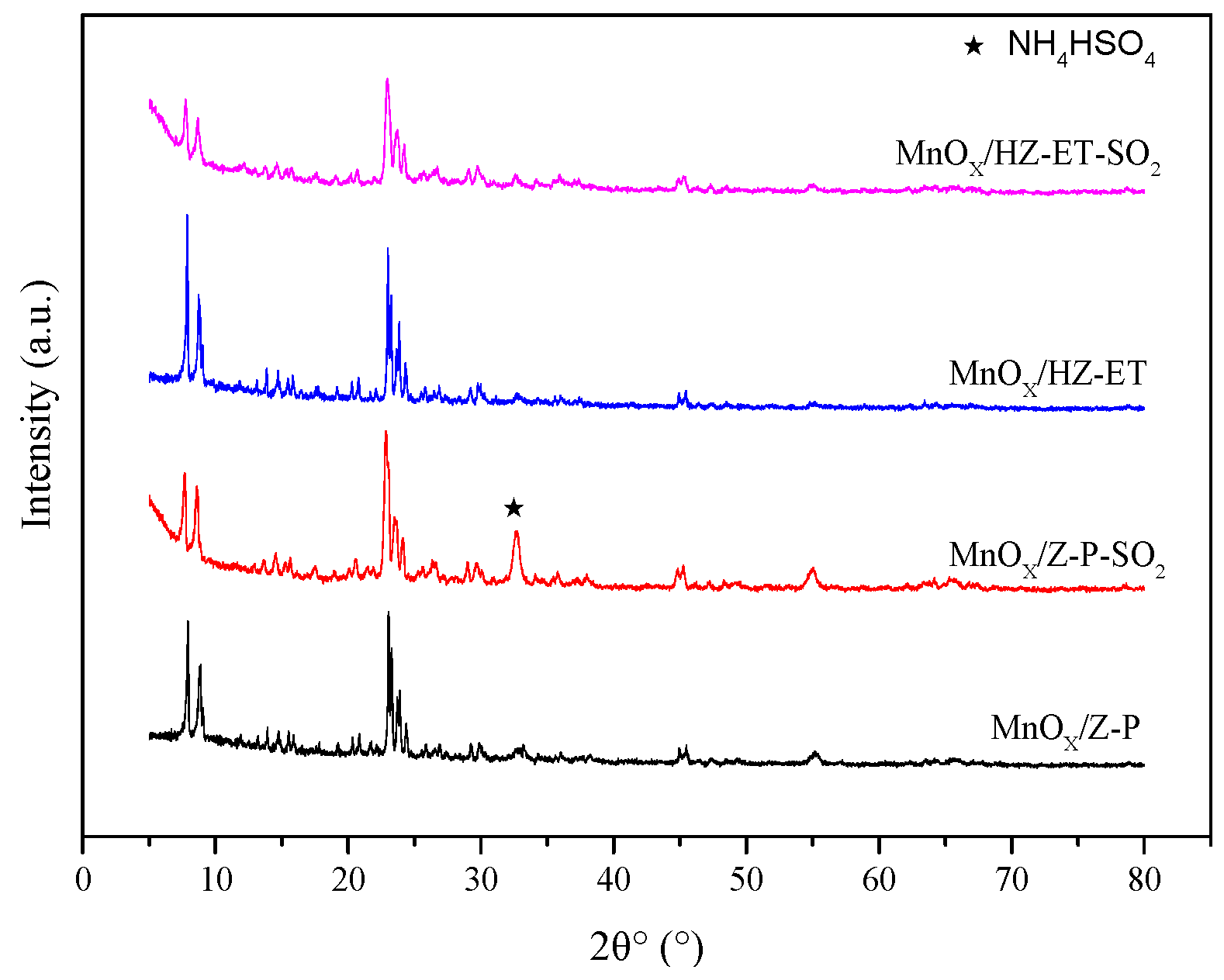
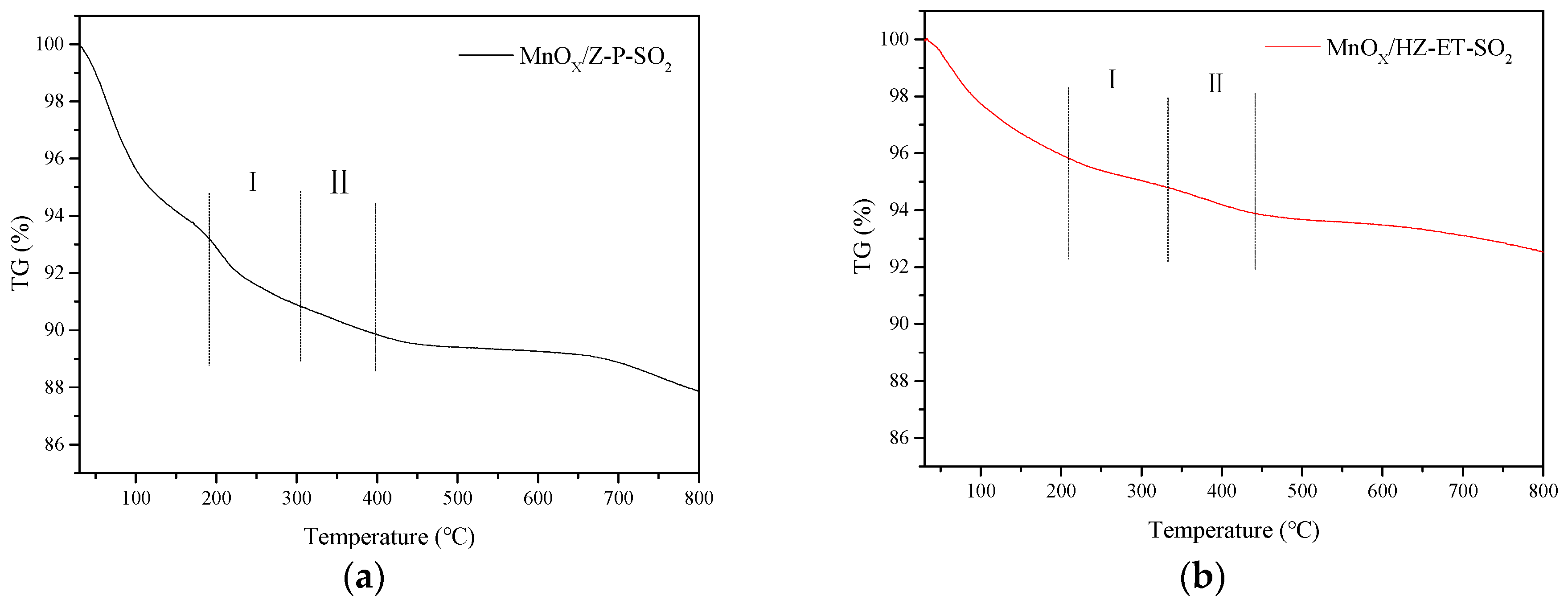
XRD and TG were applied to identity the formation of (NH4)2SO4 or NH4HSO4. Figure 9 shows the XRD patterns of poisoned MnOx/HZ-ET and MnOx/Z-P catalysts. The peak at 2θ = 33° observed over the used MnOx/Z-P could be attributed to the phase of formed NH4HSO4. No obvious peaks of NH4HSO4 could be observed in the XRD pattern of used MnOx/HZ-ET, which may be because the NH4HSO4 formed existed in amorphous species or was below the detection limit. Figure 10 presents the TG curves of the poisoned MnOx/HZ-ET and MnOx/Z-P catalysts. The first weight loss emerging at 80–110 °C could be due to the evaporation of water in the catalysts. Two other weight losses at about 250 °C and 350 °C were close to the decomposition temperature of (NH4)2SO4 or NH4HSO4 [8]. For MnOx/HZ-ET, the intensities of two weight losses was lower than that of MnOx/Z-P. The XRD and TG results showed that the MnOx/HZ-ET with a larger pore size could reduce the deposition of (NH4)2SO4 or NH4HSO4 on the catalyst surface during the NH3-SCR reaction with SO2, which was one of the reasons for the better SO2 resistance of the MnOx/HZ-ET catalyst.
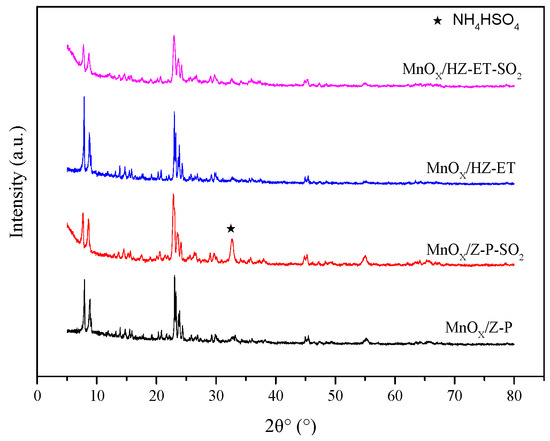
Figure 9.
XRD patterns of the catalysts used.
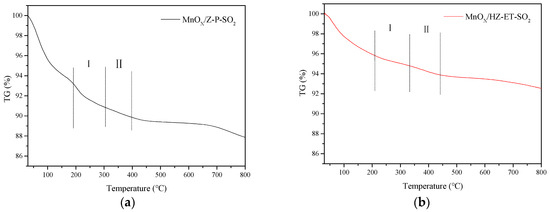
Figure 10.
TG profiles of the catalysts used. (a) MnOx/Z-P-SO2, (b) MnOx/HZ-ET-SO2.
3. Materials and Methods
3.1. Catalyst Preparation
The hierarchical ZSM-5 was prepared from commercial MFI-type zeolites by sequential desilication-recrystallization based on previous studies [16,19,26]. The parent zeolite in this study was a commercial H form ZSM-5 with a Si/Al mass ratio = 38 (XFNANO Company, Nanjing, China). Typically, three grams of ZSM-5 zeolite and 1 g cetyltrimethylammonium bromide (CTAB, Aladdin, Shanghai, China) were dispersed in 30 mL of 1 M tetraethyl ammonium hydroxide (TEAOH, Aladdin, Shanghai, China), and the mixture was stirred at ambient temperature. The solution was transferred into a Teflon-lined autoclave and treated at 150 °C for 24 h. The product was washed with distilled water, filtered, dried, and calcined at 550 °C for 4 h to remove the templates. The treated sample was denoted as HZ-ET, and for comparison, the ZSM-5 zeolite without treatment was denoted as Z-P.
The catalysts were synthesized by the ethanol dispersion method, and manganese nitrate (50 wt.% in H2O, Aladdin, Shanghai, China) was used as a precursor. Typically, one-point-nine-five grams of 50 wt.% Mn(NO3)2 (Mn loading = 15 wt.%) were dissolved in 75 mL ethanol under stirring at ambient temperature. Subsequently, two grams of HZ-ET or Z-P powder were added and stirred. Then, the solution was treated with ultrasound for 0.5 h and stirred continuously at 85 °C until the solvent was completely evaporated. The products were dried at 80 °C and calcined at 400 °C for 3 h. The catalyst was denoted as MnOx/HZ-ET. For comparison, MnOx/Z-P was also prepared according to the same method.
3.2. Low-Temperature NH3-SCR Activity Measurements
The NH3-SCR activity tests were carried out in a fixed-bed quartz glass reactor. Five-hundred milligrams of 40–60 mesh catalysts were used in the test. The reaction temperature was increased from room temperature to 240 °C with a heating rate of 5 °C/min with an isotherm step of 20 °C. The gas was composed of 800 ppm NH3, 800 ppm NO, 5.0 vol% O2, and 100 ppm SO2 (when added), balanced by Ar. The flow rate was 600 mL/min with a gas hourly space velocity (GHSV) of 40,000 h−1. The concentrations of NO/NO2 were measured by a NO–NO2–NOx analyzer (Thermal Scientific, model 42i-HL, Waltham, USA), and N2 gas chromatography was used to analyze N2-selectivity. The NOx removal efficiency and the N2 selectivity are calculated as follows:
where and represent the inlet concentration (ppm) of NOx and NH3, respectively. , , and represent the outlet concentration (ppm) of NOx and NH3 and N2O, respectively.
3.3. Characterization
X-ray diffraction (XRD) patterns were recorded on a D8 Advance diffractometer (Bruker, Karlsruhe, Germany) with Cu Kα radiation. Chemical states of all elements were analyzed by X-ray photoelectron spectroscopy (XPS, Axis Ultra DLD, Kratos, U.K.) with Al Kα radiation (hυ = 1253.6 eV). NH3-TPD analysis was performed on Tp 5080 (Xianquan Industrial and Trading Co., Ltd, Tianjin, China). A scanning electron microscope (SEM) was carried out on ZEISS Merlin instrument (Carl Zeiss AG, Jena, Germany). Transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) were performed on TECNAI G2 F20 (FEI, Hillsboro, OR, USA). N2 physisorption was performed at −196 °C using a surface analyzer (Micomeritics, ASAP 2020, Norcross, USA), and all samples were degassed in a vacuum at 30 °C for 6 h prior to measurements. The Brunauer–Emmett–Teller (BET) method was used to calculate the specific surface area. The micropore surface area and micropore volume were evaluated using a t-plot. The Barrett–Joyner–Halenda (BJH) method was used to determine the pore size distributions from desorption branches. Thermogravimetric analysis (TG) was carried out in a static N2 atmosphere using a TGA/DSC 3+instrument (Mettler, Zurich, Switzerland). Fifteen milligrams of each sample were analyzed between 30 and 800 °C at a rate of 10 °C/min.
4. Conclusions
A MnOx/HZ-ET catalyst, which possessed a hierarchical pore structure, was successfully prepared through desilication-recrystallization and ethanol dispersion methods. Compared with MnOx/Z-P prepared using traditional microporous ZSM-5 as a support, MnOx/HZ-ET showed better low-temperature NH3-SCR activity with nearly 100% NOx conversion over a broad temperature window from 120 °C to 240 °C and above 90% N2 selectivity throughout the entire temperature range. The low temperature NH3-SCR performance of MnOx/HZ-ET could be attributed to the hierarchical pore structure, higher concentration of chemisorbed oxygen and Mn4+ species, as well as appropriate acid strengths and amounts. The MnOx/HZ-ET catalyst also showed better SO2 tolerance than the MnOx/Z-P, and 60% NOx conversion could be maintained after the SO2 was added for 1 h, which could be related to its larger specific surface area and larger pore size, which may reduce the deposition of ammonium sulfate.
Author Contributions
Writing, original draft preparation, J.S.; writing, review and editing, J.S., S.C., and Z.L.; funding acquisition, B.H.; supervision, B.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China, Grant Number 51478191.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Salazar, M.; Hoffmann, S.; Tillmann, L.; Singer, V.; Becker, R.; Grünert, W. Hybrid catalysts for the selective catalytic reduction (SCR) of NO by NH3: Precipitates and physical mixtures. Appl. Catal. B 2017, 218, 793–802. [Google Scholar] [CrossRef]
- Wang, H.J.; Huang, B.C.; Yu, C.L.; Lu, M.J.; Huang, H.; Zhou, Y.L. Research progress, challenges and perspectives on the sulfur and water resistance of catalysts for low temperature selective catalytic reduction of NOx by NH3. Appl. Catal. A. 2019, 588, 117207. [Google Scholar] [CrossRef]
- Nakhostin Panahi, P. Comparative study of ZSM-5 supported transition metal (Cu, Mn, Co, and Fe) nanocatalysts in the selective catalytic reduction of NO with NH3. Environ. Prog. Sustain. Energy 2017, 36, 1049–1055. [Google Scholar] [CrossRef]
- Saeidi, M.; Hamidzadeh, M. Co-doping a metal (Cr, Mn, Fe, Co, Ni, Cu, and Zn) on Mn/ZSM-5 catalyst and its effect on the catalytic reduction of nitrogen oxides with ammonia. Res. Chem. Intermed. 2016, 43, 2143–2157. [Google Scholar] [CrossRef]
- Wijayanti, K.; Xie, K.; Kumar, A.; Kamasamudram, K.; Olsson, L. Effect of gas compositions on SO2 poisoning over Cu/SSZ-13 used for NH3-SCR. Appl. Catal. B 2017, 219, 142–154. [Google Scholar] [CrossRef]
- Kwon, D.W.; Hong, S.C. Promotional effect of tungsten-doped CeO2/TiO2 for selective catalytic reduction of NOx with ammonia. Appl. Surf. Sci. 2015, 356, 181–190. [Google Scholar] [CrossRef]
- Wang, L.S.; Huang, B.C.; Su, Y.X.; Zhou, G.Y.; Wang, K.L.; Luo, H.C.; Ye, D.Q. Manganese oxides supported on multi-walled carbon nanotubes for selective catalytic reduction of NO with NH3: Catalytic activity and characterization. Chem. Eng. J. 2012, 192, 232–241. [Google Scholar] [CrossRef]
- Yu, C.L.; Huang, B.C.; Dong, L.F.; Chen, F.; Yang, Y.; Fan, Y.M.; Yang, Y.X.; Liu, X.Q.; Wang, X.N. Effect of Pr/Ce addition on the catalytic performance and SO2 resistance of highly dispersed MnOx/SAPO-34 catalyst for NH3 -SCR at low temperature. Chem. Eng. J. 2017, 316, 1059–1068. [Google Scholar] [CrossRef]
- Fan, Y.M.; Ling, W.; Huang, B.C.; Dong, L.F.; Yu, C.L.; Xi, H.X. The synergistic effects of cerium presence in the framework and the surface resistance to SO2 and H2O in NH3 -SCR. J. Ind. Eng. Chem. 2017, 56, 108–119. [Google Scholar] [CrossRef]
- Gao, X.; Li, L.; Song, L.H.; Lu, T.; Zhao, J.X.; Liu, Z. Highly dispersed MnOx nanoparticles supported on three-dimensionally ordered macroporous carbon: A novel nanocomposite for catalytic reduction of NOx with NH3 at low temperature. RSC Adv. 2015, 5, 29577–29588. [Google Scholar] [CrossRef]
- Fang, C.; Shi, L.Y.; Hu, H.; Zhang, J.P.; Zhang, D.S. Rational design of 3D hierarchical foam-like Fe2O3@CuOx monolith catalysts for selective catalytic reduction of NO with NH3. RSC Adv. 2015, 5, 11013–11022. [Google Scholar] [CrossRef]
- Hong, Z.; Wang, Z.; Chen, D.; Sun, Q.; Li, X.B. Hollow ZSM-5 encapsulated Pt nanoparticles for selective catalytic reduction of NO by hydrogen. Appl. Surf. Sci. 2018, 440, 1037–1046. [Google Scholar] [CrossRef]
- Ma, J.; Weng, D.; Wu, X.D.; Si, Z.C.; Wu, Z.W. Highly dispersed iron species created on alkali-treated zeolite for ammonia SCR. Prog. Nat. Sci. 2013, 23, 493–500. [Google Scholar] [CrossRef]
- Zhu, N.; Lian, Z.H.; Zhang, Y.; Shan, W.P.; He, H. Improvement of low-temperature catalytic activity over hierarchical Fe-Beta catalysts for selective catalytic reduction of NOx with NH3. Chin. Chem. Lett. 2019, 30, 867–870. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.X.; Cong, Q.L.; Ma, H.Y.; Li, S.J.; Li, W. Design of a hierarchical Fe-ZSM-5@CeO2 catalyst and the enhanced performances for the selective catalytic reduction of NO with NH3. Chem. Eng. J. 2019, 369, 957–967. [Google Scholar] [CrossRef]
- Diao, Z.H.; Wang, L.; Zhang, X.W.; Liu, G.Z. Catalytic cracking of supercritical n-dodecane over meso-HZSM-5@Al-MCM-41 zeolites. Chem. Eng. Sci. 2015, 135, 452–460. [Google Scholar] [CrossRef]
- Carja, G.; Kameshima, Y.; Okada, K.; Madhusoodana, C.D. Mn–Ce/ZSM5 as a new superior catalyst for NO reduction with NH3. Appl. Catal. B. 2007, 73, 60–64. [Google Scholar] [CrossRef]
- Li, S.H.; Huang, B.C.; Yu, C.L. A CeO2-MnOx core-shell catalyst for low-temperature NH3-SCR of NO. Catal. Commun. 2017, 98, 47–51. [Google Scholar] [CrossRef]
- Na, J.D.; Liu, G.Z.; Zhou, T.Y.; Ding, G.C.; Hu, S.L.; Wang, L. Synthesis and Catalytic Performance of ZSM-5/MCM-41 Zeolites With Varying Mesopore Zize by Surfactant-Directed Recrystallization. Catal. Lett. 2013, 143, 267–275. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, L.Y.; Huang, L.; Zhang, J.P.; Gao, R.H.; Zhang, D.S. Rational Design of High-Performance DeNOx Catalysts Based on MnxCo3–xO4 Nanocages Derived from Metal–Organic Frameworks. ACS Catal. 2014, 4, 1753–1763. [Google Scholar] [CrossRef]
- Lu, X.N.; Song, C.Y.; Jia, S.H.; Tong, Z.S.; Tang, X.L.; Teng, Y.X. Low-temperature selective catalytic reduction of NOx with NH3 over cerium and manganese oxides supported on TiO2–graphene. Chem. Eng. J. 2015, 260, 776–784. [Google Scholar] [CrossRef]
- Putluru, S.S.R.; Schill, L.; Jensen, A.D.; Siret, B.; Tabaries, F.; Fehrmann, R. Mn/TiO2 and Mn–Fe/TiO2 catalysts synthesized by deposition precipitation—promising for selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. B 2015, 165, 628–635. [Google Scholar] [CrossRef]
- Putluru, S.S.R.; Schill, L.; Jensen, A.D.; Fehrmann, R.S.N. Selective Catalytic Reduction of NOx with NH3 on Cu-, Fe-, and Mn-Zeolites Prepared by Impregnation: Comparison of Activity and Hydrothermal Stability. J. Chem. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Grossale, A.; Nova, I.; Tronconi, E. Ammonia blocking of the “Fast SCR” reactivity over a commercial Fe-zeolite catalyst for Diesel exhaust aftertreatment. J. Catal. 2009, 265, 141–147. [Google Scholar] [CrossRef]
- Guo, K.; Fan, G.F.; Gu, D.; Yu, S.H.; Ma, K.L.; Liu, A.N.; Tan, W.; Wang, J.M.; Du, X.Z.; Zou, W.X.; et al. Pore Size Expansion Accelerates Ammonium Bisulfate Decomposition for Improved Sulfur Resistance in Low-Temperature NH3-SCR. ACS Appl. Mater. Interfaces 2019, 11, 4900–4907. [Google Scholar] [CrossRef]
- Dai, C.Y.; Zhang, A.F.; Liu, M.; Guo, X.W.; Song, C.H. Hollow ZSM-5 with Silicon-Rich Surface, Double Shells, and Functionalized Interior with Metallic Nanoparticles and Carbon Nanotubes. Adv. Funct. Mater. 2015, 25, 7479–7487. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).