Synthesis of a Bcl9 Alpha-Helix Mimetic for Inhibition of PPIs by a Combination of Electrooxidative Phenol Coupling and Pd-Catalyzed Cross Coupling †
Abstract
:1. Introduction
2. Results and Discussion
2.1. Electrooxidative Cross-Coupling of Phenols
2.2. Synthesis of Pyridine Boronic Acids
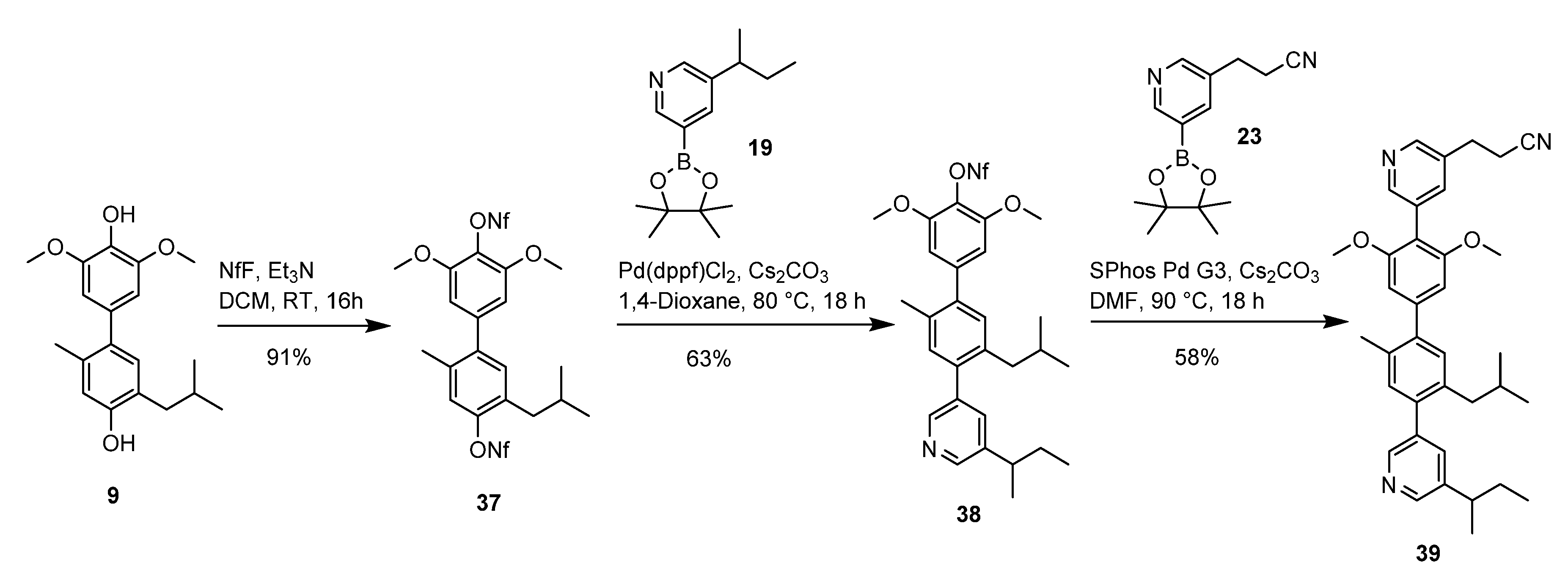
2.3. Quateraryl Assembly
2.4. Synthesis of Quateraryls as Bcl9-Mimetics
3. Materials and Methods
Electrochemical Anodic Dehydrogenative Cross-Coupling Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berg, T. Modulation of protein-protein interactions with small organic molecules. Angew. Chem. Int. Ed. 2003, 42, 2462–2481. [Google Scholar] [CrossRef]
- Villoutreix, B.O.; Kuenemann, M.A.; Poyet, J.-L.; Bruzzoni-Giovanelli, H.; Labbé, C.; Lagorce, D.; Sperandio, O.; Miteva, M.A. Drug-Like Protein-Protein Interaction Modulators: Challenges and Opportunities for Drug Discovery and Chemical Biology. Mol. Inform. 2014, 33, 414–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milroy, L.-G.; Grossmann, T.N.; Hennig, S.; Brunsveld, L.; Ottmann, C. Modulators of protein-protein interactions. Chem. Rev. 2014, 114, 4695–4748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valeur, E.; Guéret, S.M.; Adihou, H.; Gopalakrishnan, R.; Lemurell, M.; Waldmann, H.; Grossmann, T.N.; Plowright, A.T. New Modalities for Challenging Targets in Drug Discovery. Angew. Chem. Int. Ed. 2017, 56, 10294–10323. [Google Scholar] [CrossRef] [PubMed]
- Pelay-Gimeno, M.; Glas, A.; Koch, O.; Grossmann, T.N. Structure-Based Design of Inhibitors of Protein-Protein Interactions: Mimicking Peptide Binding Epitopes. Angew. Chem. Int. Ed. 2015, 54, 8896–8927. [Google Scholar] [CrossRef]
- Walensky, L.D.; Kung, A.L.; Escher, I.; Malia, T.J.; Barbuto, S.; Wright, R.D.; Wagner, G.; Verdine, G.L.; Korsmeyer, S.J. Activation of apoptosis in vivo by a hydrocarbon-stapled BH3 helix. Science 2004, 305, 1466–1470. [Google Scholar] [CrossRef] [Green Version]
- Shaginian, A.; Whitby, L.R.; Hong, S.; Hwang, I.; Farooqi, B.; Searcey, M.; Chen, J.; Vogt, P.K.; Boger, D.L. Design, synthesis, and evaluation of an alpha-helix mimetic library targeting protein-protein interactions. J. Am. Chem. Soc. 2009, 131, 5564–5572. [Google Scholar] [CrossRef] [Green Version]
- Davis, J.M.; Tsou, L.K.; Hamilton, A.D. Synthetic non-peptide mimetics of alpha-helices. Chem. Soc. Rev. 2007, 36, 326–334. [Google Scholar] [CrossRef]
- Cummings, C.G.; Hamilton, A.D. Disrupting protein-protein interactions with non-peptidic, small molecule alpha-helix mimetics. Curr. Opin. Chem. Biol. 2010, 14, 341–346. [Google Scholar] [CrossRef]
- Barnard, A.; Long, K.; Martin, H.L.; Miles, J.A.; Edwards, T.A.; Tomlinson, D.C.; Macdonald, A.; Wilson, A.J. Selective and potent proteomimetic inhibitors of intracellular protein-protein interactions. Angew. Chem. Int. Ed. 2015, 54, 2960–2965. [Google Scholar] [CrossRef] [Green Version]
- Jochim, A.L.; Arora, P.S. Assessment of helical interfaces in protein-protein interactions. Mol. Biosyst. 2009, 5, 924–926. [Google Scholar] [CrossRef] [Green Version]
- Jochim, A.L.; Arora, P.S. Systematic analysis of helical protein interfaces reveals targets for synthetic inhibitors. ACS Chem. Biol. 2010, 5, 919–923. [Google Scholar] [CrossRef] [Green Version]
- Azzarito, V.; Long, K.; Murphy, N.S.; Wilson, A.J. Inhibition of α-helix-mediated protein-protein interactions using designed molecules. Nat. Chem. 2013, 5, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Orner, B.P.; Ernst, J.T.; Hamilton, A.D. Toward proteomimetics: Terphenyl derivatives as structural and functional mimics of extended regions of an alpha-helix. J. Am. Chem. Soc. 2001, 123, 5382–5383. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.; Trobe, M.; Breinbauer, R. A modular synthesis of teraryl-based α-helix mimetics, part 2: Synthesis of 5-pyridine boronic acid pinacol ester building blocks with amino acid side chains in 3-position. Chemistry 2013, 19, 2450–2456. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.; Trobe, M.; Tan, H.; Kleineweischede, R.; Breinbauer, R. A modular synthesis of teraryl-based α-helix mimetics, part 1: Synthesis of core fragments with two electronically differentiated leaving groups. Chemistry 2013, 19, 2442–2449. [Google Scholar] [CrossRef] [PubMed]
- Trobe, M.; Breinbauer, R. Improved and scalable synthesis of building blocks for the modular synthesis of teraryl-based alpha-helix mimetics. Monatsh Chem 2016, 147, 509–521. [Google Scholar] [CrossRef]
- Trobe, M.; Peters, M.; Grimm, S.; Breinbauer, R. The Development of a Modular Synthesis of Teraryl-Based α-Helix Mimetics as Potential Inhibitors of Protein–Protein Interactions. Synlett 2014, 25, 1202–1214. [Google Scholar] [CrossRef]
- Dobrounig, P.; Trobe, M.; Breinbauer, R. Sequential and iterative Pd-catalyzed cross-coupling reactions in organic synthesis. Monatsh Chem 2017, 148, 3–35. [Google Scholar] [CrossRef] [Green Version]
- Dahms, B.; Kohlpaintner, P.J.; Wiebe, A.; Breinbauer, R.; Schollmeyer, D.; Waldvogel, S.R. Selective Formation of 4,4’-Biphenols by Anodic Dehydrogenative Cross- and Homo-Coupling Reaction. Chemistry 2019, 25, 2713–2716. [Google Scholar] [CrossRef]
- Waldvogel, S.R.; Lips, S.; Selt, M.; Riehl, B.; Kampf, C.J. Electrochemical Arylation Reaction. Chem. Rev. 2018, 118, 6706–6765. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, A.; Gieshoff, T.; Möhle, S.; Rodrigo, E.; Zirbes, M.; Waldvogel, S.R. Electrifying Organic Synthesis. Angew. Chem. Int. Ed. 2018, 57, 5594–5619. [Google Scholar] [CrossRef] [PubMed]
- Möhle, S.; Zirbes, M.; Rodrigo, E.; Gieshoff, T.; Wiebe, A.; Waldvogel, S.R. Modern Electrochemical Aspects for the Synthesis of Value-Added Organic Products. Angew. Chem. Int. Ed. 2018, 57, 6018–6041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Röckl, J.L.; Pollok, D.; Franke, R.; Waldvogel, S.R. A Decade of Electrochemical Dehydrogenative C,C-Coupling of Aryls. Acc. Chem. Res. 2020, 53, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Hahne, G.; Grossmann, T.N. Direct targeting of β-catenin: Inhibition of protein-protein interactions for the inactivation of Wnt signaling. Bioorg. Med. Chem. 2013, 21, 4020–4026. [Google Scholar] [CrossRef]
- Grossmann, T.N.; Yeh, J.T.-H.; Bowman, B.R.; Chu, Q.; Moellering, R.E.; Verdine, G.L. Inhibition of oncogenic Wnt signaling through direct targeting of β-catenin. Proc. Natl. Acad. Sci. USA 2012, 109, 17942–17947. [Google Scholar] [CrossRef] [Green Version]
- Sampietro, J.; Dahlberg, C.L.; Cho, U.S.; Hinds, T.R.; Kimelman, D.; Xu, W. Crystal structure of a beta-catenin/BCL9/Tcf4 complex. Mol. Cell 2006, 24, 293–300. [Google Scholar] [CrossRef]
- DeLano, W.L. Pymol: An open-source molecular graphics tool. In CCP4 Newsletter on Protein Crystallography; 2002; pp. 82–92, Unpublished work. [Google Scholar]
- Wiebe, A.; Riehl, B.; Lips, S.; Franke, R.; Waldvogel, S.R. Unexpected high robustness of electrochemical cross-coupling for a broad range of current density. Sci. Adv. 2017, 3, eaao3920. [Google Scholar] [CrossRef] [Green Version]
- Wiebe, A.; Schollmeyer, D.; Dyballa, K.M.; Franke, R.; Waldvogel, S.R. Selective Synthesis of Partially Protected Nonsymmetric Biphenols by Reagent- and Metal-Free Anodic Cross-Coupling Reaction. Angew. Chem. Int. Ed. 2016, 55, 11801–11805. [Google Scholar] [CrossRef]
- Lips, S.; Franke, R.; Waldvogel, S.R. Electrochemical Synthesis of 2-Hydroxy-para-terphenyls by Dehydrogenative Anodic C–C Cross-Coupling Reaction. Synlett 2019, 30, 1174–1177. [Google Scholar] [CrossRef]
- Fürstner, A.; Leitner, A.; Méndez, M.; Krause, H. Iron-catalyzed cross-coupling reactions. J. Am. Chem. Soc. 2002, 124, 13856–13863. [Google Scholar] [CrossRef] [PubMed]
- Baron, O.; Knochel, P. Preparation and selective reactions of mixed bimetallic aromatic and heteroaromatic boron-magnesium reagents. Angew. Chem. Int. Ed. 2005, 44, 3133–3135. [Google Scholar] [CrossRef] [PubMed]
- Klapars, A.; Buchwald, S.L. Copper-catalyzed halogen exchange in aryl halides: An aromatic Finkelstein reaction. J. Am. Chem. Soc. 2002, 124, 14844–14845. [Google Scholar] [CrossRef]
- Weiss, G.A.; Watanabe, C.K.; Zhong, A.; Goddard, A.; Sidhu, S.S. Rapid mapping of protein functional epitopes by combinatorial alanine scanning. Proc. Natl. Acad. Sci. USA 2000, 97, 8950–8954. [Google Scholar] [CrossRef] [Green Version]
- Högermeier, J.; Reissig, H.-U. Nine Times Fluoride can be Good for your Syntheses. Not just Cheaper: Nonafluorobutanesulfonates as Intermediates for Transition Metal-Catalyzed Reactions. Adv. Synth. Catal. 2009, 351, 2747–2763. [Google Scholar] [CrossRef]
- Bruno, N.C.; Tudge, M.T.; Buchwald, S.L. Design and Preparation of New Palladium Precatalysts for C-C and C-N Cross-Coupling Reactions. Chem. Sci. 2013, 4, 916–920. [Google Scholar] [CrossRef] [Green Version]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vareka, M.; Dahms, B.; Lang, M.; Hoang, M.H.; Trobe, M.; Weber, H.; Hielscher, M.M.; Waldvogel, S.R.; Breinbauer, R. Synthesis of a Bcl9 Alpha-Helix Mimetic for Inhibition of PPIs by a Combination of Electrooxidative Phenol Coupling and Pd-Catalyzed Cross Coupling. Catalysts 2020, 10, 340. https://doi.org/10.3390/catal10030340
Vareka M, Dahms B, Lang M, Hoang MH, Trobe M, Weber H, Hielscher MM, Waldvogel SR, Breinbauer R. Synthesis of a Bcl9 Alpha-Helix Mimetic for Inhibition of PPIs by a Combination of Electrooxidative Phenol Coupling and Pd-Catalyzed Cross Coupling. Catalysts. 2020; 10(3):340. https://doi.org/10.3390/catal10030340
Chicago/Turabian StyleVareka, Martin, Benedikt Dahms, Mario Lang, Minh Hao Hoang, Melanie Trobe, Hansjörg Weber, Maximilian M. Hielscher, Siegfried R. Waldvogel, and Rolf Breinbauer. 2020. "Synthesis of a Bcl9 Alpha-Helix Mimetic for Inhibition of PPIs by a Combination of Electrooxidative Phenol Coupling and Pd-Catalyzed Cross Coupling" Catalysts 10, no. 3: 340. https://doi.org/10.3390/catal10030340
APA StyleVareka, M., Dahms, B., Lang, M., Hoang, M. H., Trobe, M., Weber, H., Hielscher, M. M., Waldvogel, S. R., & Breinbauer, R. (2020). Synthesis of a Bcl9 Alpha-Helix Mimetic for Inhibition of PPIs by a Combination of Electrooxidative Phenol Coupling and Pd-Catalyzed Cross Coupling. Catalysts, 10(3), 340. https://doi.org/10.3390/catal10030340





