Abstract
Black TiO2 with doped nitrogen and modified carbon (b-N-TiO2/C) were successfully prepared by sol-gel method in the presence of urea as a source of nitrogen and carbon. The photocatalysts were characterized by field emission scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), Raman, electron paramagnetic resonance (EPR), and UV-vis diffuse reflectance spectra (DRS). The doped nitrogen, introduced defects, and modified carbon played a synergistic role in enhancing photocatalytic activity of b-N-TiO2/C for the degradation of chlorophyll-a in algae cells. The sample, with a proper amount of phase composition and oxygen vacancies, showed the highest efficiency to degrade chlorophyll-a, and the addition of H2O2 promoted this photocatalysis degradation. Based on the trapping experiments and electron spin resonance (ESR) signals, a photocatalytic mechanism of b-N-TiO2/C was proposed. In the photocatalytic degradation of chlorophyll-a, the major reactive species were identified as OH and O2−. This research may provide new insights into the photocatalytic inactivation of algae cells by composite photocatalysts.
1. Introduction
Harmful algal blooms (HABs) have been a serious environmental disaster in eutrophic waters due to nutrient loading and climate change [1]. The uncontrollable growth of harmful algae can destroy the ecological environment and even pose a serious threat to living animals and human beings [2]. Microcystis aeruginosa is one of the most known harmful algae in natural waters. In view of the removal of Microcystis aeruginosa, the photocatalysis can be considered as one of the most optimal technologies to inactivate algae due to its low cost, high efficiency, and environmental friendliness [3,4].
Recently, TiO2 photocatalysis has drawn much interest for the killing of various microorganisms, including harmful algae [5,6,7]. To date, much effort has been devoted to improving photocatalytic efficiency by narrowing its wide bandgap through element doping. The nitrogen element has a similar characteristic to the oxygen in TiO2, thus, nitrogen doping is considered to be one of the most effective methods in narrowing the bandgap of TiO2 [8,9]. The nitrogen 2p state can form intermediate energy to narrow the bandgap [10,11]. However, only doping nitrogen is not sufficient for extending visible light absorption in the range of more than 800 nm [12]. In order to improve the photocatalytic efficiency of doped TiO2 under visible light, engineering defects on the doped TiO2 was applied at the same time to enhance the utilization of visible light [13,14]. It has been concluded that the introduction of Ti3+ or oxygen vacancies can not only enhance visible light absorption but also lead to more effective photocatalytic performance of nitrogen-doped TiO2 [12]. To synthesize the nitrogen-doped TiO2 with oxygen vacancies, a two-step approach has been taken to incorporate nitrogen into TiO2 in the first step and then post-anneal the doped TiO2 in a vacuum or N2 atmosphere [15,16] to introduce oxygen vacancies. The two-step method was time-consuming, thus, a simple one-step approach was applied to complete the nitrogen doping and oxygen vacancies introduction at the same time through calcining nitrogen and titania precursors in an N2 atmosphere [17]. Therefore, the exploitation of the synergistic effect of doped nitrogen and introduced defects is promising to enhance the photocatalytic performance of TiO2.
In addition, carbon-modified TiO2 can also improve the visible light photocatalytic activity of TiO2 because of its unusual photoelectrochemical and electronic properties. Jia et al. reported the preparation of a carbon-coated TiO2 nanotubes film and enhancement of its catalytic application for H2 generation [18]. Wang et al. provided a facile one-pot hydrothermal treatment to successfully synthesize nitrogen-doped and carbon-modified TiO2 nanoparticles, and it has been verified that nitrogen doping narrowed the band gap and carbon modification enhanced the visible light absorption and the separation of photogenerated electrons and holes [19]. It can be indicated that the modified carbon exhibited the synergistic effect with the doped nitrogen. However, it may still be a challenge to dope nitrogen, introduce defects, and modify carbon together to improve the photocatalytic activity of TiO2.
In this study, nitrogen-doped black TiO2 with defects and modified carbon (b-N-TiO2/C) is fabricated through a one-step method in the presence of urea. After the characterization analysis of the series photocatalysts, the photocatalytic performances of as-prepared samples were examined through the degradation of Microcystis aeruginosa under visible light irradiation. Moreover, the photocatalytic mechanism of the b-N-TiO2/C is also investigated.
2. Results and Discussion
2.1. Characterization of Photocatalysts
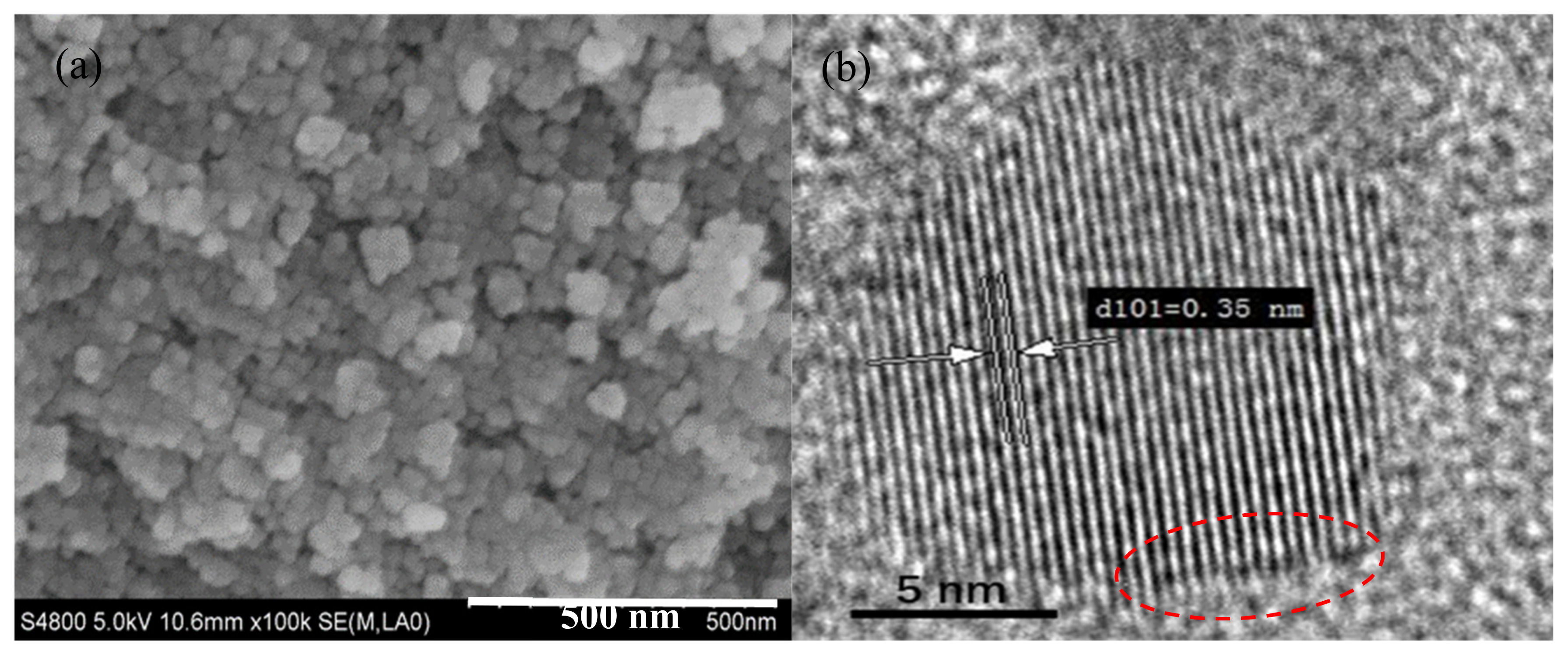
The morphology and microstructure of 0.6 N are characterized by SEM and TEM. As observed from SEM image of Figure 1a, the nanoparticles have near-spherical morphology with an average particle size of 12.08 ± 3.33 nm (as shown in Figure S2). Figure 1b displays a high-resolution TEM image of 0.6 N, and the lattice fringe spacing of 0.35 nm corresponds to the (101) planes of anatase TiO2 [20]. Compared to the pristine TiO2 nanoparticles, the morphology and microstructure seem unchanged, which may be due to the small amount of incorporated nitrogen [21]. Notably, the surface ordered shell can be observed clearly and is marked by the red line in Figure 1b, indicating that there are small surface defects after N2 calcination.
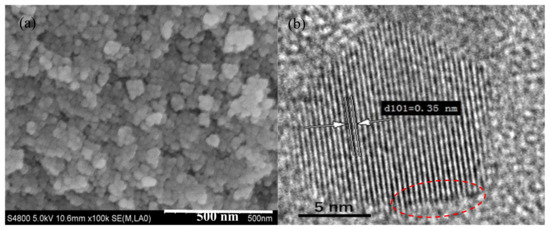
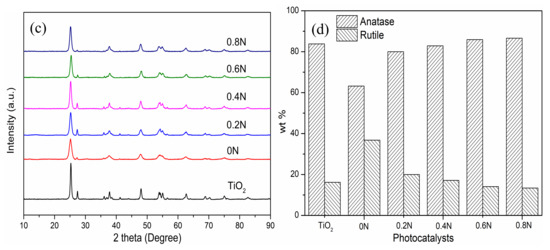
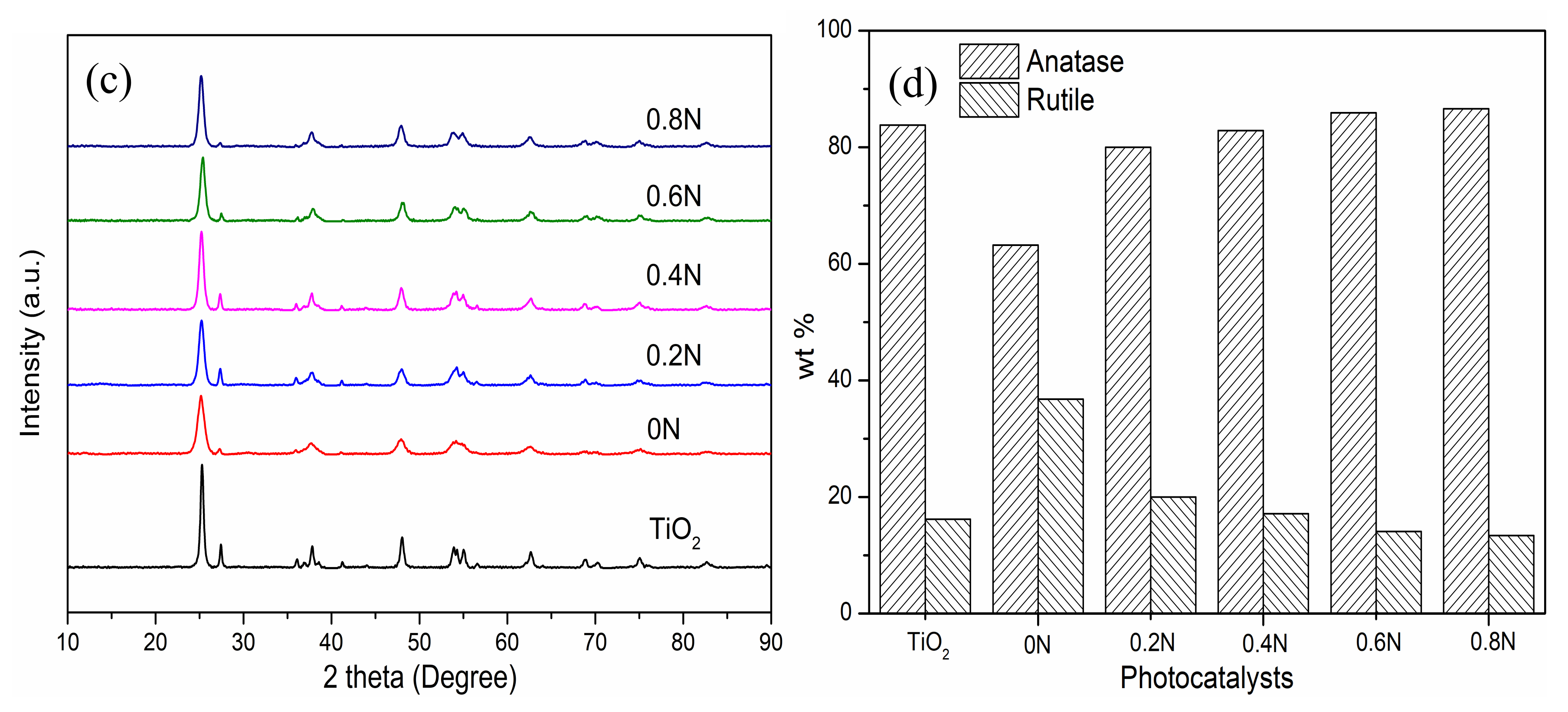
Figure 1.
(a) SEM and (b) high-resolution TEM images of 0.6N; (c) XRD patterns and (d) phase compositions of different samples of 0–0.8 N.
XRD patterns are carried out to analyze the crystallinity and crystal phase of the as-prepared samples with the addition of different amounts of urea. As shown in Figure 1c, the diffraction peaks can be well-indexed for anatase (JCPDS #21-1272) and rutile (JCPDS #21-1276) TiO2 phases, and the anatase phase is the dominant composition. Nevertheless, compared with pure TiO2 calcined in air, the doped TiO2 samples exhibit a relatively low intensity and extension of diffraction peaks, which can be ascribed to the production of Ti3+ and oxygen vacancies after incorporation [22]. Notably, the different composition percentages of anatase and rutile phase indicate the successful incorporation of nitrogen. The wt % of anatase and rutile phase TiO2 in different samples can be investigated from Figure 1d. The wt % of anatase phase is 83.8%, −63.2%, 80.0%, 82.9%, 85.9% and 86.6% in the samples of pure TiO2, 0 N, 0.2 N, 0.4 N, 0.6 N and 0.8 N, respectively. It can be concluded that the wt % of anatase phase increases with the addition of more urea, which contradicts the other report. Wei et al. reported that no phase transition occurred after the addition of more urea, and only the anatase phase existed in black TiO2 [17]. In this study, after investigating the black TiO2 samples calcined in N2 atmosphere, it is found that the addition of urea is beneficial for anatase-to-rutile phase transition, which may be due to the production of oxygen vacancies [23]. Comparing the pure TiO2 with 0N, the N2 atmosphere calcination is beneficial in transforming rutile to anatase, decreasing the wt % of anatase phase in 0 N. Meanwhile, the average crystallite sizes of as-prepared TiO2 samples were calculated based on the (101) Bragg diffraction using the Scherrer equation. The average crystallite sizes are 19.60, 12.24, 13.20, 14.68, 14.37, and 14.03 nm for the samples of pure TiO2, 0 N, 0.2 N, 0.4 N, 0.6 N and 0.8 N, respectively. On the one hand, comparing the pure TiO2 with 0N, the N2 calcination could reduce the crystallite size of TiO2. On the other hand, comparing the doped TiO2 samples calcined in N2 atmosphere, the crystallite sizes decrease at first and then increase after the addition of 0.6 g of urea. Our results show that both the calcination atmosphere and urea addition affect the phase transition and crystallite sizes in preparing TiO2 samples, which are related to the production of oxygen vacancies.
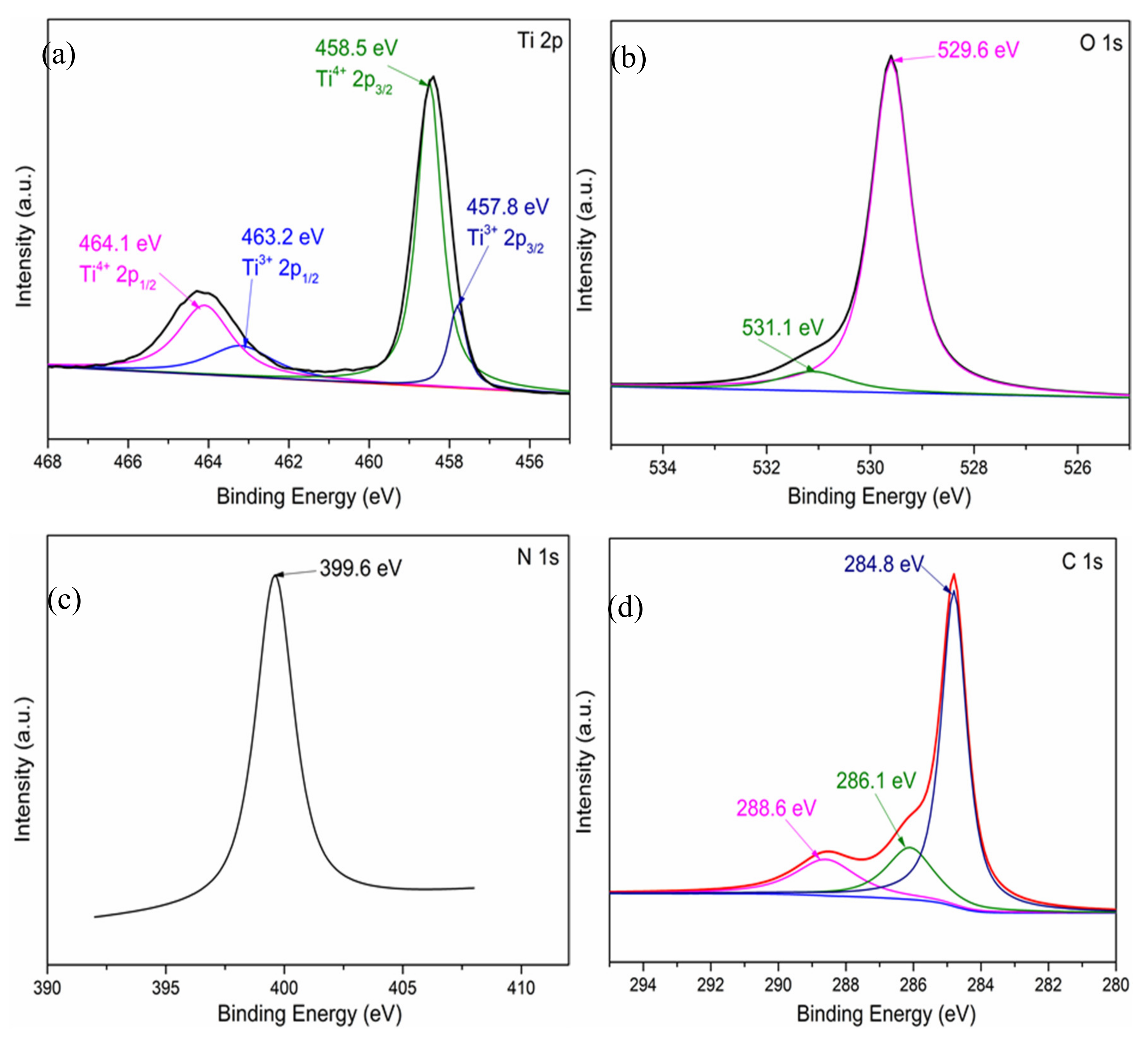
XPS was used to investigate the surface chemical composition and chemical states of elements of 0.6N. The XPS analysis of Ti 2p, O 1s, N 1s, and C 1s of the sample 0.6N are shown in Figure 2. In the Ti 2p XPS spectrum of 0.6 N (Figure 2a), the Ti 2p band can be resolved into four peaks located at 464.1, 463.2, 458.5 and 457.8 eV, referred to Ti4+ 2p1/2, Ti3+ 2p1/2, Ti4+ 2p3/2, and Ti3+ 2p3/2, respectively [24], implying that Ti3+ is created as a result of the element incorporation [19]. In the high-resolution XPS spectrum of O 1s (Figure 2b), the O 1s peak can be fitted into two peaks at 529.6 and 531.1 eV, which are attributed to Ti-O and OH, respectively [25]. Figure 2c shows the high-resolution XPS spectrum of N 1s. The main peak in binding energy of 399.6 eV can be attributed to the presence of substitutional N in the bond of O-Ti-N. No peaks at 400.9 eV are detected, indicating that there is no existence of interstitial N in Ti-O-N [26], which is in agreement with the analysis of O 1s. Curve fitting analysis of the C 1s XPS spectra affords three peaks at 284.8, 286.1 and 288.6 eV, respectively (Figure 2d). The two peaks at 286.1 and 288.6 eV are ascribed to the existence of C-O and C=O bonds, respectively [21]. The peak at 284.8 eV (around 285 eV) is assigned to the sp2 hybridized carbon [19] due to the organic precursor. No C 1s peak at 281 eV, corresponding to the Ti-C bond, was detected, implying that carbon does not dope into the TiO2 lattice [18]. Therefore, it can be proved that a carbonaceous layer was formed on the surface of TiO2 via Ti-O-C and Ti-O-CO bonds [27]. In addition, the amounts of doped elements are also confirmed by XPS and the atomic percent of Ti, C, N, and O are 29.93%, 10.67%, 1.37%, and 58.03%, respectively. The atomic percent of elements proved the existence of substitutional N in O-Ti-N, and no existence of substitutional C.

Figure 2.
High-resolution XPS spectrum of (a) Ti 2p, (b) O 1s, (c) N 1s, and (d) C 1s of 0.6 N.
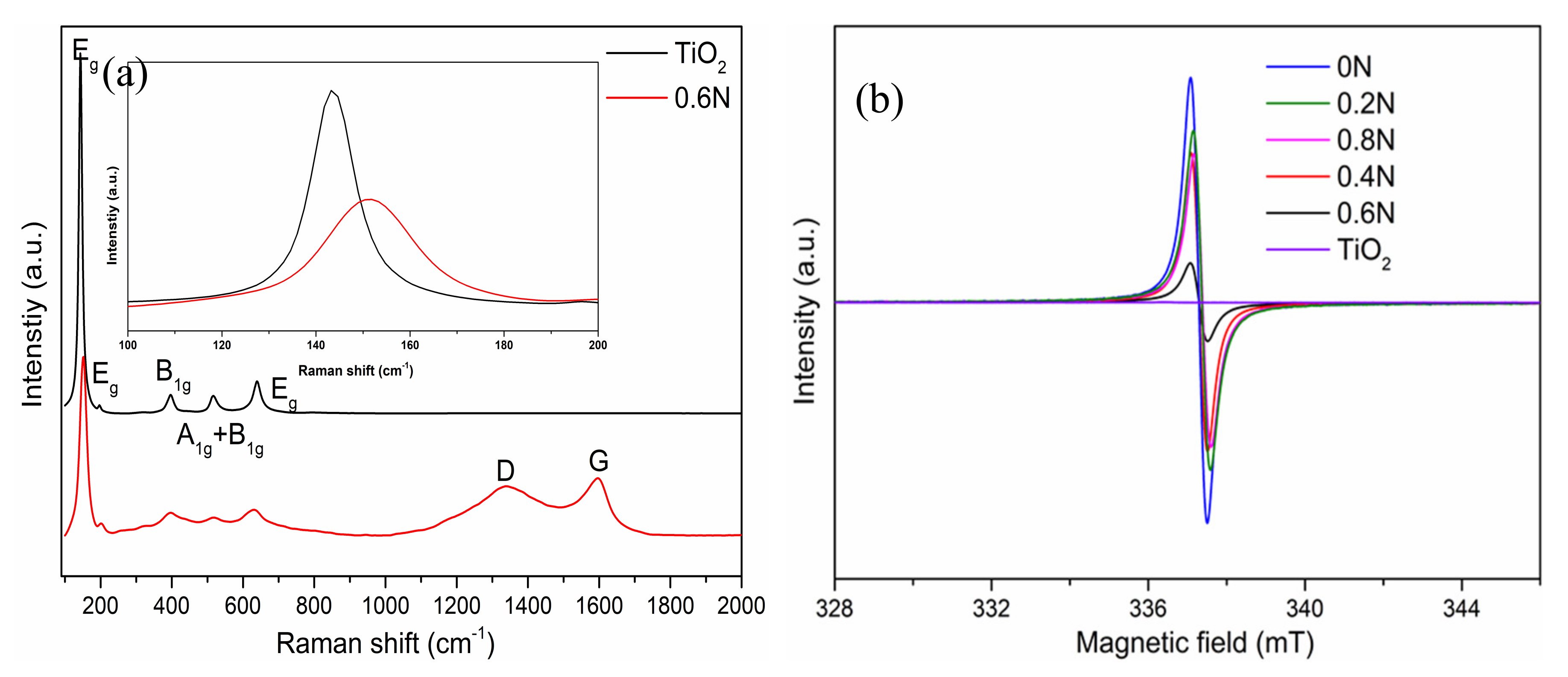
Raman spectroscopy was applied to distinguish the phase of titania and the existence of carbon. The corresponding Raman spectra of TiO2 and 0.6N are shown in Figure 3a. For the sample TiO2, there are three Eg peaks (145, 199, 640 cm−1), one B1g peak (399 cm−1), and the A1g + B1g modes at around 517 cm−1, which are the typical optical modes of anatase [28]. For the sample 0.6 N, the six active modes (3Eg + 2B1g + A1g) indicate the dominant anatase phase TiO2. However, as shown in the inset of Figure 3a, the most evident Eg peak of 0.6 N becomes wider and a blue shift, and this is due to the structural disorder which is considered to be associated with oxygen defects caused by N substitution [25,29]. In addition, there are two other important bands (D and G) located at about 1339 and 1593 cm−1, respectively, which correspond to the frequencies of stretching and breathing modes of carbon. Raman spectral information corroborated well with the XRD and XPS results. In order to prove the existence of oxygen vacancies, the EPR signals are shown in Figure 3b to represent the defect amounts using a paramagnetic spin. The sample TiO2 shows no peak in paramagnetic signals, indicating the absence of defects in the sample TiO2. The single electrons trapped by oxygen vacancies can be corresponded to the EPR signals at g = 2.001~2.005. In this study, the g value is 2.002, which confirms the formation of oxygen defects in the samples 0–0.6 N [30,31] Notably, the intensities of paramagnetic signals are different from the different amounts of urea addition, implying different amounts of oxygen vacancies. The sample 0 N has the most and 0.6 N has the least oxygen vacancies.

Figure 3.
(a) Raman spectra of pure TiO2 and 0.6N, and amplification of the evident Eg peak; (b) EPR signals of as-prepared TiO2 samples.
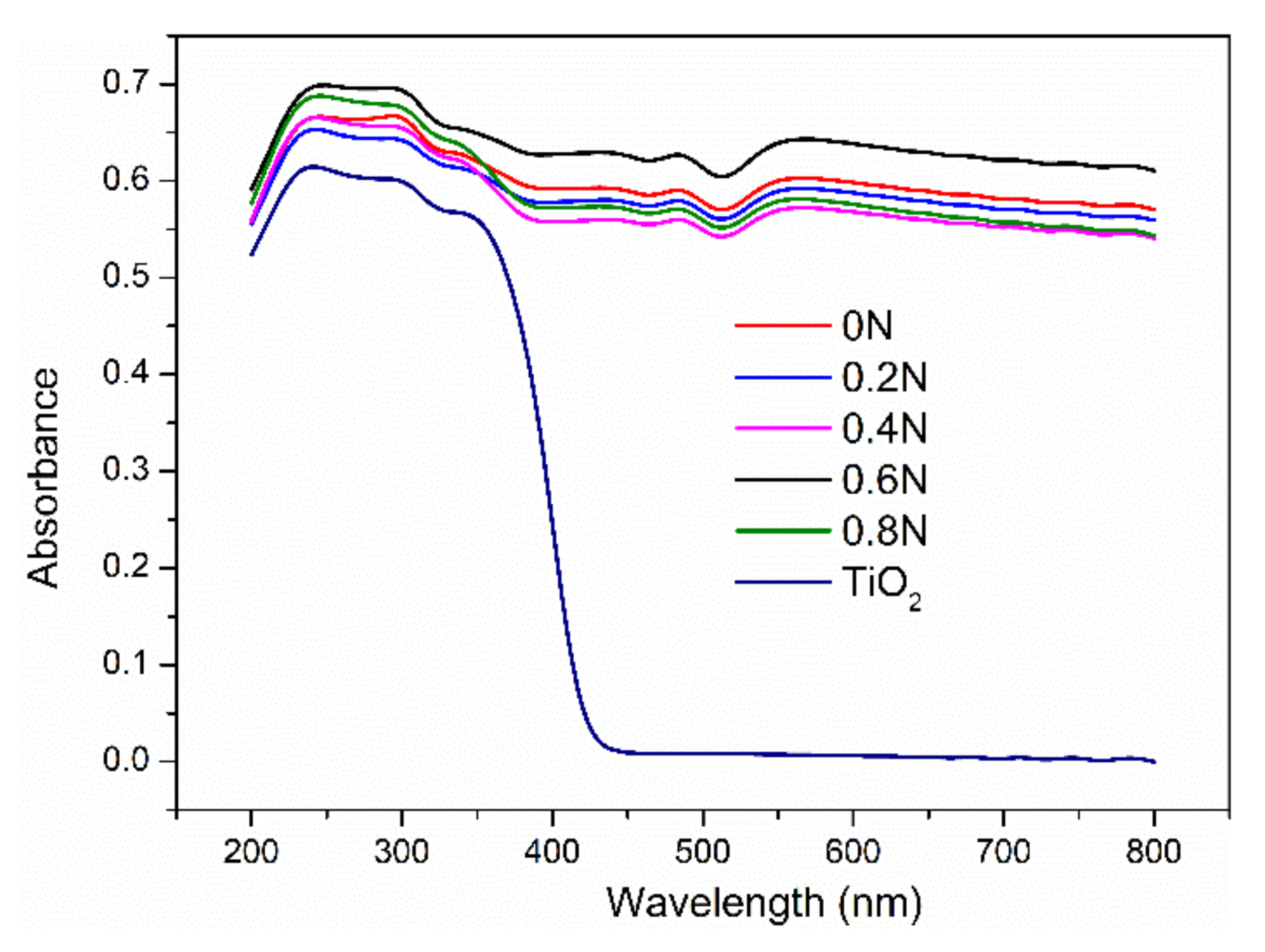
UV-vis diffuse reflectance spectra of as-prepared samples are shown in Figure 4 to evaluate the light absorption. It is clearly that the pure TiO2 mainly exhibits strong absorption in the ultraviolet (UV) region, while the black TiO2 can absorb both UV and visible light. With the addition of urea, the samples exhibit different UV and visible light absorption abilities. In the UV region, the absorption abilities are in an order of 0.6 N >0.8 N >0 N >0.4 N >0.2 N. In the visible light region, the absorption abilities are in an order of 0.6 N>0 N>0.2>0.8 N>0.4 N. The sample 0.6N exhibits the strongest absorption in both the UV and visible light regions, which is attributed to the doped nitrogen and oxygen vacancies narrowing the band gap of TiO2 [32,33]. Notably, it is not the sample of 0N with the most oxygen vacancies (as shown in Figure 3b) that has the strongest absorption in the visible light region. Even though the existence of oxygen vacancies could break the selection rule for indirect transitions of TiO2 and improve absorption for photon energy [34], the proper amount of oxygen vacancies is needed to improve the visible light absorption ability and photocatalytic activity of the photocatalyst. Moreover, the modified carbon on the surface of TiO2 acts as a sensitizer to absorb visible light and reduce the refection of light [18,35], which is another reason for enhancing the visible light absorption of black TiO2.

Figure 4.
UV-vis diffuse reflectance spectra.
2.2. Photocatalytic Performance
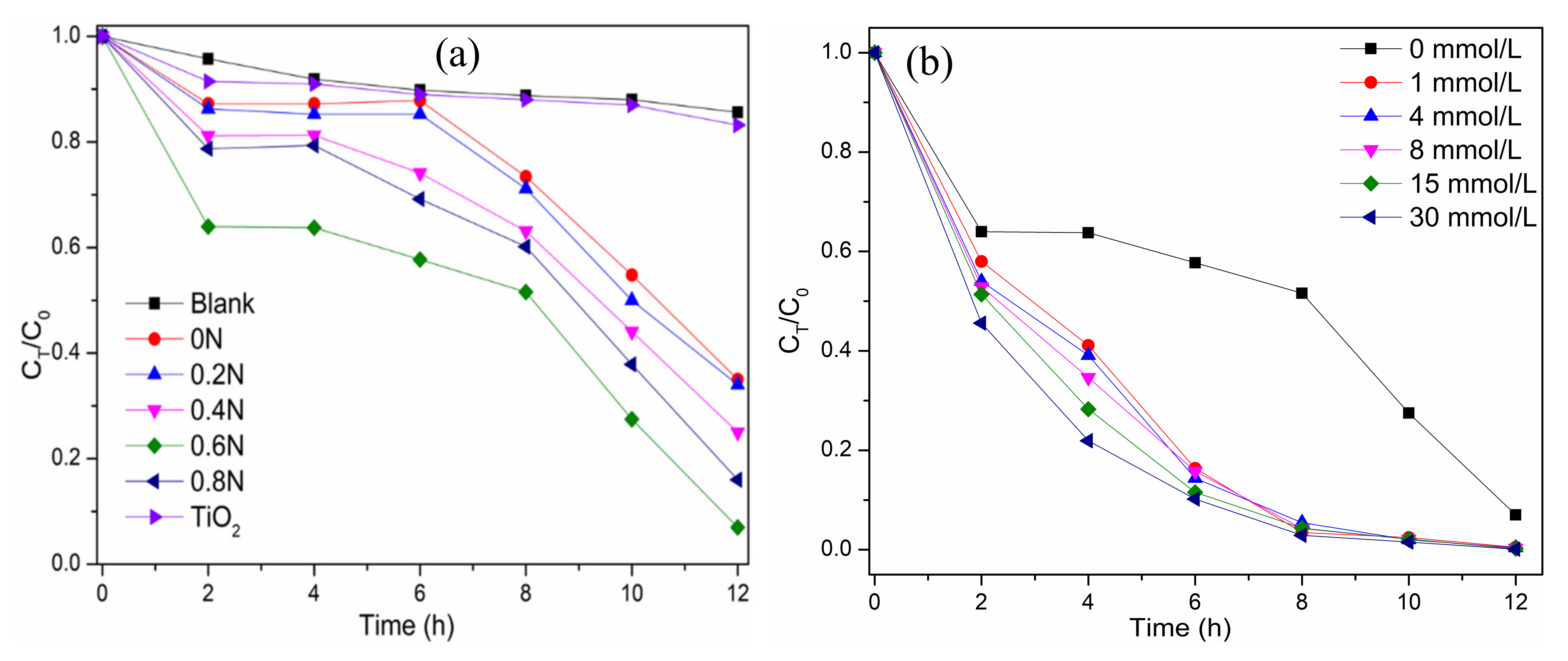
Figure 5a shows the chlorophyll-a removal in Microcystis aeruginosa cells under visible light irradiation using the as-prepared photocatalysts. The pure TiO2 shows a low photocatalytic degradation of chlorophyll-a due to the wide bandgap for weak visible light absorption. As shown in Figure 5a, the black TiO2 samples exhibit different removal rates of chlorophyll-a under visible light irradiation, which are 64.9, 65.8, 75.1, 92.7 and 83.8% for 0 N, 0.2 N, 0.4 N, 0.6 N, and 0.8 N, respectively. The sample 0.6 N has the highest photocatalytic activity to degrade chlorophyll-a. All the black TiO2 samples exhibit a similar trend to degrade chlorophyll-a, which comprises three phases, including rapid decrease-lag phase-rapidly decrease. In the first phase of 2 h, the adsorption of cells on the photocatalyst occurs, and then the cells are damaged to release chlorophyll-a in the second phase of 2–8 h. In the third phase, after 8 h irradiation, the oxidation of released chlorophyll-a is the main process. The efficiency of degrading chlorophyll-a is not very high for black TiO2 due to the lag phase from 2 to 8 h. The addition of H2O2 can enhance the degradation, avoiding the lag phase (as shown in Figure 5b). The addition of H2O2 in the range of 1–30 mmol/L can promote the degradation process, and all the removal rates of chlorophyll-a reach more than 90.0% after 8 h of visible light irradiation with a different concentration of H2O2. The first order kinetic constants during 2–8 h are 0.037 h−1 for only 0.6 N, and 0.394–0.469 h−1 for both 0.6 N and H2O2 (as shown in Figure S3). With the addition of H2O2, the reaction rate is more than 10 times that of only photocatalyst in the system. The enhancement of efficiency by H2O2 results from the produced OH by H2O2 photolysis and the increased separation efficiency of electrons and holes by the capture of electrons by H2O2 [36]. The photocatalytic degradation of chlorophyll-a in algae cells remained almost the same in the three successive experimental runs by 0.6 N (as shown in Figure S4). It can prove the high photocatalytic stability of 0.6 N during the application process.
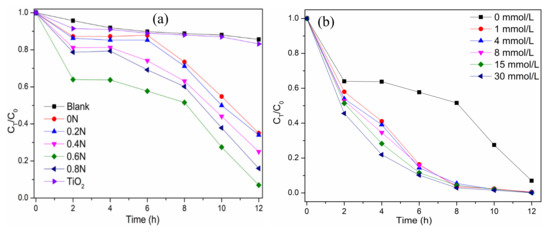
Figure 5.
(a) The degradation curves of chlorophyll-a in algae cells on different TiO2 samples under visible light irradiation; (b) Effect of H2O2 concentrations on the degradation of chlorophyll-a in algae cells over 0.6 N.
2.3. Photocatalytic Mechanism
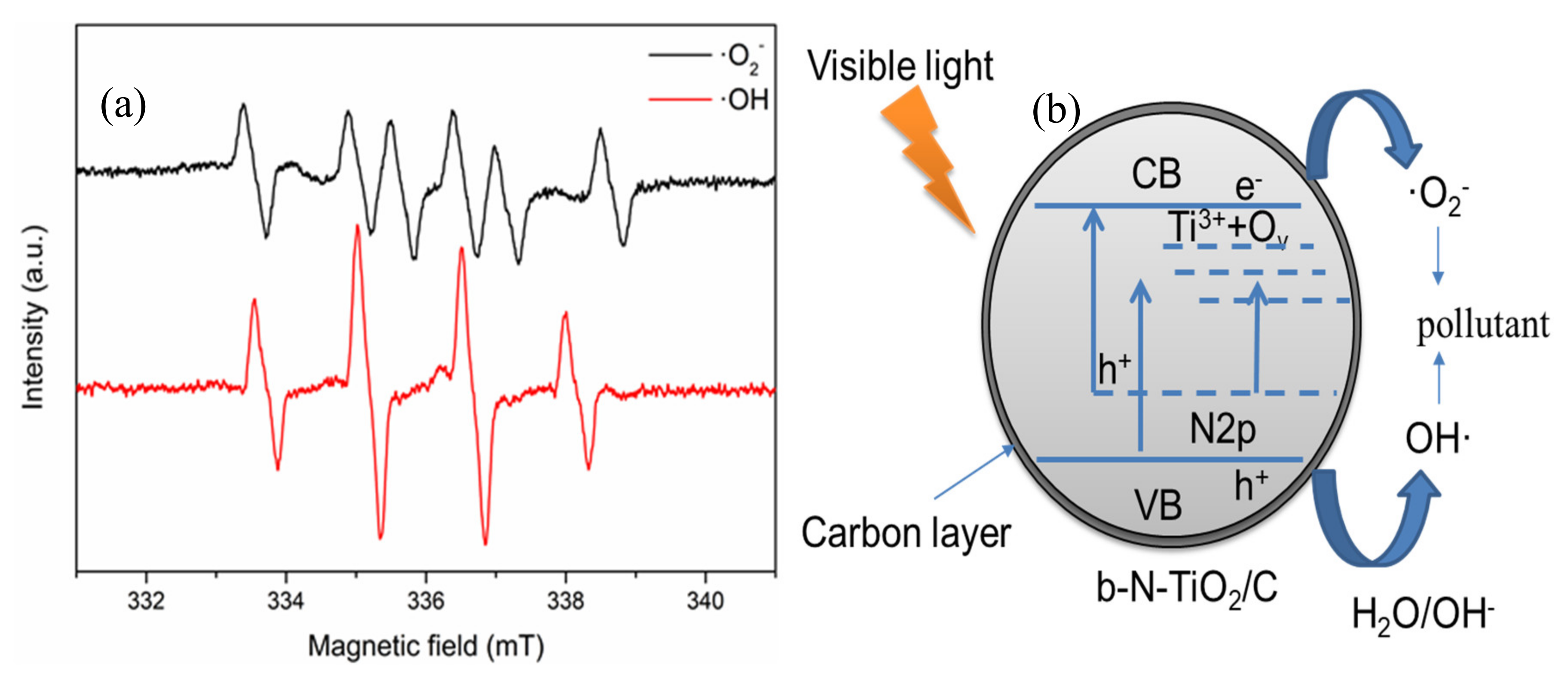
The reactive oxygen species trapping experiments as well as the ESR experiments were conducted to identify the main active species. In the Figure S5, the addition of benzoquinone as ·O2− scavenger and isopropanol as ·OH scavenger suppresses the degradation of chlorophyll-a, especially after 8 h of irradiation. The photodegradation rate of chlorophyll-a is slightly inhibited by the addition of EDTA-Na2 as a hole scavenger. This indicates that photogenerated·O2− and OH are the dominant active species in black TiO2 photocatalysis. To confirm the existence of active species, ESR spectra are shown using DMPO as spin-trap reagent in the methanol solution (for DMPO-·O2−) and the aqueous solution (for DMPO-·OH) (as shown in Figure 6a). The characteristic peaks in DMPO-·O2− and DMPO-·OH appeared in 0.6N dispersion under visible light irradiation for 5 min [37].
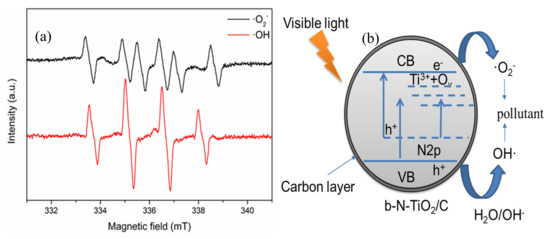
Figure 6.
(a) ESR spectra of radical adducts trapped by 5,5-dimethyl-1-pyrroline N-oxide (DMPO) in 0.6N dispersion under visible light irradiation for 5 min; (b) Schematic illustration of the proposed mechanism for the degradation of pollutants by b -N-TiO2/C under visible light irradiation.
According to the above analysis, a possible mechanism over b-N-TiO2/C is proposed in Figure 6b. It is widely accepted that the overlap of O 2p and N 2p or a new nitrogen doping level can be formed due to the nitrogen doped in the TiO2 lattice [38]. On the other hand, N doping causes the formation of Ti3+ and oxygen vacancies, which induce localized states (Ti3+ and OV) below the conduction band (CB) minimum [39,40]. The doped energy bands can not only narrow the bandgap but also improve the visible-light absorption ability. Under visible light irradiation, the electrons existing in the VB and N 2p levels could be excited by the CB or defect states. The defect states as electron acceptors can increase the driving force of electron transfer [41]. The excited electrons can reduce O2 to O2− and oxide pollutants. Meanwhile, the remaining holes in the VB can react with H2O or OH− to generate OH radicals to further degrade pollutants. Nevertheless, the holes existing in the local N 2p states have low mobility due to the highly localized characteristic, causing low oxidative activity [42]. Moreover, the carbon on the surface can promote the separation of the photogenerated electrons and holes and keep them highly reactive [19,27].
3. Materials and Methods
3.1. Chemicals and Materials
All chemicals, tetrabutyl titanate (≥98%), hydrochloric acid (36.0%–38.0%), urea (≥99.5%), hydrogen peroxide (30%), and absolute ethanol (≥99.7%), used in the experiments were purchased from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China) and were used without purification. Microcystis aeruginosa (FACHB-905) was provided by the Freshwater Algae Culture Collection at the institute of Hydrobiology (Wuhan, China). The algae were cultured in BG11 medium at 25 ℃ under a circadian (12 h light: 12 h dark).
3.2. Preparation of Photocatalysts
A set amount of urea was mixed completely with 20 mL absolute ethanol and 14 mL tetrabutyl titanate to form solution A; solution B contained 10 mL absolute ethanol, 1 mL hydrochloric acid, and 5 mL distilled water. Solution B was added dropwise to solution A without stirring. The white colloid formed was maintained in a water bath at 35 °C for 30 min and then dried overnight at 80 °C and ground into a powder. Finally, the white powder was calcinated at 500 °C in N2 for 3 h. The samples with 0, 0.2, 0.4, 0.6, and 0.8 g urea addition were labeled as 0 N, 0.2 N, 0.4 N, 0.6 N, and 0.8 N, respectively. The pure TiO2 was prepared according to the same procedures without the addition of urea and with calcination in air.
3.3. Photocatalytic Degradation
To examine the photocatalytic activities of as-prepared TiO2 samples, the photocatalytic degradation of algae cells was carried out in a multi-position photocatalytic reactor (CEL-LAB500). The reactor (as shown in Figure S1) was purchased from Beijing China Education Au-light Co. Ltd., Beijing, China. In the process of the photocatalytic experiment, the conditions were maintained as follows: the visible-light intensity in the range of 400–1000 nm was 36.7 mW/cm2, the initial algae concentration was ~3.0 × 106 cells/mL and the corresponding optical density (OD) at 680 nm was ~0.2, and the photocatalyst concentration was 0.2 g/L. During the photocatalytic process, a certain amount of samples was taken at a predetermined time to measure the concentration of chlorophyll-a after extraction. The extracting agent is ethanol (95%, v/v) and the measuring wavelengths of the spectrophotometer are 649 and 665 nm. For the photocatalysis experiment with H2O2, the same experimental procedures were followed with the addition of a different concentration of H2O2 (1–30 mmol/L). In the trapping experiment, 1 mmol/L isopropanol, benzoquinone, and disodium ethylenediaminetetraacetate (EDTA-Na2) were employed to act as scavengers of·OH,·O2− and h+, respectively.
3.4. Analytical Methods
The morphology was characterized by a field emission scanning electron microscope (SEM, JEOL-JSM-6700, Tokyo, Japan) and a transmission electron microscope (TEM, JEOL-JEM-2100, Tokyo, Japan) with an accelerating voltage of 200 kV. The crystal structures were measured by an X-ray diffraction (XRD-D8, Bruker, Karlsruhe, Germany) with Cu-Ka radiation source (λ = 0.15406 nm) in the range 10–90° at 40 kV and 40 mA. X-ray photoelectron spectroscopy (XPS) was conducted by an upgraded RBD PHI-5000C ESCA system (PerkinElmer, Waltham, MA, USA) with Mg-Ka radiation (h = 1253.6 eV). The Raman spectra were recorded in a high-spectral-resolution confocal Raman Microscope (HORIBA LabRam HR Evolution) equipped with a 514 nm laser excitation source. UV-vis DRS in the range of 200-800 nm was recorded by a LAMBDA 950 spectrometer (PerkinElmer) with an integrating sphere and BaSO4 used as a reference. Electron paramagnetic resonance (EPR) spectra were obtained using a Bruker E500 spectra at room temperature with a resonance frequency of 9.43 GHz and microwave power of 1.0 mW. The electron spin resonance (ESR) signals of radicals spin-trapped by spin-trap reagent 5,5-dimethyl-1-pyrroline N-oxide (DMPO) were recorded at 77 K with an ESR spectrometer (Bruker E500).
4. Conclusions
In summary, black TiO2 with doped nitrogen and modified carbon (b-N-TiO2/C) were successfully prepared by a one-step sol-gel method in the presence of urea as a source of nitrogen and carbon. It is proved that the nitrogen can substitute oxygen and form Ti3+ and oxygen vacancies. The carbon was modified on the surface of black TiO2. The doped nitrogen and modified carbon can enhance the visible light absorption and separation of photogenerated electrons and holes. The sample 0.8N had the highest wt % of anatase phase, and the sample 0.6N exhibited the best light absorption ability. It is the sample 0.6N that showed the highest photocatalytic activity to degrade chlorophyll-a in algae cells, and the addition of H2O2 promoted the degradation process, avoiding the lag phase. In the process of degrading chlorophyll-a by b-N-TiO2/C, the ·OH and ·O2− are the dominant reactive species, which has been proved by the results of trapping experiments and ESR spectra. Photocatalytic oxidation is one of the technologies with the highest potential to remove harmful algae in the aquatic environment. It is crucial to synthesize effective photocatalysts using a one-step method for large-scale application. The photocatalyst b-N-TiO2/C may provide new insights into harmful algae inactivation in practical applications.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/10/5/579/s1, Figure S1: a multi-position photochemical reactor with a xenon lamp, UV-cutoff filters, cooling system, and magnetic stirrers., Figure S2: particle size distribution of 0.6N, Figure S3: variations of ln(C0/CT) versus visible light irradiation time with different concentration of H2O2, Figure S4: photocatalytic degradation of chlorophyll-a in algae cells for three times by 0.6N, Figure S5: trapping experiments of reactive oxygen species for the sample 0.6N in the process to degrade chlorophyll-a in algae cells.
Author Contributions
Data curation, writing—original draft preparation, X.Z. and M.C.; conceptualization, supervision, funding acquisition, G.Z. and G.C., investigation, methodology, N.C. and L.Z., writing—review and editing, G.Z. and L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Shanghai Municipal Agricultural Commission (Grant No. 20180126), Shanghai Municipal Science and Technology Commission (Grant No. 19295801000) and the State Key Laboratory of Pollution Control and Resource Reuse Foundation (Grant No. PCRRF18001).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Paerl, H.W.; Gardner, W.S.; Havens, K.E.; Joyner, A.R.; McCarthy, M.J.; Newell, S.E.; Qin, B.; Scott, J.T. Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae 2016, 54, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, X.; Zhao, J.; Song, J.; Su, C.; Wang, Z. Surface modified TiO2 floating photocatalyst with PDDA for efficient adsorption and photocatalytic inactivation of Microcystis aeruginosa. Water Res. 2018, 131, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wang, X.; Ma, J.; Wang, X.; Wang, J.; Zhao, J. Visible-light-driven in situ inactivation of Microcystis aeruginosa with the use of floating g-C3N4 heterojunction photocatalyst: Performance, mechanisms and implications. Appl. Catal. B Environ. 2018, 226, 83–92. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Zhao, J.; Song, J.; Zhou, L.; Ma, R.; Wang, J.; Tong, X.; Chen, Y. Efficient visible light-driven in situ photocatalytic destruction of harmful alga by worm-like N,P co-doped TiO2/expanded graphite carbon layer (NPT-EGC) floating composites. Catal. Sci. Technol. 2017, 7, 2335–2346. [Google Scholar] [CrossRef]
- Wang, X.; Song, J.; Zhao, J.; Wang, Z.; Wang, X. In-situ active formation of carbides coated with NP-TiO2 nanoparticles for efficient adsorption-photocatalytic inactivation of harmful algae in eutrophic water. Chemosphere 2019, 228, 351–359. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, S.; Xu, H.; Ma, C.; Sun, J.; Li, H.; Pei, H. Application of N-TiO2 for visible-light photocatalytic degradation of Cylindrospermopsis raciborskii—More difficult than that for photodegradation of Microcystis aeruginosa? Environ. Pollut. 2019, 245, 642–650. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, K.; Liu, X.; Shi, Y. High efficiency inactivation of microalgae in ballast water by a new proposed dual-wave UV-photocatalysis system (UVA/UVC-TiO2). Environ. Sci. Pollut. R. 2019, 26, 7785–7792. [Google Scholar] [CrossRef]
- Chen, X.; Burda, C. The Electronic Origin of the Visible-Light Absorption Properties of C-, N- and S-Doped TiO2 Nanomaterials. J. Am. Chem. Soc. 2008, 130, 5018–5019. [Google Scholar] [CrossRef]
- Miao, X.; Yue, X.; Ji, Z.; Shen, X.; Zhou, H.; Liu, M.; Xu, K.; Zhu, J.; Zhu, G.; Kong, L.; et al. Nitrogen-doped carbon dots decorated on g-C3N4/Ag3PO4 photocatalyst with improved visible light photocatalytic activity and mechanism insight. Appl. Catal. B Environ. 2018, 227, 459–469. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Wang, W.; Tade, M.O.; Shao, Z. Nitrogen-doped simple and complex oxides for photocatalysis: A review. Prog. Mater. Sci. 2018, 92, 33–63. [Google Scholar] [CrossRef]
- Cao, Y.; Xing, Z.; Shen, Y.; Li, Z.; Wu, X.; Yan, X.; Zou, J.; Yang, S.; Zhou, W. Mesoporous black Ti3+/N-TiO2 spheres for efficient visible-light-driven photocatalytic performance. Chem. Eng. J. 2017, 325, 199–207. [Google Scholar] [CrossRef]
- Zhang, K.; Park, J.H. Surface Localization of Defects in Black TiO2: Enhancing Photoactivity or Reactivity. J. Phys. Chem. Lett. 2017, 8, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, H.; Hashimoto, M.; Shiraishi, Y.; Hirai, T. Photocatalytic Conversion of Nitrogen to Ammonia with Water on Surface Oxygen Vacancies of Titanium Dioxide. J. Am. Chem. Soc. 2017, 139, 10929–10936. [Google Scholar] [CrossRef]
- Gómez-Polo, C.; Larumbe, S.; Monge, M. Room temperature ferromagnetism and absorption red-shift in nitrogen-doped TiO2 nanoparticles. J. Alloys Compd. 2014, 612, 450–455. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, S.; Zhang, S.; Wang, R.; Wang, K. Preparation and visible-light photocatalytic activity of N-doped TiO2 by plasma-assisted sol-gel method. Catal. Today 2019, 337, 37–43. [Google Scholar] [CrossRef]
- Wei, S.; Wu, R.; Xu, X.; Jian, J.; Wang, H.; Sun, Y. One-step synthetic approach for core-shelled black anatase titania with high visible light photocatalytic performance. Chem. Eng. J. 2016, 299, 120–125. [Google Scholar] [CrossRef]
- Jia, F.; Yao, Z.; Jiang, Z.; Li, C. Preparation of carbon coated TiO2 nanotubes film and its catalytic application for H2 generation. Catal. Commun. 2011, 12, 497–501. [Google Scholar] [CrossRef]
- Wang, D.-H.; Jia, L.; Wu, X.-L.; Lu, L.-Q.; Xu, A.-W. One-step hydrothermal synthesis of N-doped TiO2/C nanocomposites with high visible light photocatalytic activity. Nanoscale 2012, 4, 576–584. [Google Scholar] [CrossRef]
- Liu, X.; Xing, Z.; Zhang, Y.; Li, Z.; Wu, X.; Tan, S.; Yu, X.; Zhu, Q.; Zhou, W. Fabrication of 3D flower-like black N-TiO2-x@MoS2 for unprecedented-high visible-light-driven photocatalytic performance. Appl. Catal. B Environ. 2017, 201, 119–127. [Google Scholar] [CrossRef]
- Yan, X.; Xing, Z.; Cao, Y.; Hu, M.; Li, Z.; Wu, X.; Zhu, Q.; Yang, S.; Zhou, W. In-Situ C-N-S-tridoped single crystal black TiO2 nanosheets with exposed {001} facets as efficient visible-light-driven photocatalysts. Appl. Catal. B Environ. 2017, 219, 572–579. [Google Scholar] [CrossRef]
- Hu, M.; Xing, Z.; Cao, Y.; Li, Z.; Yan, X.; Xiu, Z.; Zhao, T.; Yang, S.; Zhou, W. Ti3+ self-doped mesoporous black TiO2/SiO2/g-C3N4 sheets heterojunctions as remarkable visible-lightdriven photocatalysts. Appl. Catal. B Environ. 2018, 226, 499–508. [Google Scholar] [CrossRef]
- Nolan, N.T.; Synnott, D.W.; Seery, M.K.; Hinder, S.J.; Van Wassenhoven, A.; Pillai, S.C. Effect of N-doping on the photocatalytic activity of sol-gel TiO2. J. Hazard. Mater. 2012, 211, 88–94. [Google Scholar] [CrossRef]
- Kumar, R.; Govindarajan, S.; Siri Kiran Janardhana, R.K.; Rao, T.N.; Joshi, S.V.; Anandan, S. Facile One-Step Route for the Development of in Situ Cocatalyst-Modified Ti3+ Self-Doped TiO2 for Improved Visible-Light Photocatalytic Activity. ACS Appl. Mater. Inter. 2016, 8, 27642–27653. [Google Scholar] [CrossRef] [PubMed]
- Naldoni, A.; Allieta, M.; Santangelo, S.; Marelli, M.; Fabbri, F.; Cappelli, S.; Bianchi, C.L.; Psaro, R.; Dal Santo, V. Effect of Nature and Location of Defects on Bandgap Narrowing in Black TiO2 Nanoparticles. J. Am. Chem. Soc. 2012, 134, 7600–7603. [Google Scholar] [CrossRef] [PubMed]
- Asahi, R.; Morikawa, T. Nitrogen complex species and its chemical nature in TiO2 for visible-light sensitized photocatalysis. Chem. Phys. 2007, 339, 57–63. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, X.; Wang, X.; Zhang, Y.; Wei, W.; Sun, Y.; Antonietti, M.; Titirici, M.-M. One-Step Solvothermal Synthesis of a Carbon@TiO2 Dyade Structure Effectively Promoting Visible-Light Photocatalysis. Adv. Mater. 2010, 22, 3317–3321. [Google Scholar] [CrossRef]
- Zhou, W.; Sun, F.; Pan, K.; Tian, G.; Jiang, B.; Ren, Z.; Tian, C.; Fu, H. Well-Ordered Large-Pore Mesoporous Anatase TiO2 with Remarkably High Thermal Stability and Improved Crystallinity: Preparation, Characterization, and Photocatalytic Performance. Adv. Funct. Mater. 2011, 21, 1922–1930. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef]
- Zhang, S.; Li, W.; Jin, Z.; Yang, J.; Zhang, J.; Du, Z.; Zhang, Z. Study on ESR and inter-related properties of vacuum-dehydrated nanotubed titanic acid. J. Solid State Chem. 2004, 177, 1365–1371. [Google Scholar] [CrossRef]
- Lin, Z.A.; Lu, W.C.; Wu, C.Y.; Chang, K.S. Facile fabrication and tuning of TiO2 nanoarchitectured morphology using magnetron sputtering and its applications to photocatalysis. Ceram. Int. 2014, 40, 15523–15529. [Google Scholar] [CrossRef]
- Di Valentin, C.; Pacchioni, G.; Selloni, A. Origin of the different photoactivity of N-doped anatase and rutile TiO2. Phys. Rev. B 2004, 70, 085116. [Google Scholar] [CrossRef]
- Li, D.; Ohashi, N.; Hishita, S.; Kolodiazhnyi, T.; Haneda, H. Origin of visible-light-driven photocatalysis: A comparative study on N/F-doped and N-F-codoped TiO2 powders by means of experimental characterizations and theoretical calculations. J. Solid State Chem. 2005, 178, 3293–3302. [Google Scholar] [CrossRef]
- Zuo, F.; Wang, L.; Wu, T.; Zhang, Z.; Borchardt, D.; Feng, P. Self-Doped Ti3+ Enhanced Photocatalyst for Hydrogen Production under Visible Light. J. Am. Chem. Soc. 2010, 132, 11856–11857. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Cai, W.; Long, M.; Zhou, B.; Wu, Y.; Wu, D.; Feng, Y. Synthesis of Visible-Light Responsive Graphene Oxide/TiO2 Composites with p/n Heterojunction. ACS Nano 2010, 4, 6425–6432. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Xu, J.; Bai, X.; Zong, R.; Zhu, Y. Degradation and mineralization mechanism of phenol by BiPO4 photocatalysis assisted with H2O2. Appl. Catal. B Environ. 2013, 142–143, 561–567. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, K.; Feng, Y.; Chang, Y.; Yang, T.; Xuan, Y.; Lei, D.; Lou, L.-L.; Liu, S. Novel 3DOM-SrTiO3/Ag/Ag3PO4 ternary Z-scheme photocatalysts with remarkably improved activity and durability for contaminant degradation. Appl. Catal. B Environ. 2017, 210, 77–87. [Google Scholar] [CrossRef]
- Hoang, S.; Berglund, S.P.; Hahn, N.T.; Bard, A.J.; Mullins, C.B. Enhancing Visible Light Photo-oxidation of Water with TiO2 Nanowire Arrays via Cotreatment with H2 and NH3: Synergistic Effects between Ti3+ and N. J. Am. Chem. Soc. 2012, 134, 3659–3662. [Google Scholar] [CrossRef]
- Lei, Y.; Zhang, L.D.; Meng, G.W.; Li, G.H.; Zhang, X.Y.; Liang, C.H.; Chen, W.; Wang, S.X. Preparation and photoluminescence of highly ordered TiO2 nanowire arrays. Appl. Phys. Lett. 2001, 78, 1125–1127. [Google Scholar] [CrossRef]
- Jing, L.; Qu, Y.; Wang, B.; Li, S.; Jiang, B.; Yang, L.; Fu, W.; Fu, H.; Sun, J. Review of photoluminescence performance of nano-sized semiconductor materials and its relationships with photocatalytic activity. Sol. Energy Mater. Sol. Cells 2006, 90, 1773–1787. [Google Scholar] [CrossRef]
- Llansola-Portoles, M.J.; Bergkamp, J.J.; Finkelstein-Shapiro, D.; Sherman, B.D.; Kodis, G.; Dimitrijevic, N.M.; Gust, D.; Moore, T.A.; Moore, A.L. Controlling Surface Defects and Photophysics in TiO2 Nanoparticles. J. Phys. Chem. A 2014, 118, 10631–10638. [Google Scholar] [CrossRef] [PubMed]
- Tafen, D.N.; Wang, J.; Wu, N.; Lewis, J.P. Visible light photocatalytic activity in nitrogen-doped TiO2 nanobelts. Appl. Phys. Lett. 2009, 94, 093101. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).