Nanosized V-Ce Oxides Supported on TiO2 as a Superior Catalyst for the Selective Catalytic Reduction of NO
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphology and Structure Investigations
2.2. Surface Analysis
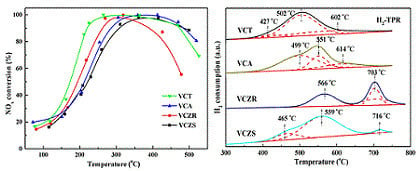
2.3. Reducibility
2.4. Surface Acidity
2.5. Catalytic Performance
3. Materials and Methods
3.1. Catalyst Preparation
3.2. SCR Activity Experiments and Measurement
3.3. Catalyst Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Han, L.; Cai, S.; Gao, M.; Hasegawa, J.-Y.; Wang, P.; Zhang, J.; Shi, L.; Zhang, D. Selective Catalytic Reduction of NOx with NH3 by Using Novel Catalysts: State of the Art and Future Prospects. Chem. Rev. 2019, 119, 10916–10976. [Google Scholar] [CrossRef]
- Kwon, D.W.; Park, K.H.; Ha, H.P.; Hong, S.C. The role of molybdenum on the enhanced performance and SO2 resistance of V/Mo-Ti catalysts for NH3-SCR. Appl. Surf. Sci. 2019, 481, 1167–1177. [Google Scholar] [CrossRef]
- Li, J.; Chen, R.; Cen, W.; Yan, P.; Li, K.; Wang, P.; Shu, S.; Chu, Y.; Dong, F. Quantifying the activation energies of ROS-induced NOx conversion: Suppressed toxic intermediates generation and clarified reaction mechanism. Chem. Eng. J. 2019, 375, 122026. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, J.; Chen, J.; Meng, X.; Chen, Y.; Chi, H. Promotive Effect of SO2 on the Activity of a Deactivated Commercial Selective Catalytic Reduction Catalyst: An in Situ DRIFT Study. Ind. Eng. Chem. Res. 2014, 53, 16229–16234. [Google Scholar] [CrossRef]
- Kwon, D.W.; Kim, J.; Ha, H.P. Establishment of surface/bulk-like species functionalization by controlling the sulfation temperature of Sb/V/Ce/Ti for NH3-SCR. Appl. Surf. Sci. 2019, 481, 1503–1514. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, S.; Junhua, L.I.; Zhu, J.; Lingling, M.A. Novel V2O5-CeO2/TiO2 catalyst with low vanadium loading for the selective catalytic reduction of NOx by NH3. Appl. Catal. B 2014, 158–159, 11–19. [Google Scholar] [CrossRef]
- Geng, Y.; Jin, K.; Mei, J.; Su, G.; Ma, L.; Yang, S. CeO2 grafted with different heteropoly acids for selective catalytic reduction of NOx with NH3. J. Hazard. Mater. 2019, 382, 121032–121043. [Google Scholar] [CrossRef]
- Silas, K.; Ghani, W.A.W.A.K.; Choong, T.S.Y.; Rashid, U. Breakthrough studies of Co3O4 supported activated carbon monolith for simultaneous SO2/NOx removal from flue gas. Fuel Process. Technol. 2018, 180, 155–165. [Google Scholar] [CrossRef]
- Lian, Z.; Liu, F.; Hong, H. Enhanced Activity of Ti-Modified V2O5/CeO2 Catalyst for the Selective Catalytic Reduction of NOx with NH3. Ind. Eng. Chem. Res. 2014, 53, 19506–19511. [Google Scholar] [CrossRef]
- Yao, W.; Wang, X.; Liu, Y.; Wu, Z. Ce-O-P material supported CeO2 catalysts: A novel catalyst for selective catalytic reduction of NO with NH3 at low temperature. Appl. Surf. Sci. 2019, 467–468, 439–445. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, L.; Li, Z.; Li, P.; Yuan, F.; Niu, X.; Zhu, Y. Activity and SO2 resistance of amorphous Ce-TiOx catalysts for the selective catalytic reduction of NO with NH3: In Situ DRIFT studies. Catal. Sci. Technol. 2016, 6, 7151–7162. [Google Scholar] [CrossRef]
- Vuong, T.H.; Radnik, J.; Kondratenko, E.; Schneider, M.; Armbruster, U.; Brückner, A. Structure-reactivity relationships in VOx/CexZr1−xO2 catalysts used for low-temperature NH3-SCR of NO. Appl. Catal. B 2016, 197, 159–167. [Google Scholar] [CrossRef]
- Ma, J.; Liu, Z.; Liu, Q.; Guo, S.; Huang, Z.; Xiao, Y. SO2 and NO removal from flue gas over V2O5/AC at lower temperatures—Role of V2O5 on SO2 removal. Fuel Process. Technol. 2008, 89, 242–248. [Google Scholar] [CrossRef]
- Chen, H.; Xia, Y.; Fang, R.; Huang, H.; Gan, Y.; Liang, C.; Zhang, J.; Zhang, W.; Liu, X. The effects of tungsten and hydrothermal aging in promoting NH3-SCR activity on V2O5/WO3-TiO2 catalysts. Appl. Surf. Sci. 2018, 459, 639–646. [Google Scholar] [CrossRef]
- Andreoli, S.; Deorsola, F.A.; Pirone, R. MnOx-CeO2 catalysts synthesized by solution combustion synthesis for the low-temperature NH3-SCR. Catal. Today 2015, 253, 199–206. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Wang, Z.; Liu, F. A skeletal reaction scheme for selective catalytic reduction of NOx with NH3 over CeO2/TiO2 catalyst. Fuel Process. Technol. 2018, 174, 17–25. [Google Scholar] [CrossRef]
- Cheng, H.; Tan, J.; Ren, Y.; Zhao, M.; Liu, J.; Wang, H.; Liu, J.; Zhao, Z. Mechanochemical Synthesis of Highly Porous CeMnOx Catalyst for the Removal of NOx. Ind. Eng. Chem. Res. 2019, 58, 16472–16478. [Google Scholar] [CrossRef]
- Sun, X.; Guo, R.-T.; Liu, S.-W.; Liu, J.; Pan, W.-G.; Shi, X.; Qin, H.; Wang, Z.-Y.; Qiu, Z.-Z.; Liu, X.-Y. The promoted performance of CeO2 catalyst for NH3-SCR reaction by NH3 treatment. Appl. Surf. Sci. 2018, 462, 187–193. [Google Scholar] [CrossRef]
- Yang, S.; Guo, Y.; Chang, H.; Ma, L.; Peng, Y.; Qu, Z.; Yan, N.; Wang, C.; Li, J. Novel effect of SO2 on the SCR reaction over CeO2: Mechanism and significance. Appl. Catal. B 2013, 136, 19–28. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, F.; Jiang, S.; Qiu, K.; Kuang, M.; Whiddon, R.; Cen, K. Ceria substrate–oxide composites as catalyst for highly efficient catalytic oxidation of NO by O2. Fuel 2016, 166, 352–360. [Google Scholar] [CrossRef]
- Jiang, S.-Y.; Zhou, R.-X. Ce doping effect on performance of the Fe/β catalyst for NOx reduction by NH3. Fuel Process. Technol. 2015, 133, 220–226. [Google Scholar] [CrossRef]
- Sohot, M.R.; Jais, U.S.; Sulaiman, M.R. Microstructure of Porous V2O5-CeO2-SiO2 Catalyst for SCR of NO with NH3 at Low Temperature. Adv. Mater. Res. 2014, 875–877, 213–217. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, W.; Wang, L.; Min, S.; Yang, L.; Kai, S.; Xu, H.; Zhou, C. Characterization and activity of V2O5-CeO2/TiO2-ZrO2 catalysts for NH3 selective catalytic reduction of NOx. Chin. J. Catal. 2015, 36, 1701–1710. [Google Scholar] [CrossRef]
- Shen, M.; Xu, L.; Wang, Q. Effect of synthesis methods on activity of V2O5/CeO2/WO3-TiO2 catalyst for selective catalytic reduction of NOx with NH3. J. Rare Earths 2016, 34, 259–267. [Google Scholar] [CrossRef]
- Yang, P.; Fan, S.; Chen, Z.; Bao, G.; Zuo, S.; Qi, C. Synthesis of Nb2O5 based solid superacid materials for catalytic combustion of chlorinated VOCs. Appl. Catal. B 2018, 239, 114–124. [Google Scholar] [CrossRef]
- Chen, H.; Li, H. Denitration performance of supported Mn-Fe/γ-Al2O3 catalyst at low temperature. Chem. Ind. Eng. Prog. 2016, 35, 1107–1112. [Google Scholar]
- Wang, H.; Chen, X.; Gao, S.; Wu, Z. Deactivation mechanism of Ce/TiO2 selective catalytic reduction catalysts by the loading of sodium and calcium salts. Catal. Sci. Technol. 2013, 3, 715–722. [Google Scholar] [CrossRef]
- Yin, M.; Qiu, W.; Song, L.; Zhu, H. Promoting Effect of Organic Ligand on the Performance of Ceria for the Selective Catalytic Reduction of NO by NH3. ChemistrySelect 2018, 3, 2683–2691. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, G.; Dong, F.; Tang, Z. An environmentally friendly wide temperature CeWTiOx catalyst with superior performance for the selective catalytic reduction NOx with NH3. J. Ind. Eng. Chem. 2019, 69, 66–76. [Google Scholar]
- Zhao, L.; Li, C.; Li, S.; Yan, W.; Zhang, J.; Teng, W.; Zeng, G. Simultaneous removal of elemental mercury and NO in simulated flue gas over V2O5/ZrO2-CeO2 catalyst. Appl. Catal. B 2016, 198, 420–430. [Google Scholar] [CrossRef]
- Zhang, G.; Han, W.; Zhao, H.; Zong, L.; Tang, Z. Solvothermal synthesis of well-designed ceria-tin-titanium catalysts with enhanced catalytic performance for wide temperature NH3-SCR reaction. Appl. Catal. B 2018, 226, 117–126. [Google Scholar] [CrossRef]
- Lu, H. Cu-Mn-Ce ternary mixed-oxide catalysts for catalytic combustion of toluene. J. Environ. Sci. 2015, 32, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Chen, L.; Cao, J.; Yang, F.; Tan, W.; Dong, L. Morphology and Crystal-Plane Effects of CeO2 on TiO2/CeO2 Catalysts during NH3-SCR Reaction. Ind. Eng. Chem. Res. 2018, 57, 12407–12419. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Liu, S.; Ren, X.; Ma, J.; Su, W.; Peng, Y. Ultra hydrothermal stability of CeO2-WO3/TiO2 for NH3-SCR of NO compared to traditional V2O5-WO3/TiO2 catalyst. Catal. Today 2015, 258, 11–16. [Google Scholar] [CrossRef]
- Chen, H.; Xia, Y.; Huang, H.; Gan, Y.; Tao, X.; Liang, C.; Luo, J.; Fang, R.; Zhang, J.; Zhang, W.; et al. Highly dispersed surface active species of Mn/Ce/TiW catalysts for high performance at low temperature NH3-SCR. Chem. Eng. J. 2017, 330, 1195–1202. [Google Scholar] [CrossRef]
- Wang, P.; Wang, H.; Chen, X.; Wu, Z. Design Strategies for a Denitrification Catalyst with Improved Resistance against Alkali Poisoning: The Significance of Nanoconfining Spaces and Acid-Base Balance. Chemcatchem 2016, 8, 787–797. [Google Scholar] [CrossRef]
- Hou, Y.; Li, Y.; Li, Q.; Liu, Y.; Huang, Z. Insight into the role of TiO2 modified activated carbon fibers for the enhanced performance in low-temperature NH3-SCR. Fuel 2019, 245, 554–562. [Google Scholar] [CrossRef]
- Gan, L.; Chen, J.; Yue, P.; Jian, Y.; Tran, T.; Li, K.; Dong, W.; Xu, G.; Li, J. NOx removal over V2O5/WO3-TiO2 prepared by a grinding method: Influence of the precursor on vanadium dispersion. Ind. Eng. Chem. Res. 2017, 57, 150–157. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, Q.; Ran, G.; Kong, M.; Ren, S.; Yang, J.; Li, J. V2O5-modified Mn-Ce/AC catalyst with high SO2 tolerance for low-temperature NH3-SCR of NO. Chem. Eng. J. 2019, 370, 810–821. [Google Scholar] [CrossRef]
- Wang, H.; Wang, P.; Chen, X.; Wu, Z. Uniformly active phase loaded selective catalytic reduction catalysts (V2O5/TNTs) with superior alkaline resistance performance. J. Hazard. Mater. 2017, 324, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Yao, X.; Yang, F.; Chen, L.; Fu, M.; Tang, C.; Dong, L. Improving the denitration performance and K-poisoning resistance of the V2O5-WO3/TiO2 catalyst by Ce4+ and Zr4+ co-doping. Chin. J. Catal. 2019, 40, 95–104. [Google Scholar] [CrossRef]
- Peng, Y.; Li, J.; Huang, X.; Li, X.; Hao, J. Deactivation Mechanism of Potassium on the V2O5/CeO2 Catalysts for SCR Reaction: Acidity, Reducibility and Adsorbed-NOx. Environ. Sci. Technol. 2014, 48, 4515–4520. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.E.; Li, C.; Zeng, G.; Yang, Z.; Zhang, X.; Xie, Y.E. The selective catalytic reduction of NO with NH3 over a novel Ce-Sn-Ti mixed oxides catalyst: Promotional effect of SnO2. Appl. Surf. Sci. 2015, 342, 174–182. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, H.; Lin, D. Ceria-based catalysts for low-temperature selective catalytic reduction of NO with NH3. Catal. Sci. Technol. 2016, 6, 1248–1264. [Google Scholar] [CrossRef]
- Jiang, Y.; Bao, C.; Liu, S.; Liang, G.; Lu, M.; Lai, C.; Shi, W.; Ma, S. Enhanced Activity of Nb-modified CeO2/TiO2 Catalyst for the Selective Catalytic Reduction of NO with NH3. Aerosol Air Qual. Res. 2018, 18, 2121–2130. [Google Scholar] [CrossRef]
- Yao, X.; Chen, L.; Cao, J.; Chen, Y.; Tian, M.; Yang, F.; Sun, J.; Tang, C.; Dong, L. Enhancing the deNOx performance of MnOx/CeO2-ZrO2 nanorod catalyst for low-temperature NH3-SCR by TiO2 modification. Chem. Eng. J. 2019, 369, 46–56. [Google Scholar] [CrossRef]
- Yan, X.; Tang, C.; Yao, X.; Lei, Z.; Li, L.; Wang, X.; Yu, D.; Fei, G.; Lin, D. Effect of metal ions doping (M=Ti4+, Sn4+) on the catalytic performance of MnOx /CeO2 catalyst for low temperature selective catalytic reduction of NO with NH3. Appl. Catal. A 2015, 495, 206–216. [Google Scholar]










| Samples | SBET (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| VCT | 53.0 | 0.86 | 21.66 |
| VCA | 143.1 | 0.54 | 16.33 |
| VCZR | 5.5 | 0.03 | 25.39 |
| VCZS | 334.4 | 0.20 | 4.51 |
| Samples | Atomic Ratio (at.%) | ||
|---|---|---|---|
| Oα/(Oα + Oβ + Oγ) | Ce3+/(Ce3+ + Ce4+) | V4+/(V4+ + V5+) | |
| VCT | 36 | 36 | 51 |
| VCA | 37 | 29 | 44 |
| VCZR | 32 | 27 | 36 |
| VCZS | 5 | 26 | 36 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, L.; Wang, X.; Hu, C.; Liu, Y.; Chen, X.; Fang, P.; Chen, D.; Cen, C. Nanosized V-Ce Oxides Supported on TiO2 as a Superior Catalyst for the Selective Catalytic Reduction of NO. Catalysts 2020, 10, 202. https://doi.org/10.3390/catal10020202
Lu L, Wang X, Hu C, Liu Y, Chen X, Fang P, Chen D, Cen C. Nanosized V-Ce Oxides Supported on TiO2 as a Superior Catalyst for the Selective Catalytic Reduction of NO. Catalysts. 2020; 10(2):202. https://doi.org/10.3390/catal10020202
Chicago/Turabian StyleLu, Long, Xueman Wang, Chunhua Hu, Ying Liu, Xiongbo Chen, Ping Fang, Dingsheng Chen, and Chaoping Cen. 2020. "Nanosized V-Ce Oxides Supported on TiO2 as a Superior Catalyst for the Selective Catalytic Reduction of NO" Catalysts 10, no. 2: 202. https://doi.org/10.3390/catal10020202
APA StyleLu, L., Wang, X., Hu, C., Liu, Y., Chen, X., Fang, P., Chen, D., & Cen, C. (2020). Nanosized V-Ce Oxides Supported on TiO2 as a Superior Catalyst for the Selective Catalytic Reduction of NO. Catalysts, 10(2), 202. https://doi.org/10.3390/catal10020202