Kinetic and Mechanistic Study of Rhodamine B Degradation by H2O2 and Cu/Al2O3/g-C3N4 Composite
Abstract
1. Introduction
2. Results and Discussion
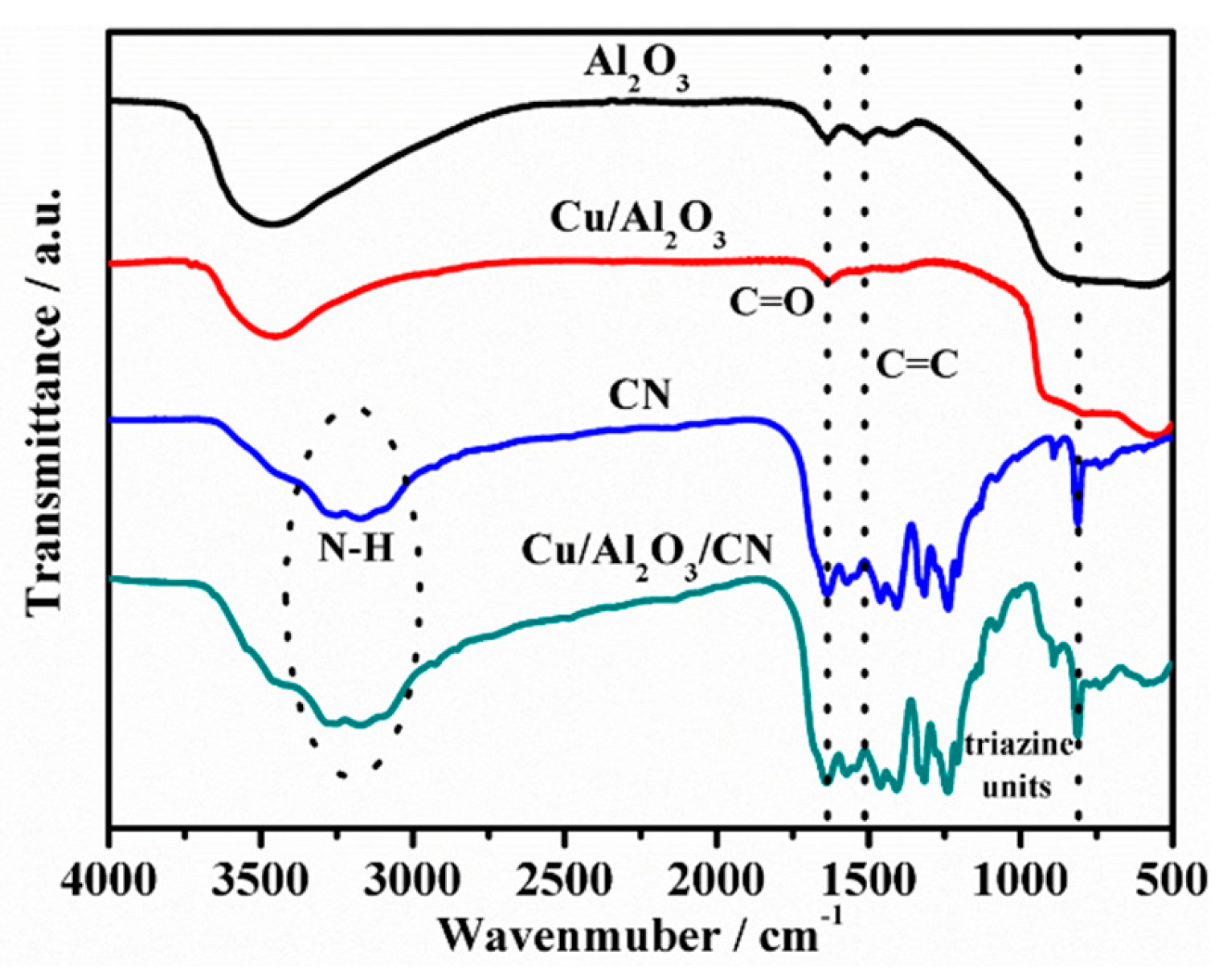
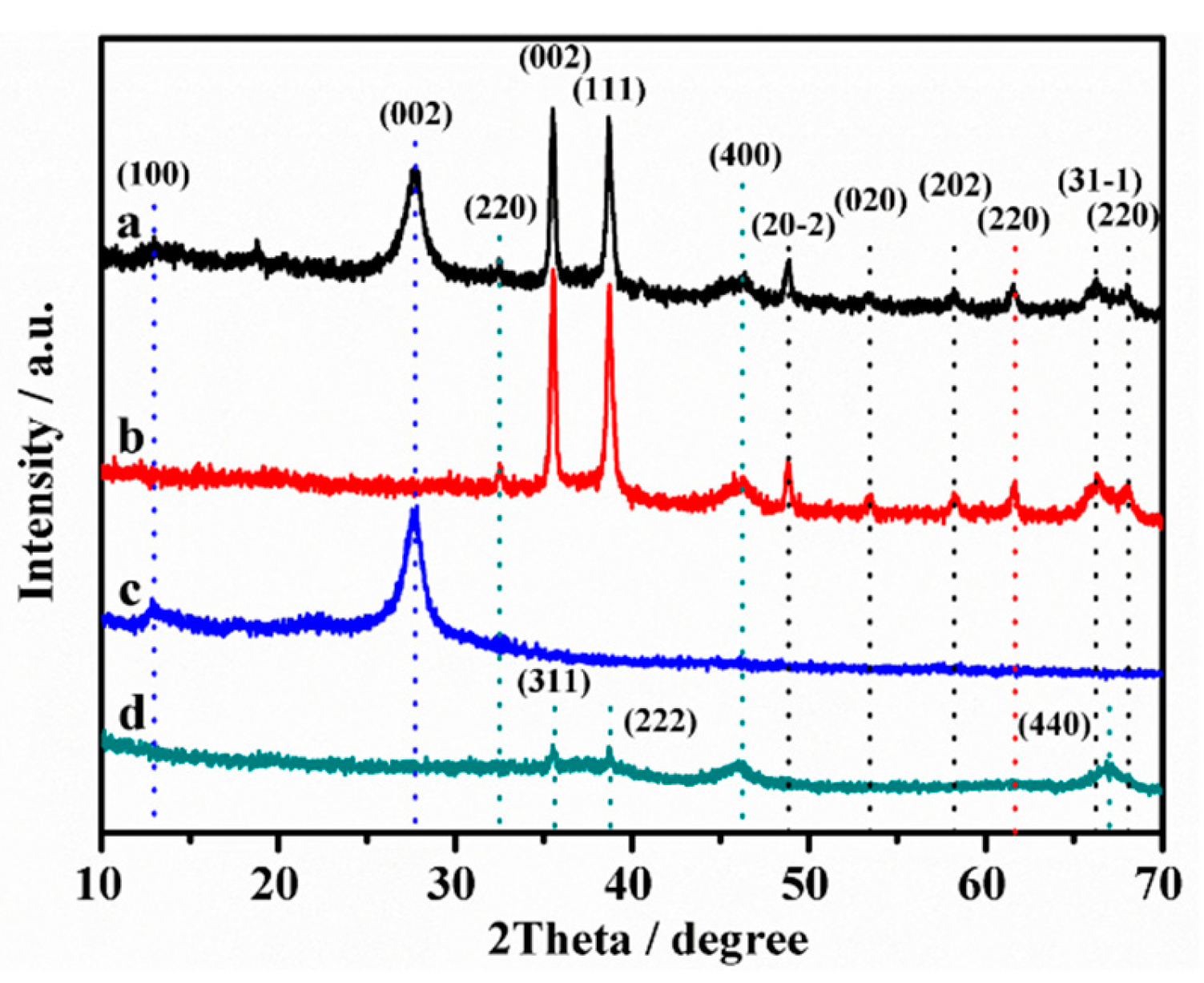
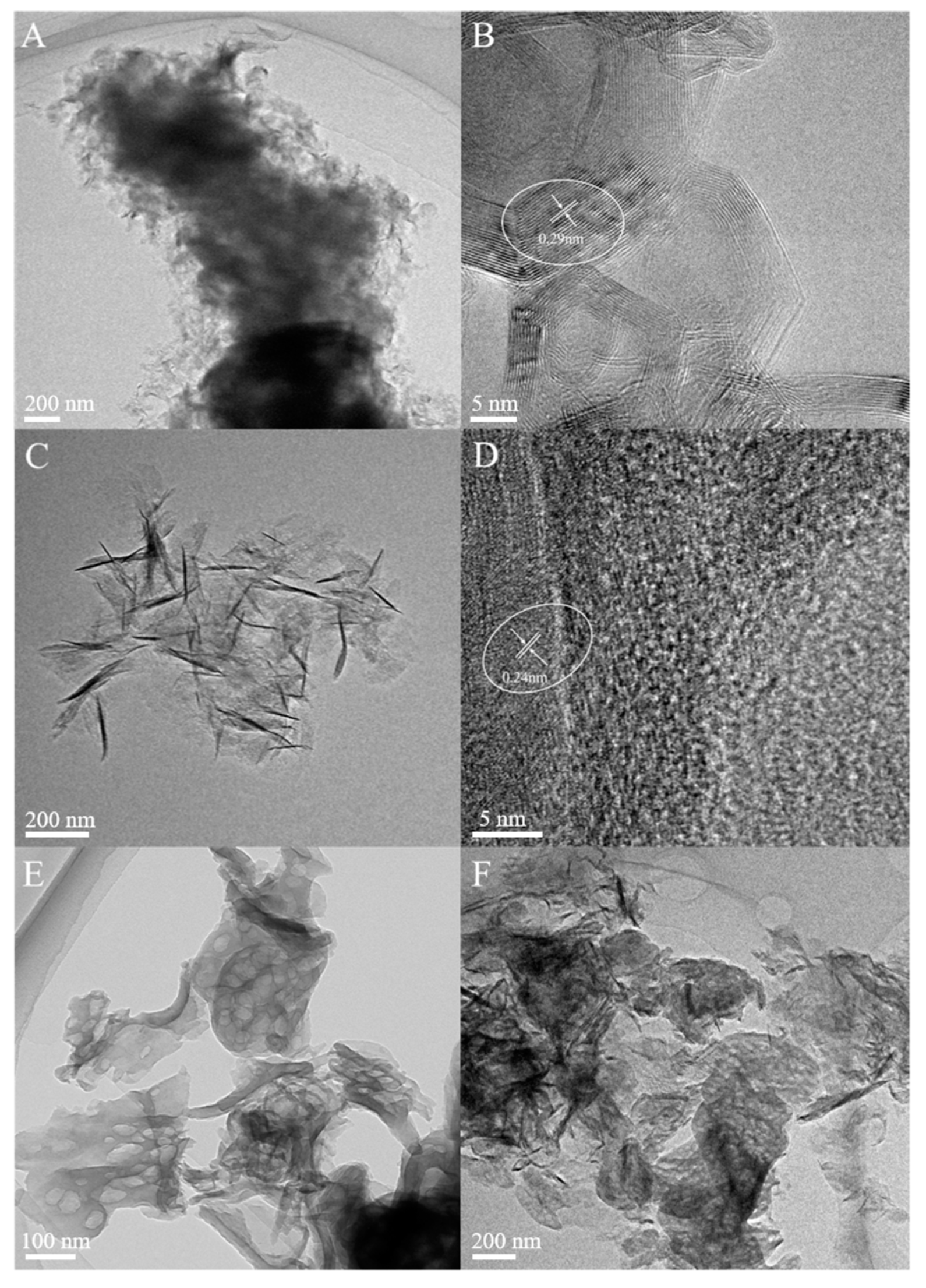
2.1. Structural Characterization of Composites
2.2. Catalytic Performance of Composites
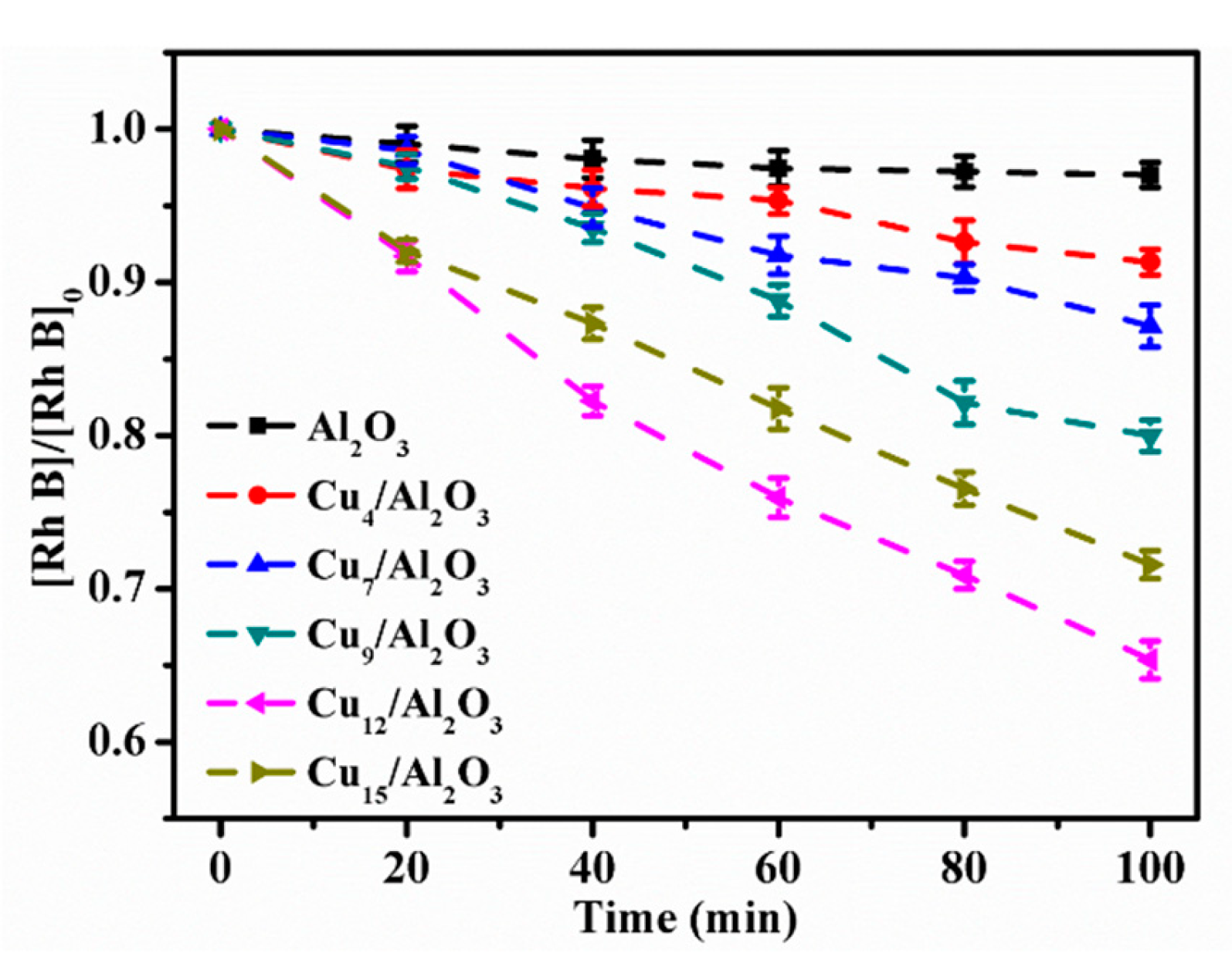
2.2.1. Effect of Cu Content
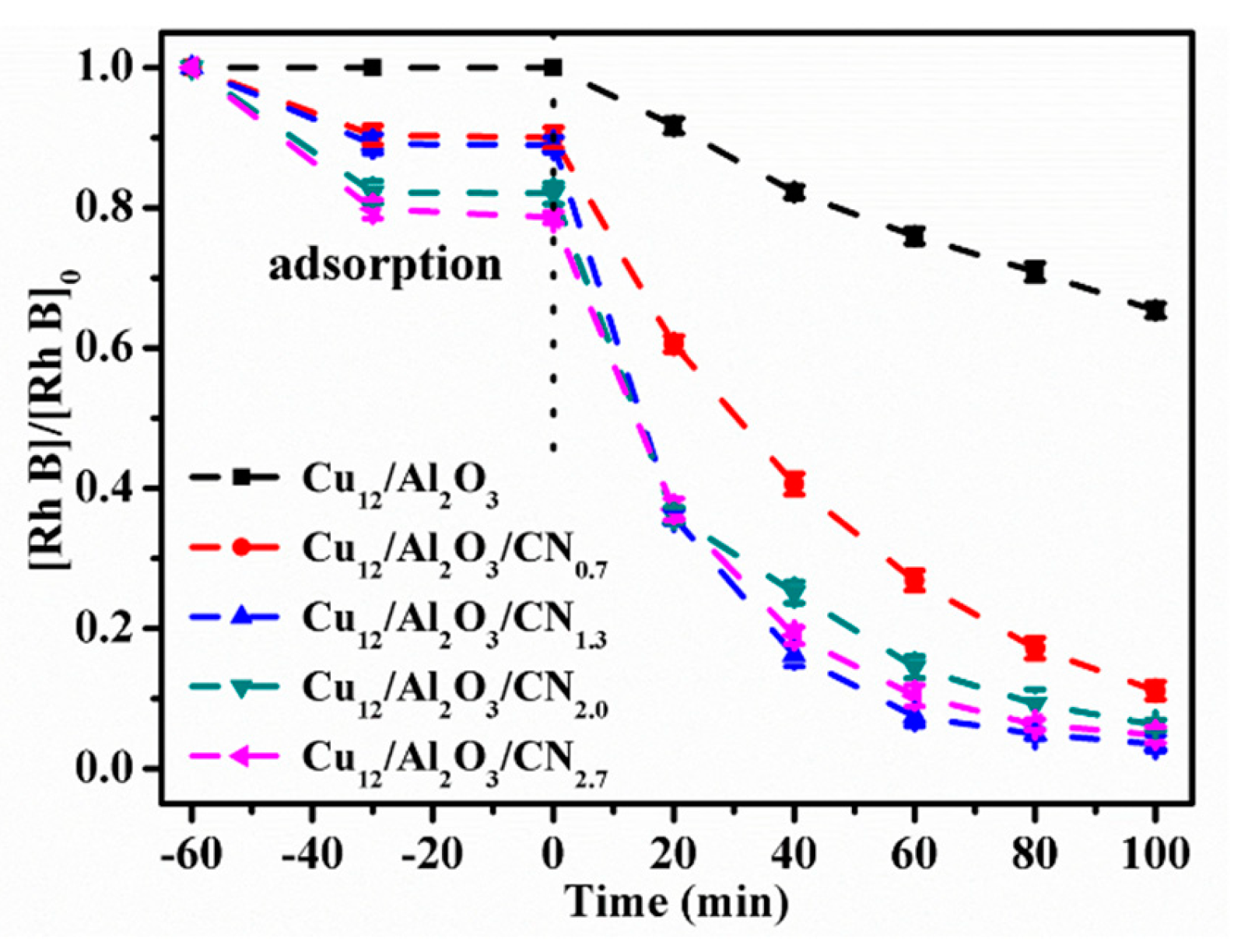
2.2.2. Effect of CN Content
2.2.3. Synergistic Effect
2.2.4. Effect of pH
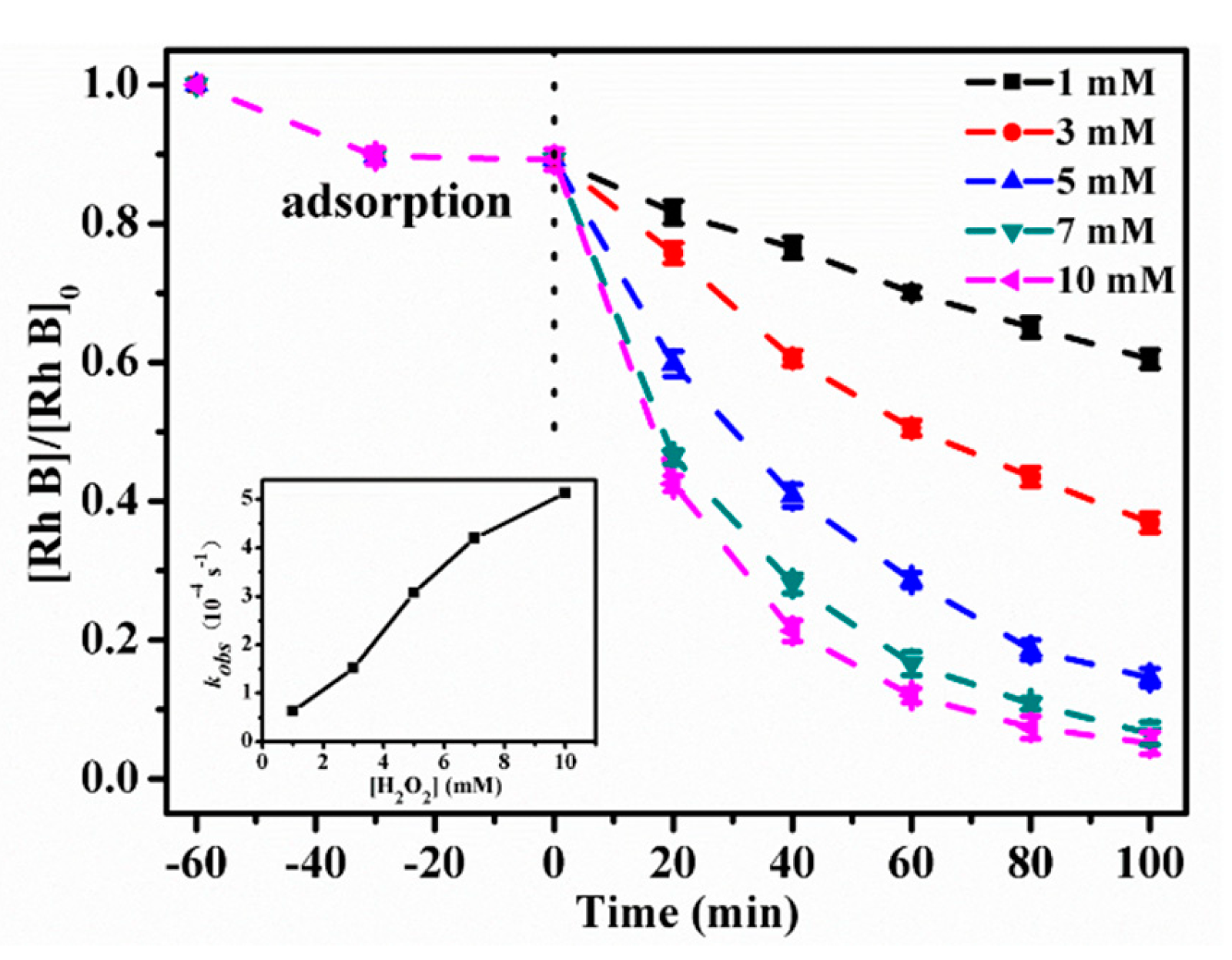
2.2.5. Effect of H2O2 Concentration
2.2.6. The Effect of the Catalyst Dose
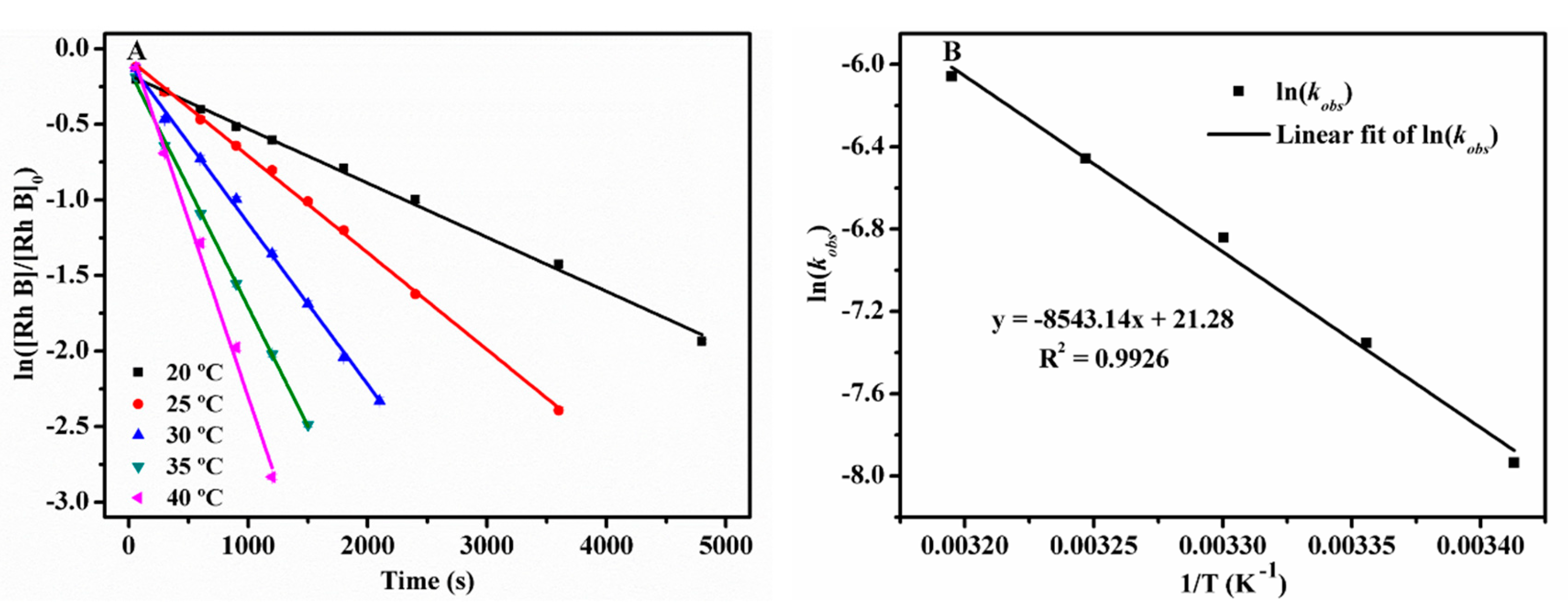
2.2.7. Activation Energy
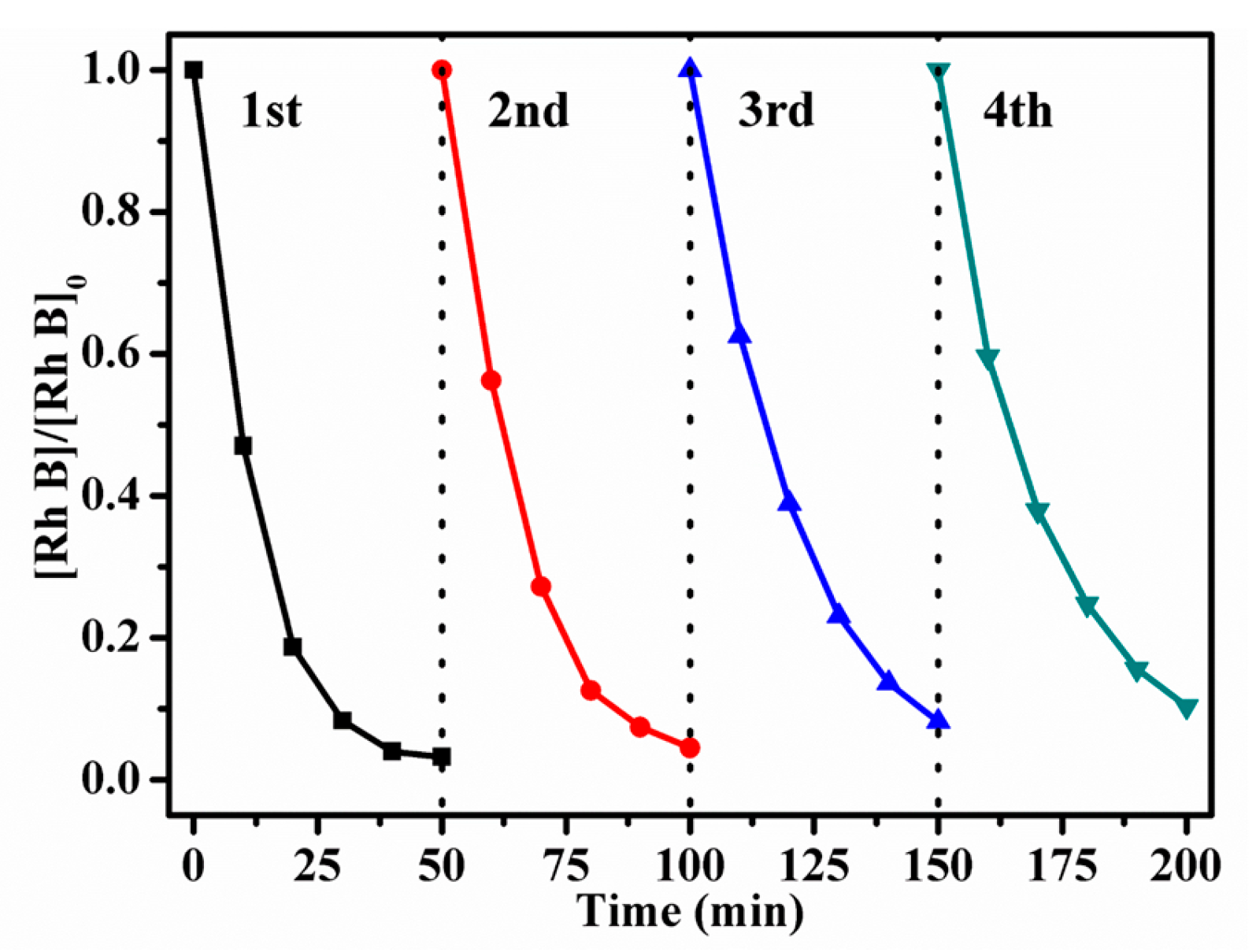
2.2.8. Recycling Experiments
2.3. Catalytic Mechanism
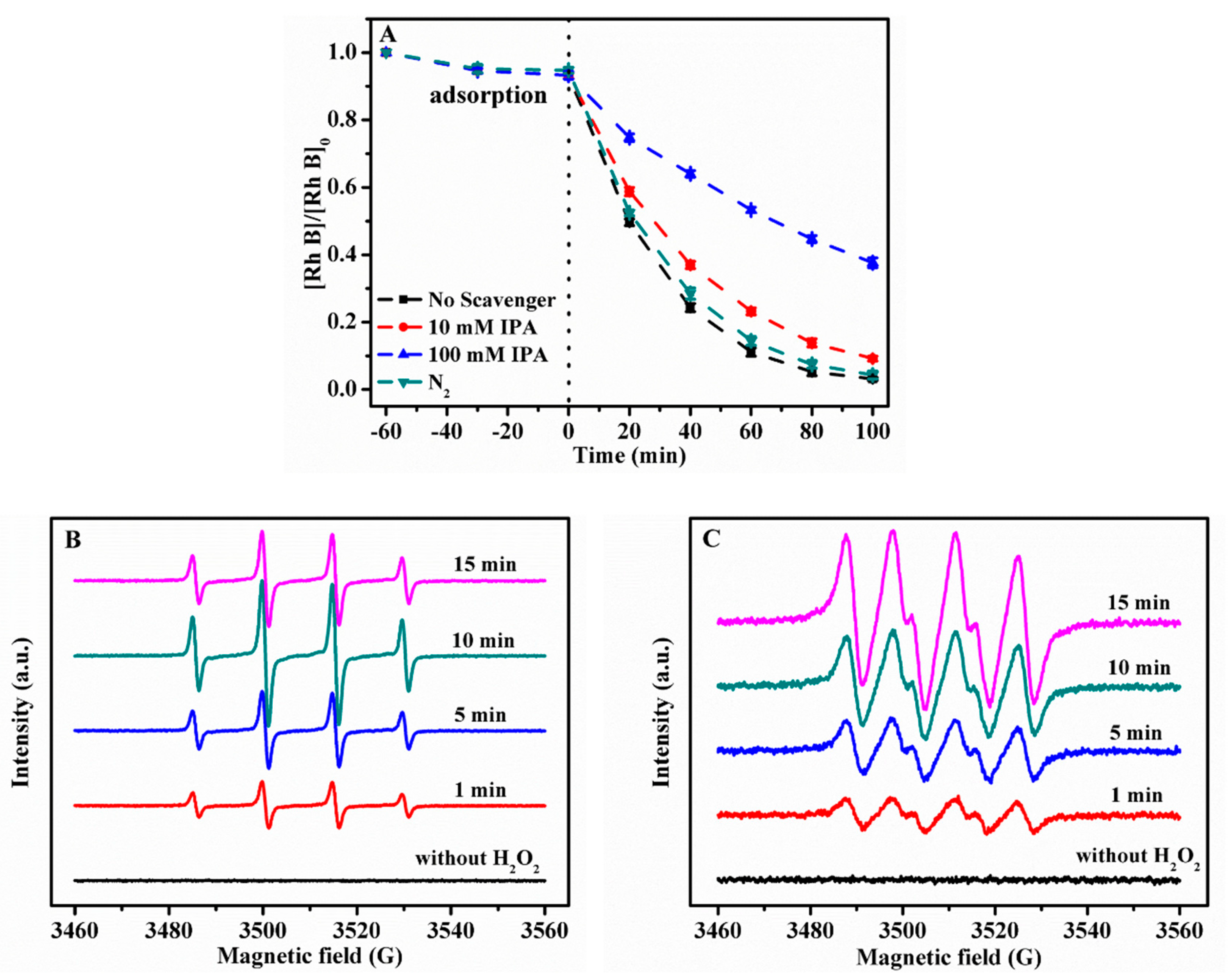
2.3.1. Scavenging Experiments
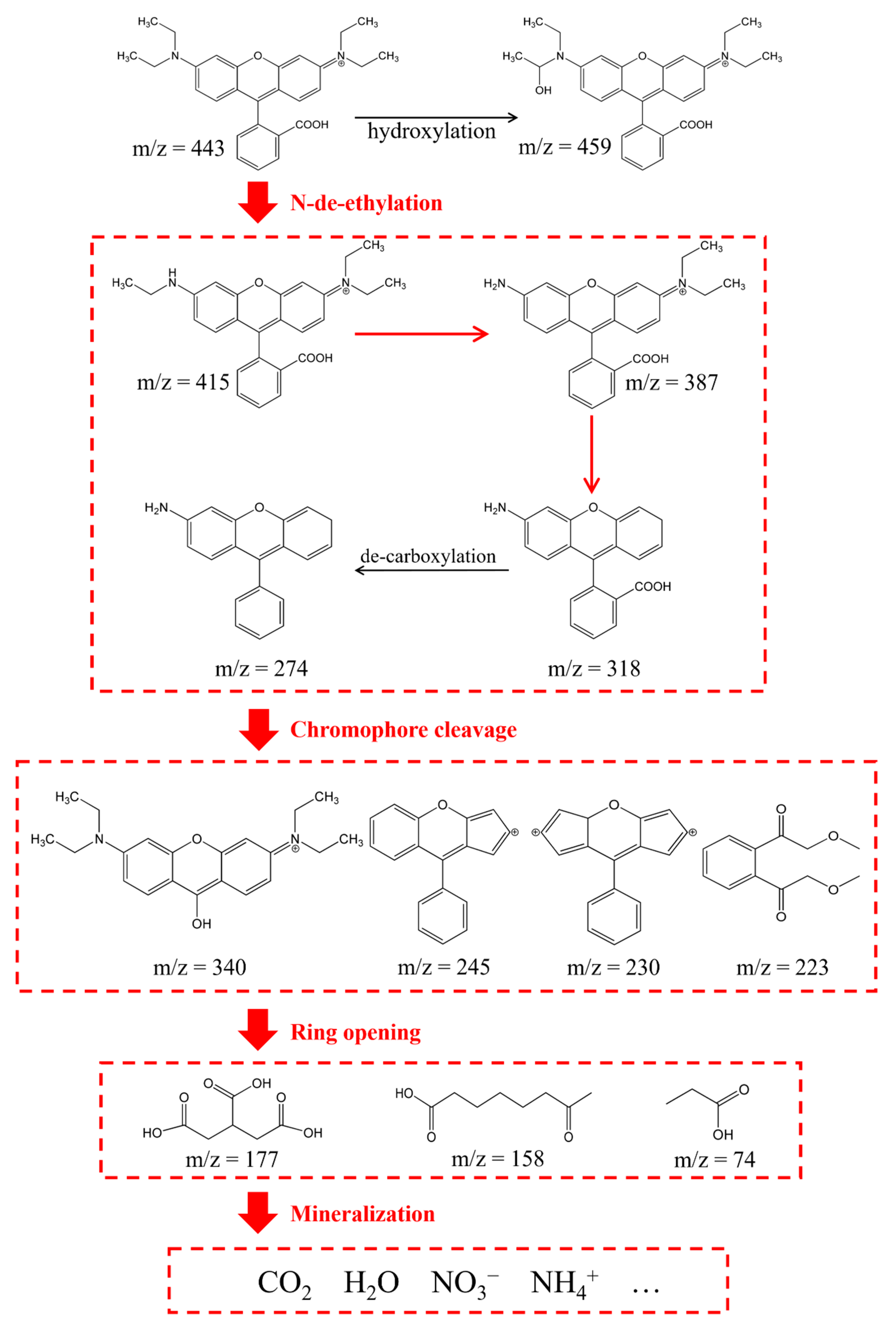
2.3.2. HPLC-MS Analysis
3. Experimental
3.1. Materials
3.2. Preparation of Composites
3.2.1. Preparation of Cu/Al2O3
3.2.2. Preparation of g-C3N4
3.2.3. Preparation of Cu/Al2O3/CN
3.3. Characterization of Prepared Composites
3.4. Catalytic Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, J.L.; Wang, S.Z. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, K.G.; Kumar, P.S.; Jaikumar, V.; Rajan, P.S. Removal of colorants from wastewater: A review on sources and treatment strategies. J. Ind. Eng. Chem. 2019, 75, 1–19. [Google Scholar] [CrossRef]
- Li, H.P.; Liu, J.Y.; Hou, W.G.; Du, N.; Zhang, R.J.; Tao, X.T. Synthesis and characterization of g-C3N4/Bi2MoO6 heterojunctions with enhanced visible light photocatalytic activity. Appl. Catal. B 2014, 160, 89–97. [Google Scholar] [CrossRef]
- Adityosulindro, S.; Barthe, L.; González-Labrada, K.; Jáuregui Haza, U.J.; Delmas, H.; Julcour, C. Sonolysis and sono-Fenton oxidation for removal of ibuprofen in (waste)water. Ultrason. Sonochem. 2017, 39, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.P.; Xiong, X.F.; Gong, W.Q.; Feng, D.X.; Xian, M.; Ge, Z.X.; Xu, N.A. Removal of rhodamine B by ozone-based advanced oxidation process. Desalination 2011, 278, 84–90. [Google Scholar]
- Masomboon, N.; Ratanatamskul, C.; Lu, M.C. Kinetics of 2,6-dimethylaniline oxidation by various Fenton processes. J. Hazard. Mater. 2011, 192, 347–353. [Google Scholar] [CrossRef]
- Patil, S.P.; Bethi, B.; Sonawane, G.H.; Shrivastava, V.S.; Sonawane, S. Efficient adsorption and photocatalytic degradation of Rhodamine B dye over Bi2O3-bentonite nanocomposites: A kinetic study. J. Ind. Eng. Chem. 2016, 34, 356–363. [Google Scholar] [CrossRef]
- Madhavan, J.; Grieser, F.; Ashokkumar, M. Combined advanced oxidation processes for the synergistic degradation of ibuprofen in aqueous environments. J. Hazard. Mater. 2010, 178, 202–208. [Google Scholar] [CrossRef]
- Rao, Y.F.; Xue, D.; Pan, H.M.; Feng, J.T.; Li, Y.J. Degradation of ibuprofen by a synergistic UV/Fe(III)/Oxone process. Chem. Eng. J. 2016, 283, 65–75. [Google Scholar] [CrossRef]
- Al-Hamadani, Y.A.J.; Park, C.M.; Assi, L.N.; Chu, K.H.; Hoque, S.; Jang, M.; Yoon, Y.; Ziehl, P. Sonocatalytic removal of ibuprofen and sulfamethoxazole in the presence of different fly ash sources. Ultrason. Sonochem. 2017, 39, 354–362. [Google Scholar] [CrossRef]
- Cheng, M.; Zeng, G.M.; Huang, D.L.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: A review. Chem. Eng. J. 2016, 284, 582–598. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Saleh, R.; Taufik, A. Degradation of methylene blue and congo-red dyes using Fenton, photo-Fenton, sono-Fenton, and sonophoto-Fenton methods in the presence of iron (II,III) oxide/zinc oxide/graphene (Fe3O4/ZnO/graphene) composites. Sep. Purif. Technol. 2019, 210, 563–573. [Google Scholar] [CrossRef]
- Bello, M.M.; Raman, A.A.A.; Asghar, A. A review on approaches for addressing the limitations of Fenton oxidation for recalcitrant wastewater treatment. Process Saf. Environ. Prot. 2019, 126, 119–140. [Google Scholar] [CrossRef]
- Wang, P.; Wang, N.N.; Zheng, T.; Zhang, G.S. A review on Fenton-like processes for organic wastewater treatment. J. Environ. Chem. Eng. 2016, 4, 762–787. [Google Scholar] [CrossRef]
- de Luna, M.D.G.; Colades, J.I.; Su, C.C.; Lu, M.C. Comparison of dimethyl sulfoxide degradation by different Fenton processes. Chem. Eng. J. 2013, 232, 418–424. [Google Scholar] [CrossRef]
- Karpinska, A.; Sokol, A.; Karpinska, J. Studies on the kinetics of carbamazepine degradation in aqueous matrix in the course of modified Fenton’s reactions. J. Pharm. Biomed. Anal. 2015, 106, 46–51. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Kurosu, S.; Suzuki, M.; Kawase, Y. Hydroxyl radical generation by zero-valent iron/Cu (ZVI/Cu) bimetallic catalyst in wastewater treatment: Heterogeneous Fenton/Fenton-like reactions by Fenton reagents formed in-situ under oxic conditions. Chem. Eng. J. 2018, 334, 1537–1549. [Google Scholar] [CrossRef]
- Xia, D.H.; Xu, W.J.; Hu, L.L.; He, C.; Leung, D.Y.C.; Wang, W.J.; Wong, P.K. Synergistically catalytic oxidation of toluene over Mn modified g-C3N4/ZSM-4 under vacuum UV irradiation. J. Hazard. Mater. 2018, 349, 91–100. [Google Scholar] [CrossRef]
- Su, Y.H.; Chen, P.; Wang, F.L.; Zhang, Q.X.; Chen, T.S.; Wang, Y.F.; Yao, K.; Lv, W.Y.; Liu, G.G. Decoration of TiO2/g-C3N4 Z-scheme by carbon dots as a novel photocatalyst with improved visible-light photocatalytic performance for the degradation of enrofloxacin. RSC Adv. 2017, 7, 34096–34103. [Google Scholar] [CrossRef]
- Nieto-Juarez, J.I.; Pierzchla, K.; Sienkiewicz, A.; Kohn, T. Inactivation of MS2 coliphage in Fenton and Fenton-like systems: Role of transition metals, hydrogen peroxide and sunlight. Environ. Sci. Technol. 2010, 44, 3351–3356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Xu, D.; Hu, C.; Shi, Y.L. Framework Cu-doped AlPO4 as an effective Fenton-like catalyst for bisphenol A degradation. Appl. Catal. B 2017, 207, 9–16. [Google Scholar] [CrossRef]
- Li, Z.F.; Soroka, I.L.; Min, F.Y.; Jonsson, M. pH-Control as a way to fine-tune the Cu/Cu2O ratio in radiation induced synthesis of Cu2O particles. Dalton Trans. 2018, 47, 16139–16144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, B.D.; Zhang, G.Y.; Gan, Y.H.; Zhang, S.J. Enhanced decomplexation of Cu(II)-EDTA: The role of acetylacetone in Cu-mediated photo-Fenton reactions. Chem. Eng. J. 2019, 358, 1218–1226. [Google Scholar] [CrossRef]
- Xu, J.H.; Wang, W.Z.; Gao, E.P.; Ren, J.; Wang, L. Bi2WO6/Cu-0: A novel coupled system with enhanced photocatalytic activity by Fenton-like synergistic effect. Catal. Commun. 2011, 12, 834–838. [Google Scholar] [CrossRef]
- Mao, J.; Quan, X.; Wang, J.; Gao, C.; Chen, S.; Yu, H.T.; Zhang, Y.B. Enhanced heterogeneous Fenton-like activity by Cu-doped BiFeO3 perovskite for degradation of organic pollutants. Front. Environ. Sci. Eng. Chin. 2018, 12, 1–10. [Google Scholar] [CrossRef]
- Zhang, L.L.; Nie, Y.L.; Hu, C.; Qu, J.H. Enhanced Fenton degradation of Rhodamine B over nanoscaled Cu-doped LaTiO3 perovskite. Appl. Catal. B 2012, 125, 418–424. [Google Scholar] [CrossRef]
- Xiao, P.; Li, H.L.; Wang, T.; Xu, X.L.; Li, J.L.; Zhu, J.J. Efficient Fenton-like La-Cu-O/SBA-15 catalyst for the degradation of organic dyes under ambient conditions. RSC Adv. 2014, 4, 12601–12604. [Google Scholar] [CrossRef]
- Bonfim, D.P.F.; Santana, C.S.; Batista, M.S.; Fabiano, D.P. Catalytic Evaluation of CuO/[Si]MCM-41 in Fenton-like Reactions. Chem. Eng. Technol. 2019, 42, 882–888. [Google Scholar] [CrossRef]
- Zuo, S.Y.; Xu, H.M.; Liao, W.; Yuan, X.J.; Sun, L.; Li, Q.; Zan, J.; Li, D.Y.; Xia, D.S. Molten-salt synthesis of g-C3N4-Cu2O heterojunctions with highly enhanced photocatalytic performance. Colloids Surf. A 2018, 546, 307–315. [Google Scholar] [CrossRef]
- Peng, B.Y.; Zhang, S.S.; Yang, S.Y.; Wang, H.J.; Yu, H.; Zhang, S.Q.; Peng, F. Synthesis and characterization of g-C3N4/Cu2O composite catalyst with enhanced photocatalytic activity under visible light irradiation. Mater. Res. Bull. 2014, 56, 19–24. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, Y.R.; Chen, Z.H.; Zhang, Z.G.; Fang, X.M. Constructing a novel ternary Fe(III)/graphene/g-C3N4 composite photocatalyst with enhanced visible-light driven photocatalytic activity via interfacial charge transfer effect. Appl. Catal. B 2016, 183, 231–241. [Google Scholar] [CrossRef]
- Pomilla, F.R.; Cortes, M.A.L.R.M.; Hamilton, J.W.J.; Molinari, R.; Barbieri, G.; Marci, G.; Palmisano, L.; Sharma, P.K.; Brown, A.; Byrne, J.A. An Investigation into the Stability of Graphitic C3N4 as a Photocatalyst for CO2 Reduction. J. Phys. Chem. C 2018, 122, 28727–28738. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Liu, N.Y.; Han, Y.Z.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.T.; Zhong, J.; Kang, Z.H. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.D.; Shen, Q.M.; Zhou, C.S.; Fang, L.J.; Yang, M.; Xia, T. Kinetic and Mechanistic Study on Catalytic Decomposition of Hydrogen Peroxide on Carbon-Nanodots/Graphitic Carbon Nitride Composite. Catalysts 2018, 8, 445. [Google Scholar] [CrossRef]
- Fang, L.J.; Liu, Z.D.; Zhou, C.S.; Guo, Y.L.; Feng, Y.P.; Yang, M. Degradation Mechanism of Methylene Blue by H2O2 and Synthesized Carbon Nanodots/Graphitic Carbon Nitride/Fe(II) Composite. J. Phys. Chem. C 2019, 123, 26921–26931. [Google Scholar] [CrossRef]
- Lyu, L.; Zhang, L.L.; He, G.Z.; He, H.; Hu, C. Selective H2O2 conversion to hydroxyl radicals in the electron-rich area of hydroxylated C-g-C3N4/ CuCo-Al2O3. J. Mater. Chem. A 2017, 5, 7153–7164. [Google Scholar] [CrossRef]
- Zhu, J.J.; Xiao, P.; Li, H.L.; Carabineiro, S.A.C. Graphitic Carbon Nitride: Synthesis, Properties, and Applications in Catalysis. ACS Appl. Mater. Interfaces 2014, 6, 16449–16465. [Google Scholar] [CrossRef]
- Jia, J.K.; Huang, W.X.; Feng, C.S.; Zhang, Z.; Zuojiao, K.C.; Liu, J.X.; Jiang, C.Y.; Wang, Y.P. Fabrication of g-C3N4/Ag3PO4-H2O2 heterojunction system with enhanced visible-light photocatalytic activity and mechanism insight. J. Alloys Compd. 2019, 790, 616–625. [Google Scholar] [CrossRef]
- Xu, S.Q.; Zhu, H.X.; Cao, W.R.; Wen, Z.B.; Wang, J.N.; Francois-Xavier, C.P.; Wintgens, T. Cu-Al2O3-g-C3N4 and Cu-Al2O3-C-dots with dual-reaction centres for simultaneous enhancement of Fenton-like catalytic activity and selective H2O2 conversion to hydroxyl radicals. Appl. Catal. B 2018, 234, 223–233. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, L.L.; Liu, L.F. Fenton-like Catalytic Removal of Organic Pollutants in Water by Framework Cu in Cu-Al2O3. Huanjing Kexue 2017, 38, 1054–1060. [Google Scholar]
- Ma, X.Z.; Zhang, J.T.; Wang, B.; Li, Q.G.; Chu, S. Hierarchical Cu2O foam/g-C3N4 photocathode for photoelectrochemical hydrogen production. Appl. Surf. Sci. 2018, 427, 907–916. [Google Scholar] [CrossRef]
- Dikdim, J.M.D.; Gong, Y.; Noumi, G.B.; Sieliechi, J.M.; Zhao, X.; Ma, N.; Yang, M.; Tchatchueng, J.B. Peroxymonosulfate improved photocatalytic degradation of atrazine by activated carbon/graphitic carbon nitride composite under visible light irradiation. Chemosphere 2019, 217, 833–842. [Google Scholar] [CrossRef]
- Engelhart, W.; Dreher, W.; Eibl, O.; Schier, V. Deposition of alumina thin film by dual magnetron sputtering: Is it gamma-Al2O3? Acta Mater. 2011, 59, 7757–7767. [Google Scholar] [CrossRef]
- Sareen, S.; Mutreja, V.; Singh, S.; Pal, B. Highly dispersed Au, Ag and Cu nanoparticles in mesoporous SBA-15 for highly selective catalytic reduction of nitroaromatics. RSC Adv. 2015, 5, 184–190. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Sun, J.Y.; Wang, Y.B.; Zhao, X. Electrospun flexible self-standing Cu-Al2O3 fibrous membranes as Fenton catalysts for bisphenol A degradation. J. Mater. Chem. A 2017, 5, 19151–19158. [Google Scholar] [CrossRef]
- Lyu, L.; Zhang, L.L.; Hu, C. Enhanced Fenton-like degradation of pharmaceuticals over framework copper species in copper-doped mesoporous silica microspheres. Chem. Eng. J. 2015, 274, 298–306. [Google Scholar] [CrossRef]
- Sheng, Y.Y.; Sun, Y.; Xu, J.; Zhang, J.; Han, Y.F. Fenton-like degradation of rhodamine B over highly durable Cu-embedded alumina: Kinetics and mechanism. AlChE J. 2018, 64, 538–549. [Google Scholar] [CrossRef]
- Merouani, S.; Hamdaoui, O.; Saoudi, F.; Chiha, M. Sonochemical degradation of Rhodamine B in aqueous phase: Effects of additives. Chem. Eng. J. 2010, 158, 550–557. [Google Scholar] [CrossRef]
- Nieto, L.M.; Hodaifa, G.; Rodriguez, S.; Gimenez, J.A.; Ochando, J. Degradation of organic matter in olive-oil mill wastewater through homogeneous Fenton-like reaction. Chem. Eng. J. 2011, 173, 503–510. [Google Scholar] [CrossRef]
- Hodaifa, G.; Ochando-Pulido, J.M.; Rodriguez-Vives, S.; Martinez-Ferez, A. Optimization of continuous reactor at pilot scale for olive-oil mill wastewater treatment by Fenton-like process. Chem. Eng. J. 2013, 220, 117–124. [Google Scholar] [CrossRef]
- Ma, Q.B.; Hofmann, J.P.; Litke, A.; Hensen, E.J.M. Cu2O photoelectrodes for solar water splitting: Tuning photoelectrochemical performance by controlled faceting. Sol. Energy Mater. Sol. Cells 2015, 141, 178–186. [Google Scholar] [CrossRef]
- Aylmore, M.G.; Muir, D.M. Thermodynamic analysis of gold leaching by ammoniacal thiosulfate using Eh/pH and speciation diagrams. Miner. Metall. Process 2001, 18, 221–227. [Google Scholar] [CrossRef]
- Lodha, S.; Jain, A.; Punjabi, P.B. A novel route for waste water treatment: Photocatalytic degradation of rhodamine B. Arab. J. Chem. 2011, 4, 383–387. [Google Scholar] [CrossRef]
- Matheson, L.J.; Tratnyek, P.G. Reductive Dehalogenation of Chlorinated Methanes by Iron Metal. Environ. Sci. Technol. 2002, 28, 2045–2053. [Google Scholar] [CrossRef]
- Bjorkbacka, A.; Yang, M.; Gasparrini, C.; Leygraf, C.; Jonsson, M. Kinetics and mechanisms of reactions between H2O2 and copper and copper oxides. Dalton Trans. 2015, 44, 16045–16051. [Google Scholar] [CrossRef]
- Lousada, C.M.; Jonsson, M. Kinetics, Mechanism, and Activation Energy of H2O2 Decomposition on the Surface of ZrO2. J. Phys. Chem. C 2010, 114, 11202–11208. [Google Scholar] [CrossRef]
- Lousada, C.M.; Yang, M.; Nilsson, K.; Jonsson, M. Catalytic decomposition of hydrogen peroxide on transition metal and lanthanide oxides. J. Mol. Catal. A Chem. 2013, 379, 178–184. [Google Scholar] [CrossRef]
- Kanel, S.R.; Greneche, J.M.; Choi, H. Arsenic(V) removal kom groundwater using nano scale zero-valent iron as a colloidal reactive barrier material. Environ. Sci. Technol. 2006, 40, 2045–2050. [Google Scholar] [CrossRef]
- Cope, D.B.; Benson, C.H. Grey-Iron Foundry Slags As Reactive Media for Removing Trichloroethylene from Groundwater. Environ. Sci. Technol. 2009, 43, 169–175. [Google Scholar] [CrossRef]
- Agrawal, A.; Tratnyek, P.G. Reduction of Nitro Aromatic Compounds by Zero-Valent Iron Metal. Environ. Sci. Technol. 1995, 30, 153–160. [Google Scholar] [CrossRef]
- Sun, Y.K.; Li, J.X.; Huang, T.L.; Guan, X.H. The influences of iron characteristics, operating conditions and solution chemistry on contaminants removal by zero-valent iron: A review. Water Res. 2016, 100, 277–295. [Google Scholar] [CrossRef]
- Li, H.; Liao, J.Y.; Zeng, T. A facile synthesis of CuO nanowires and nanorods, and their catalytic activity in the oxidative degradation of Rhodamine B with hydrogen peroxide. Catal. Commun. 2014, 46, 169–173. [Google Scholar] [CrossRef]
- Zaman, S.; Zainelabdin, A.; Amin, G.; Nur, O.; Willander, M. Efficient catalytic effect of CuO nanostructures on the degradation of organic dyes. J. Phys. Chem. Solids 2012, 73, 1320–1325. [Google Scholar] [CrossRef]
- Dragoi, M.; Samide, A.; Moanta, A. Discoloration of Waters Containing Azo Dye Congo Red by Fenton Oxidation Process Estimation of activation parameters. Rev. Chim. 2011, 62, 1195–1198. [Google Scholar]
- Martin, R.; Navalon, S.; Delgado, J.J.; Calvino, J.J.; Alvaro, M.; Garcia, H. Influence of the Preparation Procedure on the Catalytic Activity of Gold Supported on Diamond Nanoparticles for Phenol Peroxidation. Chem. Eur. J. 2011, 17, 9494–9502. [Google Scholar] [CrossRef]
- Yao, Z.P.; Chen, C.J.; Wang, J.K.; Xia, Q.X.; Li, C.X.; Jiang, Z.H. Iron Oxide Coating Fenton-like Catalysts: Preparation and Degradation of Phenol. Chin. J. Inorg. Chem. 2017, 33, 1797–1804. [Google Scholar]
- Yang, M.; Zhang, X.; Grosjean, A.; Soroka, I.L.; Jonsson, M. Kinetics and Mechanism of the Reaction between H2O2 and Tungsten Powder in Water. J. Phys. Chem. C 2015, 119, 22560–22569. [Google Scholar] [CrossRef]
- Ma, J.Q.; Yang, Q.F.; Wen, Y.Z.; Liu, W.P. Fe-g-C3N4/graphitized mesoporous carbon composite as an effective Fenton-like catalyst in a wide pH range. Appl. Catal. B 2017, 201, 232–240. [Google Scholar] [CrossRef]
- Zhu, J.N.; Zhu, X.Q.; Cheng, F.F.; Li, P.; Wang, F.; Xiao, Y.W.; Xiong, W.W. Preparing copper doped carbon nitride from melamine templated crystalline copper chloride for Fenton-like catalysis. Appl. Catal. B 2019, 256, 117830. [Google Scholar] [CrossRef]
- Ma, J.Q.; Jia, N.Z.F.; Shen, C.S.; Liu, W.P.; Wen, Y.Z. Stable cuprous active sites in Cu+-graphitic carbon nitride: Structure analysis and performance in Fenton-like reactions. J. Hazard. Mater. 2019, 378, 120782. [Google Scholar] [CrossRef]
- Fan, J.W.; Jiang, X.; Min, H.Y.; Li, D.D.; Ran, X.Q.; Zou, L.Y.; Sun, Y.; Li, W.; Yang, J.P.; Teng, W.; et al. Facile preparation of Cu-Mn/CeO2/SBA-15 catalysts using ceria as an auxiliary for advanced oxidation processes. J. Mater. Chem. A 2014, 2, 10654–10661. [Google Scholar] [CrossRef]
- Sun, Y.; Tian, P.F.; Ding, D.D.; Yang, Z.X.; Wang, W.Z.; Xin, H.; Xu, J.; Han, Y.F. Revealing the active species of Cu-based catalysts for heterogeneous Fenton reaction. Appl. Catal. B 2019, 258, 117985. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, L.X.; Geng, F.L.; Guo, L.H.; Wan, B.; Yang, Y. Carbon dots decorated graphitic carbon nitride as an efficient metal-free photocatalyst for phenol degradation. Appl. Catal. B 2016, 180, 656–662. [Google Scholar] [CrossRef]
- Yu, D.Y.; Li, L.B.; Wu, M.; Crittenden, J.C. Enhanced photocatalytic ozonation of organic pollutants using an iron-based metal-organic framework. Appl. Catal. B 2019, 251, 66–75. [Google Scholar] [CrossRef]
- He, Z.; Sun, C.; Yang, S.G.; Ding, Y.C.; He, H.; Wang, Z.L. Photocatalytic degradation of rhodamine B by Bi2WO6 with electron accepting agent under microwave irradiation: Mechanism and pathway. J. Hazard. Mater. 2009, 162, 1477–1486. [Google Scholar] [CrossRef]
- Yu, K.; Yang, S.G.; He, H.; Sun, C.; Gu, C.G.; Ju, Y.M. Visible Light-Driven Photocatalytic Degradation of Rhodamine B over NaBiO3: Pathways and Mechanism. J. Phys. Chem. A 2009, 113, 10024–10032. [Google Scholar] [CrossRef]
- Martinez-de la Cruz, A.; Perez, U.M.G. Photocatalytic properties of BiVO4 prepared by the co-precipitation method: Degradation of rhodamine B and possible reaction mechanisms under visible irradiation. Mater. Res. Bull. 2010, 45, 135–141. [Google Scholar] [CrossRef]
- Guo, J.X.; Zhou, H.R.; Ting, S.; Luo, H.D.; Liang, J.; Yuan, S.D. Investigation of catalytic activity and mechanism for RhB degradation by LaMnO3 perovskites prepared via the citric acid method. New J. Chem. 2019, 43, 18146–18157. [Google Scholar] [CrossRef]
- Sun, M.; Li, D.Z.; Chen, Y.B.; Chen, W.; Li, W.J.; He, Y.H.; Fu, X.Z. Synthesis and Photocatalytic Activity of Calcium Antimony Oxide Hydroxide for the Degradation of Dyes in Water. J. Phys. Chem. C 2009, 113, 13825–13831. [Google Scholar] [CrossRef]
- Sharma, G.; Dionysiou, D.D.; Sharma, S.; Kumar, A.; Al-Muhtaseb, A.H.; Naushad, M.; Stadler, F.J. Highly efficient Sr/Ce/activated carbon bimetallic nanocomposite for photoinduced degradation of rhodamine B. Catal. Today 2019, 335, 437–451. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Thomas, M.; Natarajan, K.; Bajaj, H.C.; Tayade, R.J. Study on UV-LED/TiO2 process for degradation of Rhodamine B dye. Chem. Eng. J. 2011, 169, 126–134. [Google Scholar] [CrossRef]
- Khandekar, D.C.; Bhattacharyya, A.R.; Bandyopadhyaya, R. Role of impregnated nano-photocatalyst (SnxTi(1-x)O2) inside mesoporous silica (SBA-15) for degradation of organic pollutant (Rhodamine B) under UV light. J. Environ. Chem. Eng. 2019, 7, 103433. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, K.; Xu, H.; Yan, W. Electrochemical oxidation of rhodamine B by PbO2/Sb-SnO2/TiO2 nanotube arrays electrode. Chin. J. Catal. 2019, 40, 917–927. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, H.G.; Zhang, Y.L.; Cheng, X.; Zhou, P.; Zhang, G.C.; Wang, J.Q.; Tang, P.; Ke, T.L.; Li, W. Heterogeneous activation of persulfate for Rhodamine B degradation with 3D flower sphere-like BiOI/Fe3O4 microspheres under visible light irradiation. Sep. Purif. Technol. 2018, 192, 88–98. [Google Scholar] [CrossRef]
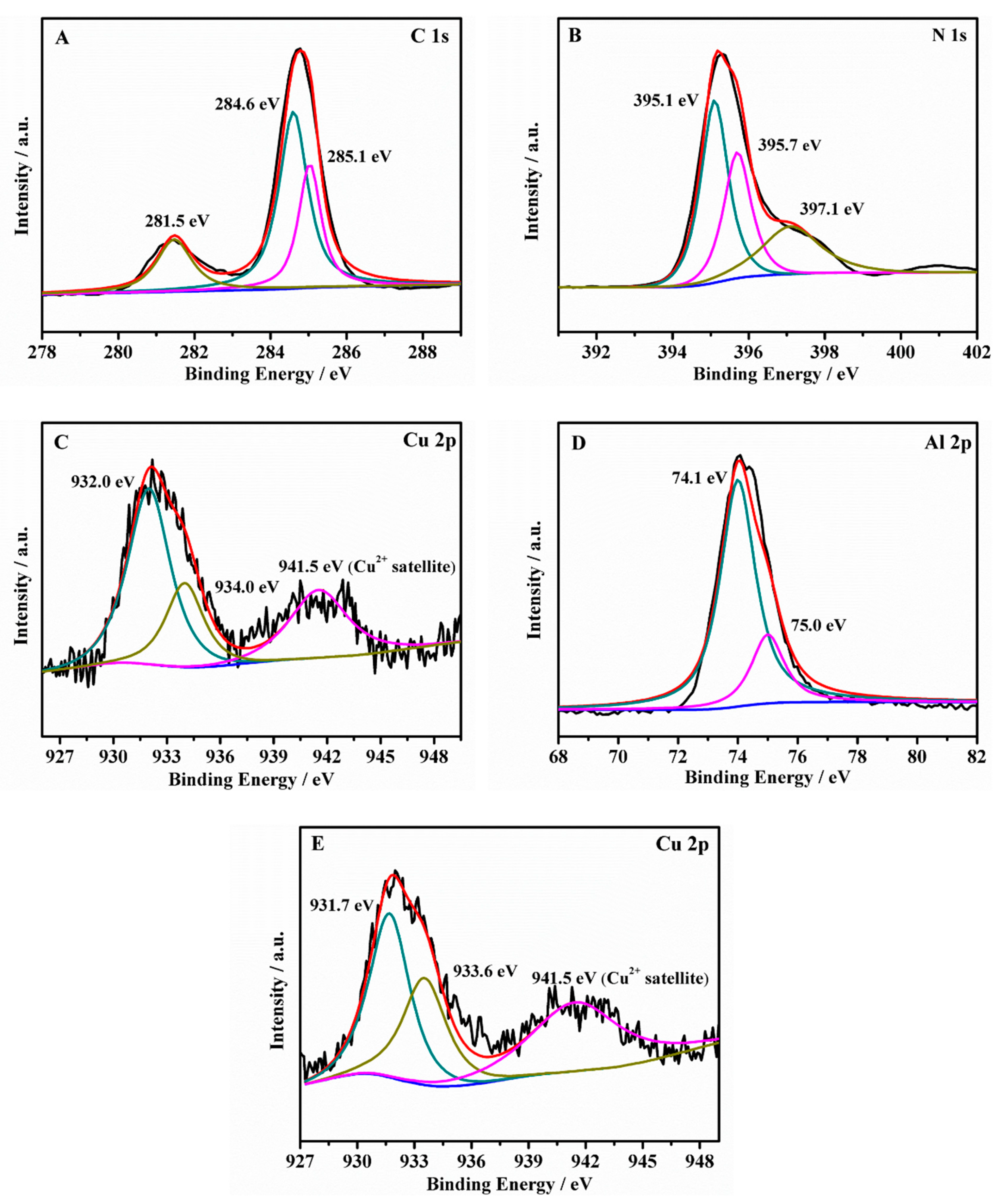




| BE/eV | Chemical Bonds | BE/eV | Chemical Bonds | ||
|---|---|---|---|---|---|
| C 1s | 281.5 | C−H | N 1s | 395.1 | C−N−C |
| 284.6 | C−C | 395.7 | N−(C)3 | ||
| 285.1 | C−O | 397.1 | C=N | ||
| Cu 2p | 932.0/931.7 | Cu+ | Al 2p | 74.1 | Al−O−Al |
| 934.0/933.6 | Cu2+ | 75.0 | Al−O−Cu |
| Catalyst Dose (g/L) | SA/V (105 m−1) | kobs (10−4 s−1) | Standard Deviation (10−5 s−1) | R2 (%) |
|---|---|---|---|---|
| 0.4 | 0.586 | 1.33 | 0.317 | 99.72 |
| 0.6 | 0.879 | 2.73 | 0.592 | 99.73 |
| 0.8 | 1.172 | 4.63 | 0.859 | 99.83 |
| 0.9 | 1.319 | 5.44 | 0.409 | 99.98 |
| 1.0 | 1.466 | 6.47 | 1.042 | 99.87 |
| Temperature (°C) | kobs (10−3 s−1) | Standard Deviation (10−5 s−1) | R2 (%) |
|---|---|---|---|
| 20 | 0.358 | 0.689 | 99.70 |
| 25 | 0.647 | 1.042 | 99.87 |
| 30 | 1.07 | 2.184 | 99.71 |
| 35 | 1.57 | 2.081 | 99.91 |
| 40 | 2.34 | 8.081 | 99.53 |
| Recycling Cycles | 1st | 2nd | 3rd | 4th |
|---|---|---|---|---|
| Removal ratio | 96.8% | 95.5% | 91.8% | 89.7% |
| kobs (10−3 s−1) | 1.37 | 1.12 | 0.83 | 0.77 |
| Cu leaching (mg/L) | 0.16 | 0.37 | 0.43 | 0.55 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Liu, Z.; Fang, L.; Guo, Y.; Feng, Y.; Yang, M. Kinetic and Mechanistic Study of Rhodamine B Degradation by H2O2 and Cu/Al2O3/g-C3N4 Composite. Catalysts 2020, 10, 317. https://doi.org/10.3390/catal10030317
Zhou C, Liu Z, Fang L, Guo Y, Feng Y, Yang M. Kinetic and Mechanistic Study of Rhodamine B Degradation by H2O2 and Cu/Al2O3/g-C3N4 Composite. Catalysts. 2020; 10(3):317. https://doi.org/10.3390/catal10030317
Chicago/Turabian StyleZhou, Chunsun, Zhongda Liu, Lijuan Fang, Yulian Guo, Yanpeng Feng, and Miao Yang. 2020. "Kinetic and Mechanistic Study of Rhodamine B Degradation by H2O2 and Cu/Al2O3/g-C3N4 Composite" Catalysts 10, no. 3: 317. https://doi.org/10.3390/catal10030317
APA StyleZhou, C., Liu, Z., Fang, L., Guo, Y., Feng, Y., & Yang, M. (2020). Kinetic and Mechanistic Study of Rhodamine B Degradation by H2O2 and Cu/Al2O3/g-C3N4 Composite. Catalysts, 10(3), 317. https://doi.org/10.3390/catal10030317




