Photocatalytic Reforming for Hydrogen Evolution: A Review
Abstract
:1. Introduction
- (i)
- Hydrogen atom is the most abundant element in the universe;
- (ii)
- The heating value of hydrogen (141.8 × 106 J/kg at 298 K) is much higher than that of most fuels (e.g., gasoline: 44 × 106 J/kg at 298 K);
- (iii)
- Water (H2O) is the only exhaust product when hydrogen is converted into energy, which is more environmentally-friendly compared to fossil fuels.
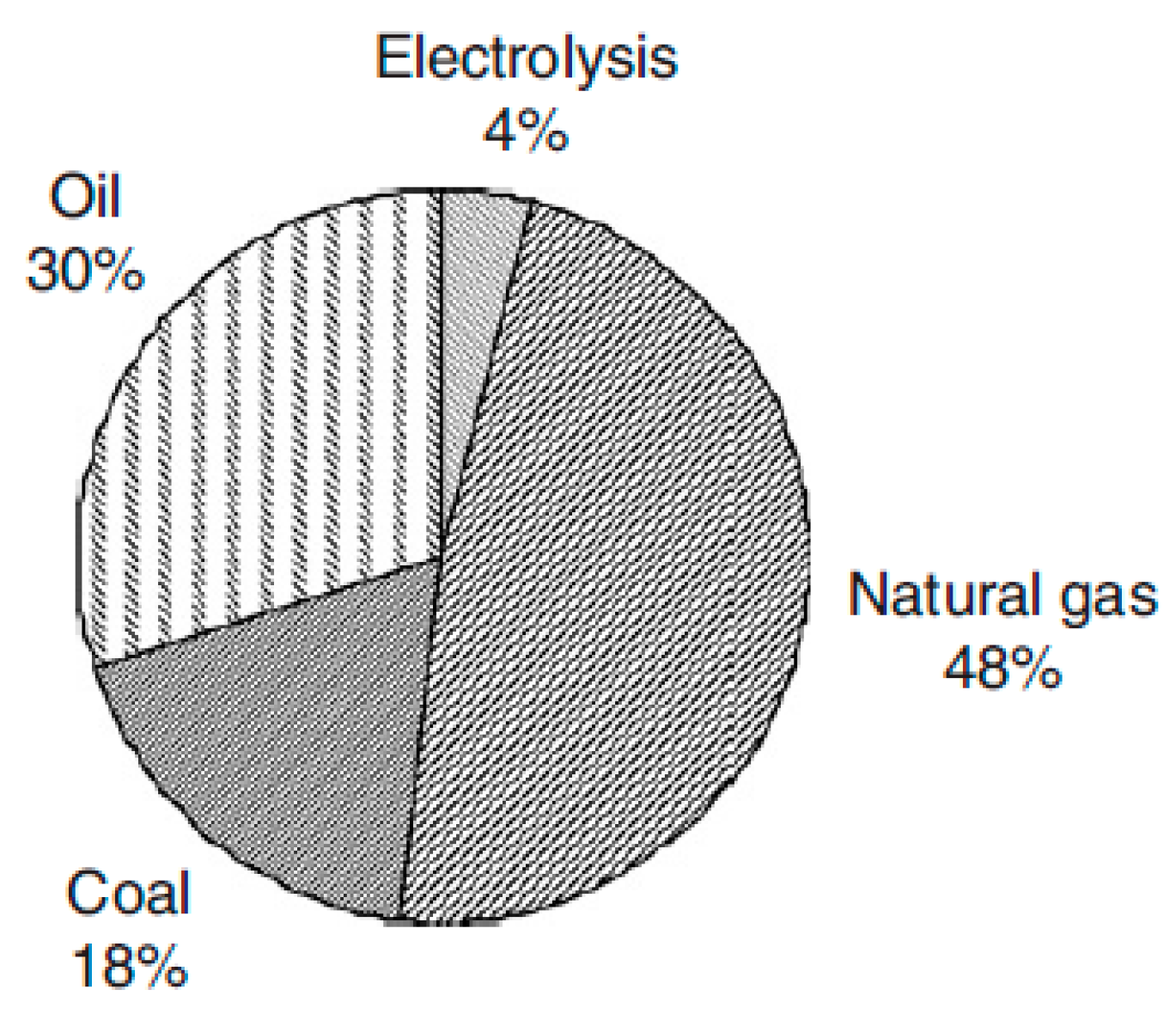
2. Industrial Hydrogen Production
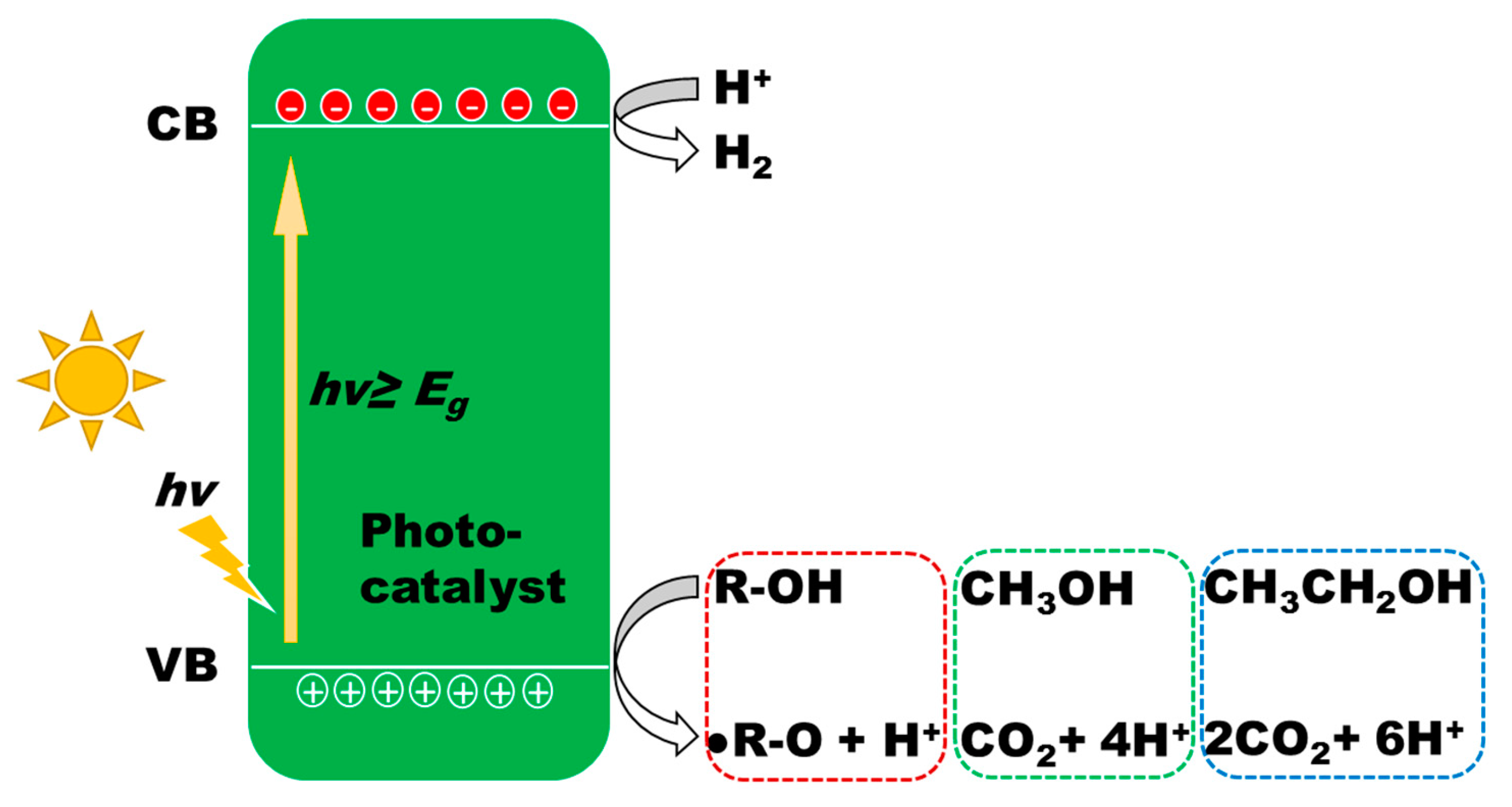
3. Photocatalysis for Hydrogen Evolution
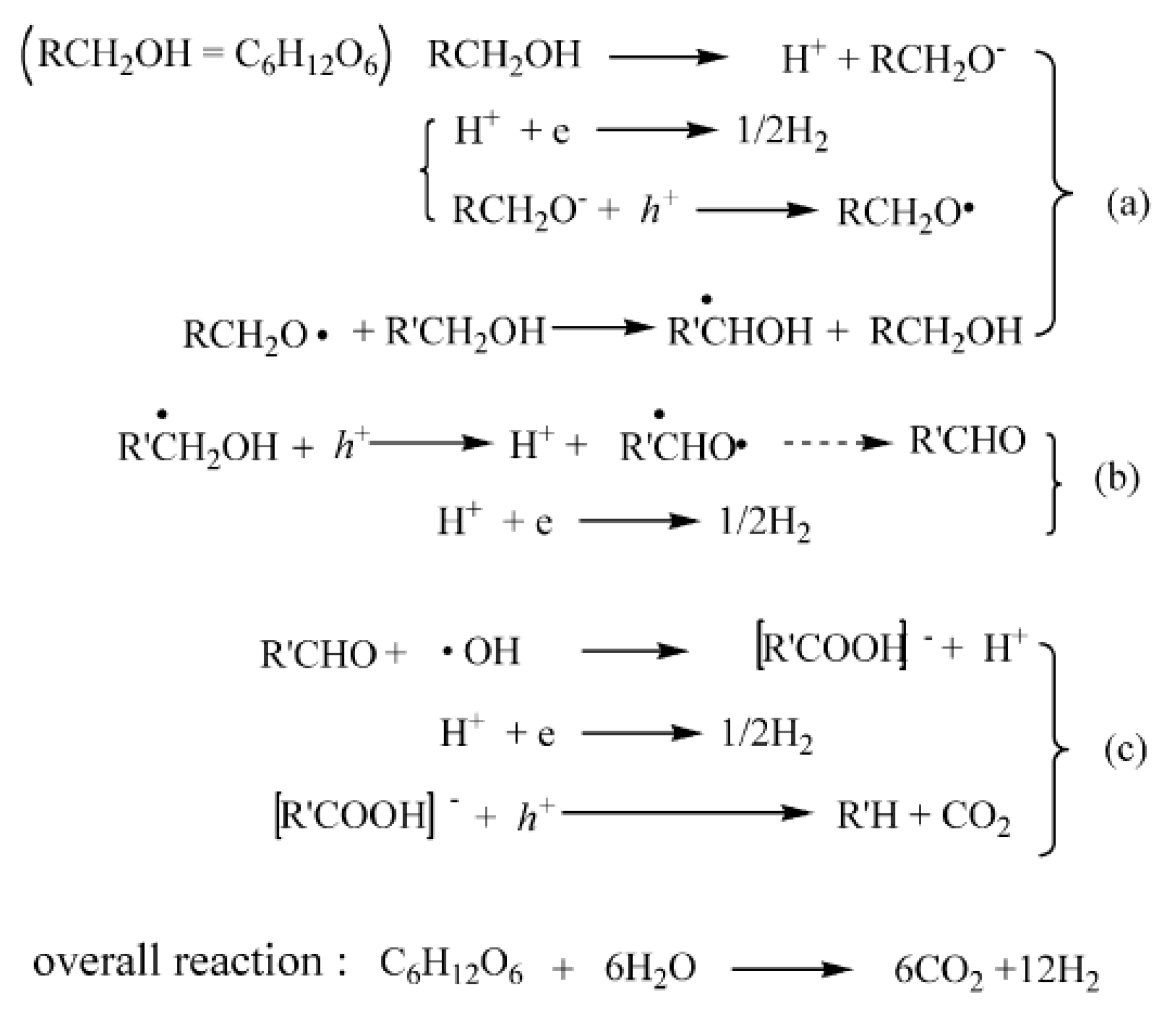
3.1. Photocatalytic Reforming of Methanol
- Photogeneration of charge carriers (electrons and holes);
- Adsorbed species on the surface of photocatalyst react with separated charge carriers: i.e., adsorbed H2O and CH3OH are oxidized by holes with the formation of OH− and various fragments including CH3O−, CH2O−, CHO−, HCOO− and HCOOH;
- As-formed HCOOH is further oxidized to CO2;
- A large quantity of protons formed from the last steps, and are reduced by the electrons either on the CB of TiO2 or Au to form H2.
3.2. Photocatalytic Reforming of Ethanol
3.3. Photocatalytic Reforming of Biomass
3.3.1. TiO2
3.3.2. Metal Sulfide
3.3.3. Graphene Analog Photocatalyst
3.3.4. Oxide Solid Solution Photocatalyst
4. Future Work and Concluding Remarks
- Low photocatalytic efficiency. Currently, the photocatalytic activity is too low to use on a large scale, which is also the most significant problem for not only photocatalytic reforming, but also all other photocatalytic applications. With the rapid development of material science, design and synthesis of novel materials are one of the most effective approaches. Meanwhile, exploration of effective modification methods to improve the photocatalytic activity is of the essence, as well.
- Low visible light-responsivity. When we aim to utilize solar light as the energy source, it is essential to expand the utilization of the solar spectrum, especially the visible-light part. Feasible approaches include the modifications of existing photocatalyst to narrow its band gap, and preparation of new materials with narrow band gap. These effective modification methods include doping, noble metal nanoparticles loading and fabrication of heterojunction. Recently, with the rapid development of material science and preparation technologies, many advanced semiconductors exhibited excellent visible-light-induced photoactivity, including g-C3N4 and bismuth-based semiconductors (BiOBr, Bi2WO6, BiVO4 etc.). All those materials are of promise to be applied in photocatalytic reforming for H2 production, whereas were rarely been reported in this research field.
- Ambiguous mechanism for photocatalytic processes. Most of the mechanism studies on photocatalysis were on the basis of band structures. However, some phenomena were difficult to explain, based on the electrical band structures. Instead, advanced material characterization techniques, as well as simulation methodologies should be adopted to acquire an insight into the photocatalytic process.
- Rare studies on photoreactor design. In the process of scaling-up photocatalysis, photoreactor design plays significant role. Photocatalytic activity can also be improved with rational photoreactor design. However, most of studies on photocatalytic reforming were still in lab scale. More contributions on the exploration of photoreactor design should be made to upscale this process.
- No standard platform. So far, there has been no standard platform that can be used to compare photoactivity for either different materials or the same material tested from a different lab. There is an urgent need to establish a standard to evaluate different materials, and guide the direction of future work.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meng, X.; Zhang, Z.; Li, X. Synergetic photoelectrocatalytic reactors for environmental remediation: A review. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 83–101. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Meng, X. Recent development on palladium enhanced photocatalytic activity: A review. J. Alloys Compd. 2020, 830, 154669. [Google Scholar] [CrossRef]
- Meng, X.; Yun, N.; Zhang, Z. Recent advances in computational photocatalysis: A review. Can. J. Chem. Eng. 2019, 97, 1982–1998. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Z. Bismuth-based Photocatalytic Semiconductors: Introduction, Challenges and Possible Approaches. J. Mol. Catal. A Chem. 2016, 423, 533–549. [Google Scholar] [CrossRef]
- Zhang, K.-L.; Liu, C.-M.; Huang, F.-Q.; Zheng, C.; Wang, W.-D. Study of the electronic structure and photocatalytic activity of the BiOCl photocatalyst. Appl. Catal. B Environ. 2006, 68, 125–129. [Google Scholar] [CrossRef]
- Cao, S.; Chan, T.-S.; Lu, Y.-R.; Shi, X.; Fu, B.; Wu, Z.; Li, H.; Liu, K.; Alzuabi, S.; Cheng, P.; et al. Photocatalytic pure water splitting with high efficiency and value by Pt/porous brookite TiO2 nanoflutes. Nano Energy 2020, 67. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, P.; Niu, M.; Maddy, J. The survey of key technologies in hydrogen energy storage. Int. J. Hydrog. Energy 2016, 41, 14535–14552. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrog. Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrog. Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Ibrahim, A.B.A.; Akilli, H. Supercritical water gasification of wastewater sludge for hydrogen production. Int. J. Hydrog. Energy 2019, 44, 10328–10349. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, B. Recent Advances in Electrochemical Hydrogen Production from Water Assisted by Alternative Oxidation Reactions. ChemElectroChem 2019, 6, 3214–3226. [Google Scholar] [CrossRef]
- Kothari, R.; Buddhi, D.; Sawhney, R.L. Comparison of environmental and economic aspects of various hydrogen production methods. Renew. Sustain. Energy Rev. 2008, 12, 553–563. [Google Scholar] [CrossRef]
- Chisalita, D.-A.; Cormos, C.-C. Techno-economic assessment of hydrogen production processes based on various natural gas chemical looping systems with carbon capture. Energy 2019, 181, 331–344. [Google Scholar] [CrossRef]
- Yamamuro, K.; Tamura, S.; Watanabe, R.; Sekine, Y. Hydrogen Production by Water Gas Shift Reaction Over Pd–K Impregnated Co Oxide Catalyst. Catal. Lett. 2013, 143, 339–344. [Google Scholar] [CrossRef]
- Seçer, A.; Küçet, N.; Fakı, E.; Hasanoğlu, A. Comparison of co–gasification efficiencies of coal, lignocellulosic biomass and biomass hydrolysate for high yield hydrogen production. Int. J. Hydrog. Energy 2018, 43, 21269–21278. [Google Scholar] [CrossRef]
- Kim, J.; Choi, H.; Lim, J.; Rhim, Y.; Chun, D.; Kim, S.; Lee, S.; Yoo, J. Hydrogen production via steam gasification of ash free coals. Int. J. Hydrog. Energy 2013, 38, 6014–6020. [Google Scholar] [CrossRef] [Green Version]
- Yuzer, B.; Selcuk, H.; Chehade, G.; Demir, M.E.; Dincer, I. Evaluation of hydrogen production via electrolysis with ion exchange membranes. Energy 2019. [Google Scholar] [CrossRef]
- Bairachnyi, V.; Rudenko, N.; Zhelavska, Y.; Pilipenko, A. Using aluminum alloys in the electrochemical hydrogen production. Mater. Today Proc. 2019, 6, 299–304. [Google Scholar] [CrossRef]
- Raptis, D.; Seferlis, A.K.; Mylona, V.; Politis, C.; Lianos, P. Electrochemical hydrogen and electricity production by using anodes made of commercial aluminum. Int. J. Hydrog. Energy 2019, 44, 1359–1365. [Google Scholar] [CrossRef]
- Wang, M.; Chen, L.; Sun, L. Recent progress in electrochemical hydrogen production with earth-abundant metal complexes as catalysts. Energy Environ. Sci. 2012, 5. [Google Scholar] [CrossRef]
- Voldsund, M.; Jordal, K.; Anantharaman, R. Hydrogen production with CO2 capture. Int. J. Hydrog. Energy 2016, 41, 4969–4992. [Google Scholar] [CrossRef]
- Li, Z.; Meng, X.; Zhang, Z. Recent development on MoS2-based photocatalysis: A review. J. Photochem. Photobiol. C Photochem. Rev. 2018, 35, 39–55. [Google Scholar] [CrossRef]
- Kim, M.K.; Sim, W.H.; Choi, M.; Lim, H.; Kwon, Y.; Jeong, H.M. Electrochemically Li-intercalated TiO2 nanoparticles for High performance photocatalytic production of hydrogen. Catal. Today 2019. [Google Scholar] [CrossRef]
- Zhu, J.; Zäch, M. Nanostructured materials for photocatalytic hydrogen production. Curr. Opin. Colloid Interface Sci. 2009, 14, 260–269. [Google Scholar] [CrossRef]
- Meng, X.; Zisheng, Z. Two Dimensional Graphitic Materials for Photoelectrocatalysis: A Short Review. Catal. Today 2018. [Google Scholar] [CrossRef]
- Li, Z.; Meng, X.; Zhang, Z. An Effective Approach to Improve the Photocatalytic Activity of Graphitic Carbon Nitride via Hydroxyl Surface Modification. Catalysts 2018, 9, 17. [Google Scholar] [CrossRef] [Green Version]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Ismail, A.A.; Bahnemann, D.W. Photochemical splitting of water for hydrogen production by photocatalysis: A review. Sol. Energy Mat. Sol. Cells 2014, 128, 85–101. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I.; Naterer, G.F. Review of photocatalytic water-splitting methods for sustainable hydrogen production. Int. J. Energy Res. 2016, 40, 1449–1473. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I.; Zamfirescu, C. A review on selected heterogeneous photocatalysts for hydrogen production. Int. J. Energy Res. 2014, 38, 1903–1920. [Google Scholar] [CrossRef]
- Shimura, K.; Yoshida, H. Heterogeneous photocatalytic hydrogen production from water and biomass derivatives. Energy Environ. Sci. 2011, 4, 2467–2481. [Google Scholar] [CrossRef]
- Dickinson, A.; James, D.; Perkins, N.; Cassidy, T.; Bowker, M. The photocatalytic reforming of methanol. J. Mol. Catal. A Chem. 1999, 146, 211–221. [Google Scholar] [CrossRef]
- Li, Z.; Ivanenko, A.; Meng, X.; Zhang, Z. Photocatalytic oxidation of methanol to formaldehyde on bismuth-based semiconductors. J. Hazard. Mater. 2019, 380, 120822. [Google Scholar] [CrossRef] [PubMed]
- Chiarello, G.L.; Selli, E. Photocatalytic production of hydrogen. In Advances in Hydrogen Production, Storage and Distribution; Woodhead Publishing: Cambridge, UK, 2014; pp. 216–247. [Google Scholar] [CrossRef]
- Wu, G.; Chen, T.; Su, W.; Zhou, G.; Zong, X.; Lei, Z.; Li, C. H2 production with ultra-low CO selectivity via photocatalytic reforming of methanol on Au/TiO2 catalyst. Int. J. Hydrog. Energy 2008, 33, 1243–1251. [Google Scholar] [CrossRef]
- Lee, C.; Park, J.; Jeon, Y.; Park, J.-I.; Einaga, H.; Truong, Y.B.; Kyratzis, I.L.; Mochida, I.; Choi, J.; Shul, Y.-G. Phosphate-Modified TiO2/ZrO2 Nanofibrous Web Composite Membrane for Enhanced Performance and Durability of High-Temperature Proton Exchange Membrane Fuel Cells. Energy Fuels 2017, 31, 7645–7652. [Google Scholar] [CrossRef]
- Ola, O.; Maroto-Valer, M.M. Review of material design and reactor engineering on TiO2 photocatalysis for CO2 reduction. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 16–42. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.S.; Kwak, S.-Y. The hydrothermal synthesis of mesoporous TiO2 with high crystallinity, thermal stability, large surface area, and enhanced photocatalytic activity. Appl. Catal. A Gen. 2007, 323, 110–118. [Google Scholar] [CrossRef]
- Sreethawong, T.; Yamada, Y.; Kobayashi, T.; Yoshikawa, S. Catalysis of nanocrystalline mesoporous TiO2 on cyclohexene epoxidation with H2O2: Effects of mesoporosity and metal oxide additives. J. Mol. Catal. A Chem. 2005, 241, 23–32. [Google Scholar] [CrossRef]
- Tschirch, J.; Bahnemann, D.; Wark, M.; Rathouský, J. A comparative study into the photocatalytic properties of thin mesoporous layers of TiO2 with controlled mesoporosity. J. Photochem. Photobiol. A Chem. 2008, 194, 181–188. [Google Scholar] [CrossRef]
- Bingham, M.; Mills, A. Photonic efficiency and selectivity study of M (M=Pt, Pd, Au and Ag)/TiO2 photocatalysts for methanol reforming in the gas phase. J. Photochem. Photobiol. A Chem. 2019. [Google Scholar] [CrossRef]
- Tálas, E.; Pászti, Z.; Korecz, L.; Domján, A.; Németh, P.; Szíjjártó, G.P.; Mihály, J.; Tompos, A. PtOx-SnOx-TiO2 catalyst system for methanol photocatalytic reforming: Influence of cocatalysts on the hydrogen production. Catal. Today 2018, 306, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Silva, C.G.; Sampaio, M.J.; Marques, R.R.N.; Ferreira, L.A.; Tavares, P.B.; Silva, A.M.T.; Faria, J.L. Photocatalytic production of hydrogen from methanol and saccharides using carbon nanotube-TiO2 catalysts. Appl. Catal. B Environ. 2015, 178, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Irie, H.; Kamiya, K.; Shibanuma, T.; Miura, S.; Tryk, D.A.; Yokoyama, T.; Hashimoto, K. Visible Light-Sensitive Cu(II)-Grafted TiO2 Photocatalysts: Activities and X-ray Absorption Fine Structure Analyses. J. Phys. Chem. C 2009, 113, 10761–10766. [Google Scholar] [CrossRef]
- Bernareggi, M.; Dozzi, M.; Bettini, L.; Ferretti, A.; Chiarello, G.; Selli, E. Flame-Made Cu/TiO2 and Cu-Pt/TiO2 Photocatalysts for Hydrogen Production. Catalysts 2017, 7, 301. [Google Scholar] [CrossRef]
- Miwa, T.; Kaneco, S.; Katsumata, H.; Suzuki, T.; Ohta, K.; Chand Verma, S.; Sugihara, K. Photocatalytic hydrogen production from aqueous methanol solution with CuO/Al2O3/TiO2 nanocomposite. Int. J. Hydrog. Energy 2010, 35, 6554–6560. [Google Scholar] [CrossRef]
- Tawalare, P.K.; Belsare, P.D.; Moharil, S.V. Semiconductor host for designing phosphors for modification of solar spectrum. Opt. Mater. 2020, 100, 109668. [Google Scholar] [CrossRef]
- Liang, Z.; Bai, X.; Hao, P.; Guo, Y.; Xue, Y.; Tian, J.; Cui, H. Full solar spectrum photocatalytic oxygen evolution by carbon-coated TiO2 hierarchical nanotubes. Appl. Catal. B Environ. 2019, 243, 711–720. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Z. Plasmonic ternary Ag–rGO–Bi2MoO6 composites with enhanced visible light-driven photocatalytic activity. J. Catal. 2016, 344, 616–630. [Google Scholar] [CrossRef]
- Meng, X.; Li, Z.; Zhang, Z. Palladium nanoparticles and rGO co-modified BiVO4 with greatly improved visible light-induced photocatalytic activity. Chemosphere 2018, 198, 1–12. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Z. Bi2MoO6 co-modified by reduced graphene oxide and palladium (Pd2+ and Pd0) with enhanced photocatalytic decomposition of phenol. Appl. Catal. B Environ. 2017, 209, 383–393. [Google Scholar] [CrossRef]
- Krissanasaeranee, M.; Wongkasemjit, S.; Cheetham, A.K.; Eder, D. Complex carbon nanotube-inorganic hybrid materials as next-generation photocatalysts. Chem. Phys. Lett. 2010, 496, 133–138. [Google Scholar] [CrossRef]
- Majrik, K.; Turcsányi, Á.; Pászti, Z.; Szabó, T.; Domján, A.; Mihály, J.; Tompos, A.; Dékány, I.; Tálas, E. Graphite Oxide-TiO2 Nanocomposite Type Photocatalyst for Methanol Photocatalytic Reforming Reaction. Top. Catal. 2018, 61, 1323–1334. [Google Scholar] [CrossRef] [Green Version]
- Ashok, J.; Dewangan, N.; Das, S.; Hongmanorom, P.; Wai, M.H.; Tomishige, K.; Kawi, S. Recent progress in the development of catalysts for steam reforming of biomass tar model reaction. Fuel Process. Technol. 2020, 199. [Google Scholar] [CrossRef]
- Chen, D.; Wang, W.; Liu, C. Ni-encapsulated graphene chainmail catalyst for ethanol steam reforming. Int. J. Hydrog. Energy 2019, 44, 6560–6572. [Google Scholar] [CrossRef]
- Cifuentes, A.; Torres, R.; Llorca, J. Modelling of the ethanol steam reforming over Rh-Pd/CeO2 catalytic wall reactors. Int. J. Hydrog. Energy 2019. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Cai, W.; Tana; Provendier, H.; Schuurman, Y.; Descorme, C.; Mirodatos, C.; Shen, W. Ageing analysis of a model Ir/CeO2 catalyst in ethanol steam reforming. Appl. Catal. B Environ. 2012, 125, 546–555. [Google Scholar] [CrossRef]
- Wu, R.-C.; Tang, C.-W.; Huang, H.-H.; Wang, C.-C.; Chang, M.-B.; Wang, C.-B. Effect of boron doping and preparation method of Ni/Ce0.5Zr0.5O2 catalysts on the performance for steam reforming of ethanol. Int. J. Hydrog. Energy 2019, 44, 14279–14289. [Google Scholar] [CrossRef]
- Freni, S.; Cavallaro, S.; Mondello, N.; Spadaro, L.; Frusteri, F. Production of hydrogen for MC fuel cell by steam reforming of ethanol over MgO supported Ni and Co catalysts. Catal. Commun. 2003, 4, 259–268. [Google Scholar] [CrossRef]
- Cavallaro, S.; Chiodo, V.; Vita, A.; Freni, S. Hydrogen production by auto-thermal reforming of ethanol on Rh/Al2O3 catalyst. J. Power Sources 2003, 123, 10–16. [Google Scholar] [CrossRef]
- Sakata, T.; Kawai, T. Heterogeneous photocatalytic production of hydrogen and methane from ethanol and water. Chem. Phys. Lett. 1981, 80, 341–344. [Google Scholar] [CrossRef]
- Sola, A.C.; Homs, N.; Ramírez de la Piscina, P. Photocatalytic H2 production from ethanol(aq) solutions: The effect of intermediate products. Int. J. Hydrog. Energy 2016, 41, 19629–19636. [Google Scholar] [CrossRef]
- Sola, A.C.; Ramírez de la Piscina, P.; Homs, N. Behaviour of Pt/TiO2 catalysts with different morphological and structural characteristics in the photocatalytic conversion of ethanol aqueous solutions. Catal. Today 2020, 341, 13–20. [Google Scholar] [CrossRef]
- Bamwenda, G.R.; Tsubota, S.; Nakamura, T.; Haruta, M. Photoassisted hydrogen production from a water-ethanol solution: A comparison of activities of Au TiO2 and Pt TiO2. J. Photochem. Photobiol. A Chem. 1995, 89, 177–189. [Google Scholar] [CrossRef]
- Strataki, N.; Bekiari, V.; Kondarides, D.I.; Lianos, P. Hydrogen production by photocatalytic alcohol reforming employing highly efficient nanocrystalline titania films. Appl. Catal. B Environ. 2007, 77, 184–189. [Google Scholar] [CrossRef]
- Zou, J.-J.; He, H.; Cui, L.; Du, H.-Y. Highly efficient Pt/TiO2 photocatalyst for hydrogen generation prepared by a cold plasma method. Int. J. Hydrog. Energy 2007, 32, 1762–1770. [Google Scholar] [CrossRef]
- Luo, B.; Song, R.; Jing, D. Particle aggregation behavior during photocatalytic ethanol reforming reaction and its correlation with the activity of H2 production. Colloids Surf. A Physicochem. Eng. Asp. 2017, 535, 114–120. [Google Scholar] [CrossRef]
- Meng, X.; Li, Z.; Zhang, Z. Pd-nanoparticle-decorated peanut-shaped BiVO4 with improved visible light-driven photocatalytic activity comparable to that of TiO2 under UV light. J. Catal. 2017, 356, 53–64. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Wang, W.; Huang, W. Engineering highly active TiO2 photocatalysts via the surface-phase junction strategy employing a titanate nanotube precursor. J. Catal. 2014, 310, 16–23. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, F.; Jiao, Y.; Yang, H.; Zhang, J. Ag0-loaded brookite/anatase composite with enhanced photocatalytic performance towards the degradation of methyl orange. J. Mol. Catal. A Chem. 2011, 348, 114–119. [Google Scholar] [CrossRef]
- Romero Ocaña, I.; Beltram, A.; Delgado Jaén, J.J.; Adami, G.; Montini, T.; Fornasiero, P. Photocatalytic H2 production by ethanol photodehydrogenation: Effect of anatase/brookite nanocomposites composition. Inorg. Chim. Acta 2015, 431, 197–205. [Google Scholar] [CrossRef]
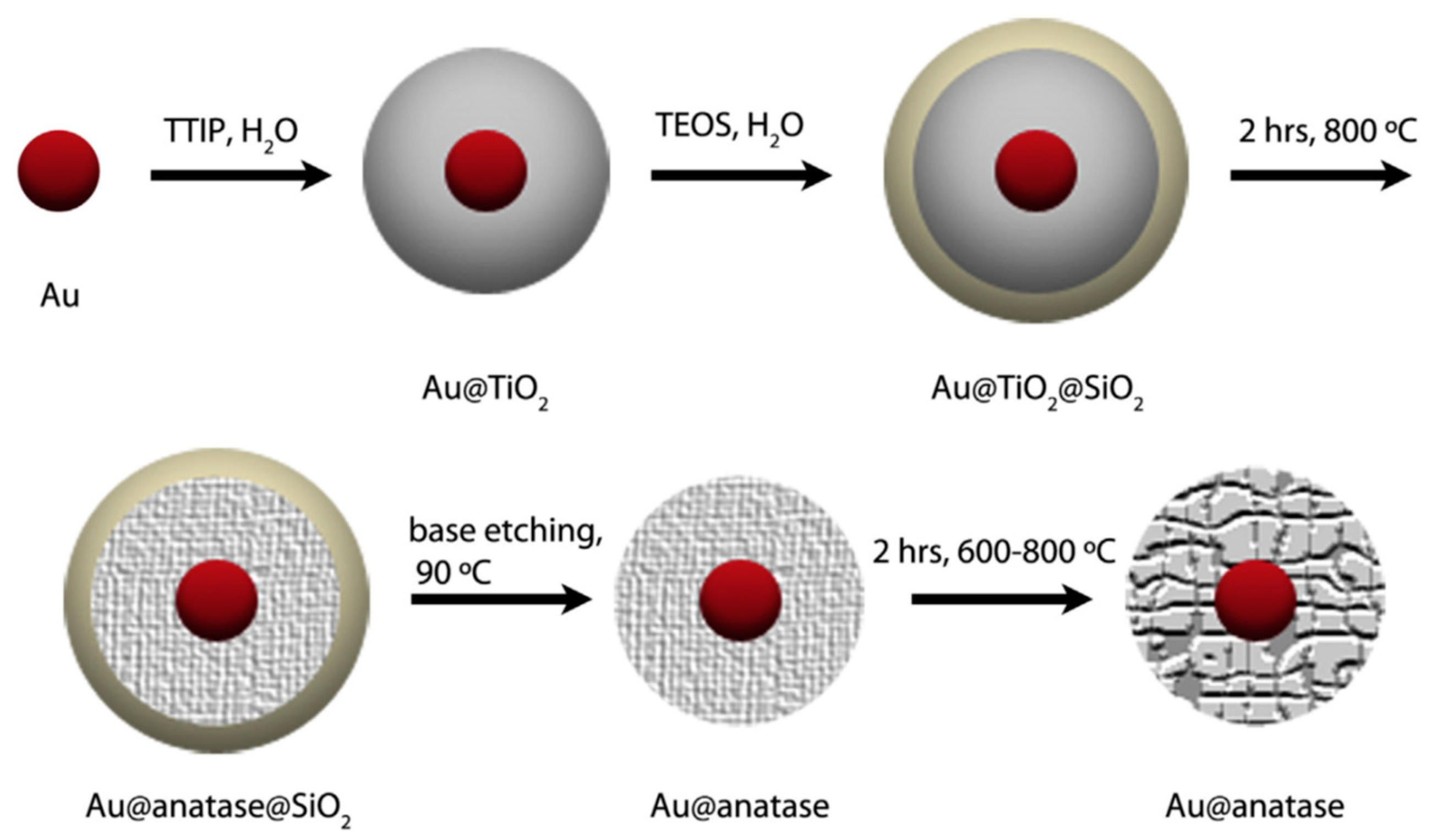
- Goebl, J.; Joo, J.B.; Dahl, M.; Yin, Y. Synthesis of tailored Au@TiO2 core–shell nanoparticles for photocatalytic reforming of ethanol. Catal. Today 2014, 225, 90–95. [Google Scholar] [CrossRef]
- Sampaio, M.J.; Oliveira, J.W.L.; Sombrio, C.I.L.; Baptista, D.L.; Teixeira, S.R.; Carabineiro, S.A.C.; Silva, C.G.; Faria, J.L. Photocatalytic performance of Au/ZnO nanocatalysts for hydrogen production from ethanol. Appl. Catal. A Gen. 2016, 518, 198–205. [Google Scholar] [CrossRef]
- Fu, X.; Leung, D.Y.C.; Wang, X.; Xue, W.; Fu, X. Photocatalytic reforming of ethanol to H2 and CH4 over ZnSn(OH)6 nanocubes. Int. J. Hydrog. Energy 2011, 36, 1524–1530. [Google Scholar] [CrossRef]
- Puga, A.V. Photocatalytic production of hydrogen from biomass-derived feedstocks. Coord. Chem. Rev. 2016, 315, 1–66. [Google Scholar] [CrossRef]
- Guo, F.; Zhao, X.; Peng, K.; Liang, S.; Jia, X.; Qian, L. Catalytic reforming of biomass primary tar from pyrolysis over waste steel slag based catalysts. Int. J. Hydrog. Energy 2019, 44, 16224–16233. [Google Scholar] [CrossRef]
- Iwasaki, W. A consideration of the economic efficiency of hydrogen production from biomass. Int. J. Hydrog. Energy 2003, 28, 939–944. [Google Scholar] [CrossRef]
- Balat, H.; Kırtay, E. Hydrogen from biomass—Present scenario and future prospects. Int. J. Hydrog. Energy 2010, 35, 7416–7426. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H.; Sumathy, K. An overview of hydrogen production from biomass. Fuel Process. Technol. 2006, 87, 461–472. [Google Scholar] [CrossRef]
- Setiabudi, H.D.; Aziz, M.A.A.; Abdullah, S.; Teh, L.P.; Jusoh, R. Hydrogen production from catalytic steam reforming of biomass pyrolysis oil or bio-oil derivatives: A review. Int. J. Hydrog. Energy 2019. [Google Scholar] [CrossRef]
- Jeong, Y.-S.; Choi, Y.-K.; Kang, B.-S.; Ryu, J.-H.; Kim, H.-S.; Kang, M.-S.; Ryu, L.-H.; Kim, J.-S. Lab-scale and pilot-scale two-stage gasification of biomass using active carbon for production of hydrogen-rich and low-tar producer gas. Fuel Process. Technol. 2020, 198, 106240. [Google Scholar] [CrossRef]
- Zhang, S.-p.; Li, X.-j.; Li, Q.-y.; Xu, Q.-l.; Yan, Y.-j. Hydrogen production from the aqueous phase derived from fast pyrolysis of biomass. J. Anal. Appl. Pyrolysis 2011, 92, 158–163. [Google Scholar] [CrossRef]
- Watanabe, M.; Inomata, H.; Arai, K. Catalytic hydrogen generation from biomass (glucose and cellulose) with ZrO2 in supercritical water. Biomass Bioenergy 2002, 22, 405–410. [Google Scholar] [CrossRef]
- Fu, X.; Long, J.; Wang, X.; Leung, D.; Ding, Z.; Wu, L.; Zhang, Z.; Li, Z.; Fu, X. Photocatalytic reforming of biomass: A systematic study of hydrogen evolution from glucose solution. Int. J. Hydrog. Energy 2008, 33, 6484–6491. [Google Scholar] [CrossRef]
- Navarro, R.M.; Sánchez-Sánchez, M.C.; Alvarez-Galvan, M.C.; Valle, F.d.; Fierro, J.L.G. Hydrogen production from renewable sources: Biomass and photocatalytic opportunities. Energy Environ. Sci. 2009, 2, 35–54. [Google Scholar] [CrossRef]
- Melo, M.d.O.; Silva, L.A. Photocatalytic production of hydrogen: An innovative use for biomass derivatives. J. Braz. Chem. Soc. 2011, 22, 1399–1406. [Google Scholar] [CrossRef] [Green Version]
- Bellardita, M.; García-López, E.I.; Marcì, G.; Nasillo, G.; Palmisano, L. Photocatalytic Solar Light H2 Production by Aqueous Glucose Reforming. Eur. J. Inorg. Chem. 2018, 2018, 4522–4532. [Google Scholar] [CrossRef] [Green Version]
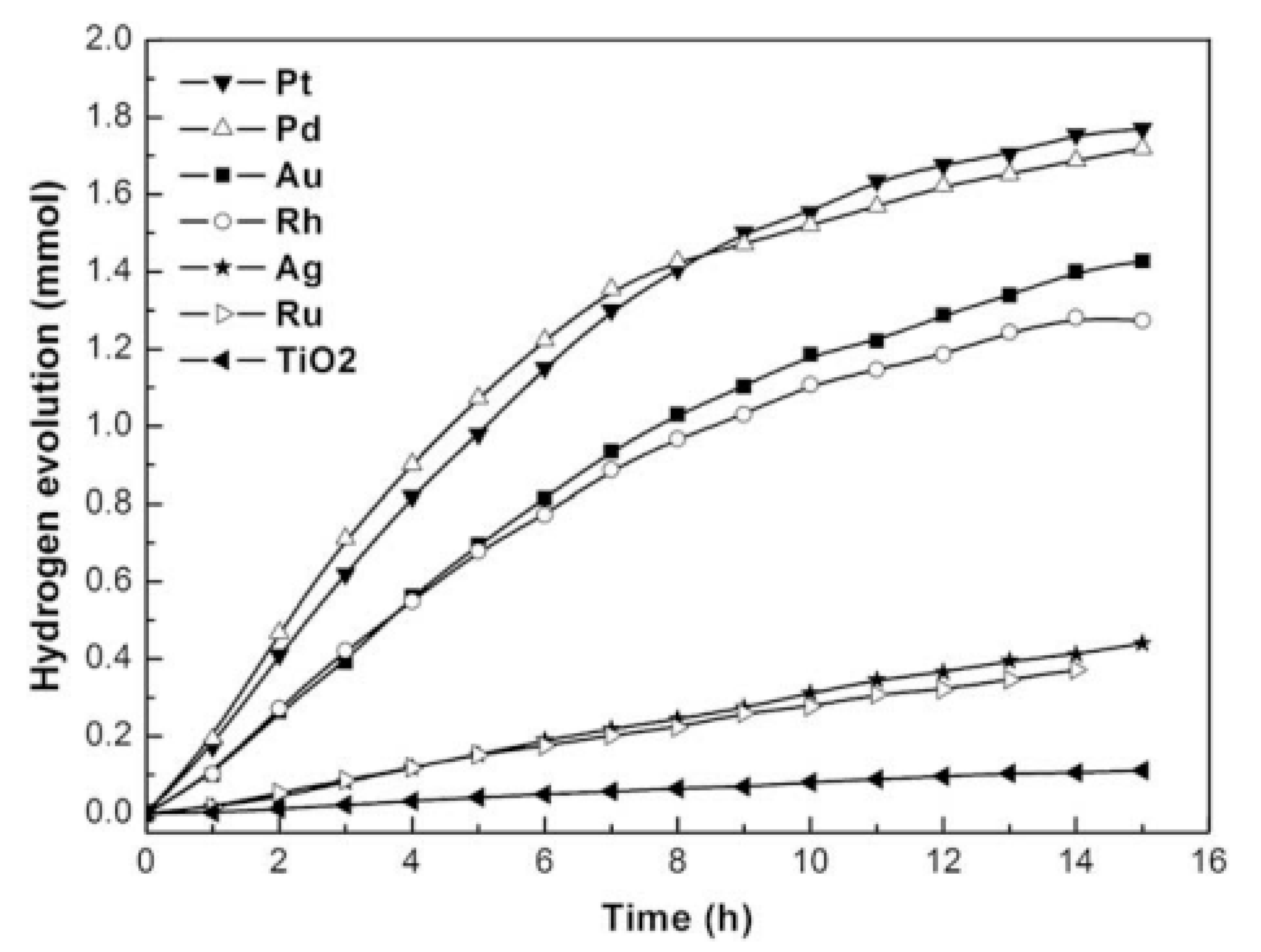
- Xu, Q.; Ma, Y.; Zhang, J.; Wang, X.; Feng, Z.; Li, C. Enhancing hydrogen production activity and suppressing CO formation from photocatalytic biomass reforming on Pt/TiO2 by optimizing anatase–rutile phase structure. J. Catal. 2011, 278, 329–335. [Google Scholar] [CrossRef]
- Wu, G.; Chen, T.; Zhou, G.; Zong, X.; Li, C. H2 production with low CO selectivity from photocatalytic reforming of glucose on metal/TiO2 catalysts. Sci. China Ser. B Chem. 2008, 51, 97–100. [Google Scholar] [CrossRef]
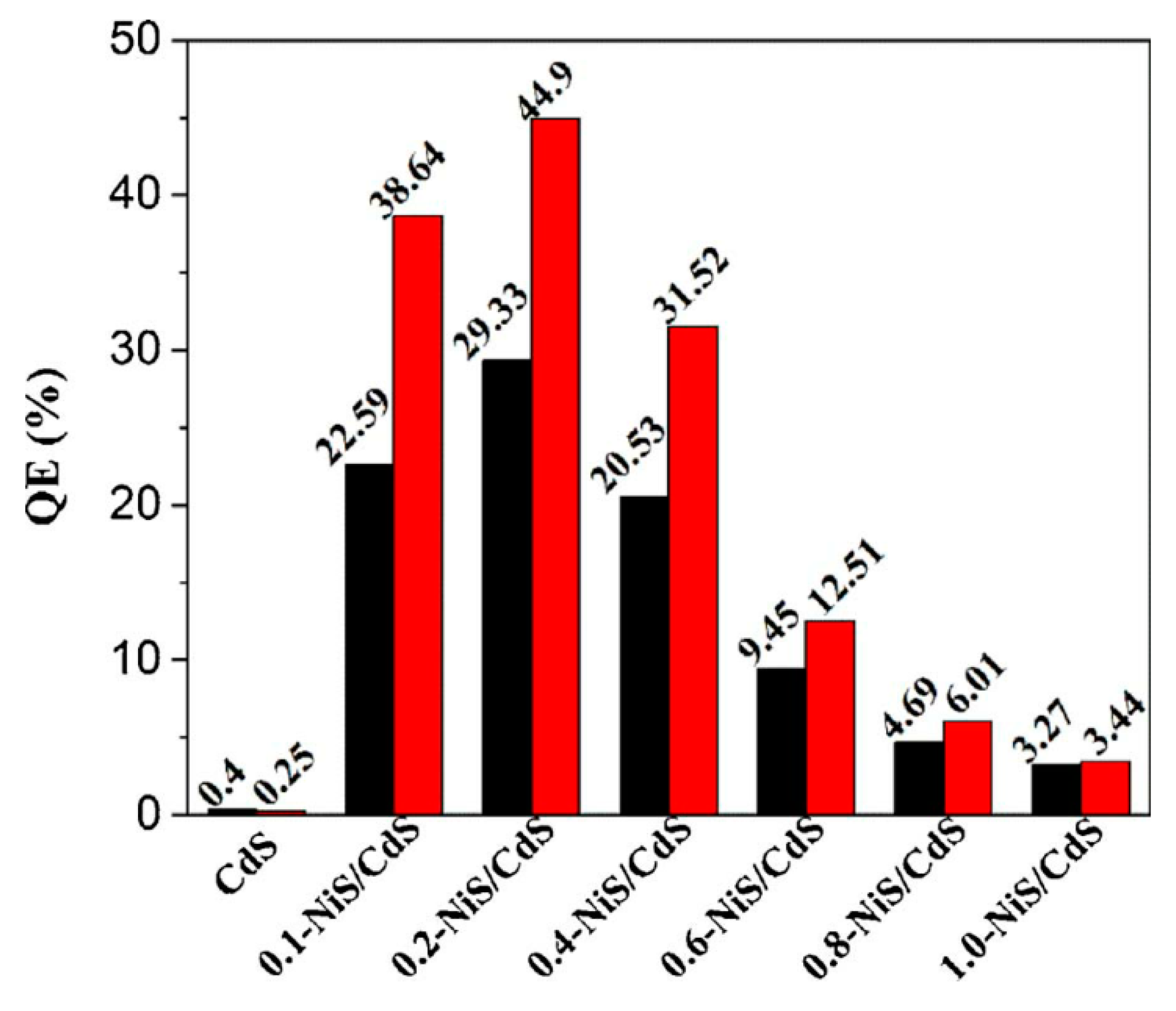
- Li, C.; Wang, H.; Naghadeh, S.B.; Zhang, J.Z.; Fang, P. Visible light driven hydrogen evolution by photocatalytic reforming of lignin and lactic acid using one-dimensional NiS/CdS nanostructures. Appl. Catal. B Environ. 2018, 227, 229–239. [Google Scholar] [CrossRef]
- Li, C.; Wang, H.; Ming, J.; Liu, M.; Fang, P. Hydrogen generation by photocatalytic reforming of glucose with heterostructured CdS/MoS2 composites under visible light irradiation. Int. J. Hydrog. Energy 2017, 42, 16968–16978. [Google Scholar] [CrossRef]
- Speltini, A.; Scalabrini, A.; Maraschi, F.; Sturini, M.; Pisanu, A.; Malavasi, L.; Profumo, A. Improved photocatalytic H2 production assisted by aqueous glucose biomass by oxidized g-C3N4. Int. J. Hydrog. Energy 2018, 43, 14925–14933. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, J.; Wang, S.; Yao, Z.; Wu, H.; Shi, L.; Yin, Y.; Wang, S.; Sun, H. Photocatalytic reforming of biomass for hydrogen production over ZnS nanoparticles modified carbon nitride nanosheets. J. Colloid Interface Sci. 2019, 555, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.-C.; Ke, N.-J.; Nam, L.D.; Nguyen, B.-S.; Xiao, Y.-K.; Lee, Y.-L.; Teng, H. Photocatalytic reforming of sugar and glucose into H2 over functionalized graphene dots. J. Mater. Chem. A 2019, 7, 8384–8393. [Google Scholar] [CrossRef]
- Jing, D.; Liu, M.; Shi, J.; Tang, W.; Guo, L. Hydrogen production under visible light by photocatalytic reforming of glucose over an oxide solid solution photocatalyst. Catal. Commun. 2010, 12, 264–267. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Q.; Feng, Z.; Li, M.; Li, C. Importance of the Relationship between Surface Phases and Photocatalytic Activity of TiO2. Angew. Chem. Int. Ed. 2008, 47, 1766–1769. [Google Scholar] [CrossRef]
- Yasuda, M.; Misriyani; Takenouchi, Y.; Kurogi, R.; Uehara, S.; Shiragami, T. Fuelization of Italian Ryegrass and Napier Grass through a Biological Treatment and Photocatalytic Reforming. J. Sustain. Bioenergy Syst. 2015, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Meng, X.; Zhang, Z. Fabrication of surface hydroxyl modified g-C3N4 with enhanced photocatalytic oxidation activity. Catal. Sci. Technol. 2019, 9, 3979–3993. [Google Scholar] [CrossRef]



| Catalyst | Biomass | Efficiency | Light | Ref |
|---|---|---|---|---|
| Pt-TiO2-W | 1.25 mmol/L glucose | 1.0 mmol h−1 gcat−1 | sunlight | [88] |
| Pt/P25 | 20 vol% methanol, 20 vol.% propanetriol, 1.3 mmol/L glucose | 0.749, 7.094, 7.784 mmol h−1 gcat−1 | 300 W Xe lamp | [89] |
| Pd/TiO2 | 250 mg/L glucose | 5.7 mmol h−1 gcat−1 | 125 W high-pressure mercury lamp | [85] |
| Rh/TiO2 | 1.25 mmol/L glucose | 1.5 mmol h−1 gcat−1 | 300 W Hg lamp | [90] |
| NiS/CdS | 0.10 g/L lignin & 2.0 vol.% lactic acid | 1.5 (both lignin and lactic acid) & 1.086 (only lactic acid) mmol h−1 gcat−1 | 300 W Xe lamp | [91] |
| CdS/MoS2 | 0.1 mol/L glucose | 55.0 mmol h−1 gcat−1 | 300 W Xe lamp | [92] |
| O-g-C3N4 | 1 mol/L glucose | 1.37 mmol h−1 gcat−1 | simulated solar light | [93] |
| ZnS-g-C3N4 | 50ppm glucose | 69.8 μmol h−1 gcat−1 | 300 W Xe lamp | [94] |
| S, N-GO | 0.35 mol/L Sugar & 0.35 mol/L glucose | 12.5 & 8.75 μmol h−1 gcat−1 | 300 W Xe lamp | [95] |
| Bi0.5Y0.5VO4 | 1 mol/L glucose | 50μmol h−1 gcat−1 | 350 W Xe lamp | [96] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Y.; Gao, X.; Li, Z.; Meng, X. Photocatalytic Reforming for Hydrogen Evolution: A Review. Catalysts 2020, 10, 335. https://doi.org/10.3390/catal10030335
Yao Y, Gao X, Li Z, Meng X. Photocatalytic Reforming for Hydrogen Evolution: A Review. Catalysts. 2020; 10(3):335. https://doi.org/10.3390/catal10030335
Chicago/Turabian StyleYao, Yuan, Xinyu Gao, Zizhen Li, and Xiangchao Meng. 2020. "Photocatalytic Reforming for Hydrogen Evolution: A Review" Catalysts 10, no. 3: 335. https://doi.org/10.3390/catal10030335