Suzuki–Miyaura Cross-Coupling of Amides Using Well-Defined, Air- and Moisture-Stable Nickel/NHC (NHC = N-Heterocyclic Carbene) Complexes
Abstract
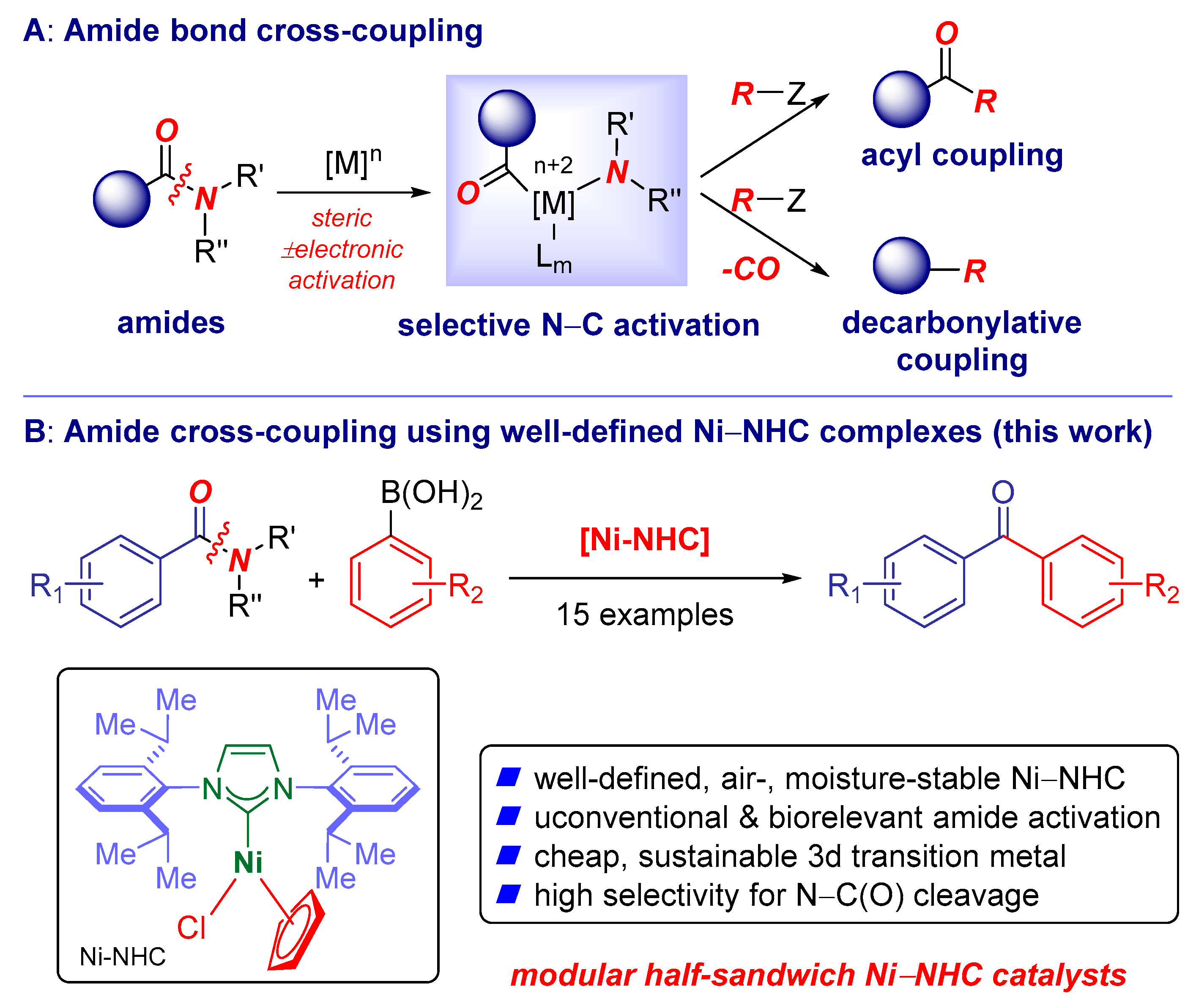
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. General Procedure for [CpNi(IPr)Cl] Catalyzed Cross-Coupling of Amides
4.3. Representative Procedure for [CpNi(IPr)Cl] Catalyzed Cross-Coupling of Amides
4.4. Characterization Data for Products 3a–l (Tables 2-3)
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tasker, S.Z.; Standley, E.A.; Jamison, T.F. Recent Advances in Homogeneous Nickel Catalysis. Nature 2014, 509, 299–309. [Google Scholar] [CrossRef] [Green Version]
- Ananikov, V.P. Nickel: The “Spirited Horse” of Transition Metal Catalysis. ACS Catal. 2015, 5, 1964–1971. [Google Scholar] [CrossRef]
- Diccianni, J.B.; Diao, T. Mechanisms of Nickel-Catalyzed Cross-Coupling Reactions. Trends Chem. 2019, 1, 830–844. [Google Scholar] [CrossRef]
- Shi, S.; Nolan, S.P.; Szostak, M. Well-Defined Palladium(II)-NHC (NHC = N-Heterocyclic Carbene) Precatalysts for Cross-Coupling Reactions of Amides and Esters by Selective Acyl CO–X (X = N, O) Cleavage. Acc. Chem. Res. 2018, 51, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, D.; Bauer, A.; Lemmerer, M.; Maulide, N. Amide Activation: An Emerging Tool for Chemoselective Synthesis. Chem. Soc. Rev. 2018, 47, 7899–7925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, G.; Szostak, M. N-Acyl-Glutarimides: Privileged Scaffolds in Amide N–C Bond Cross-Coupling. Eur. J. Org. Chem. 2018, 2018, 2352–2365. [Google Scholar] [CrossRef]
- Liu, C.; Szostak, M. Decarbonylative Cross-Coupling of Amides. Org. Biomol. Chem. 2018, 16, 7998–8010. [Google Scholar] [CrossRef]
- Takise, R.; Muto, K.; Yamaguchi, J. Cross-Coupling of Aromatic Esters and Amides. Chem. Soc. Rev. 2017, 46, 5864–5888. [Google Scholar] [CrossRef]
- Dander, J.E.; Garg, N.K. Breaking Amides using Nickel Catalysis. ACS Catal. 2017, 7, 1413–1423. [Google Scholar] [CrossRef]
- Liu, C.; Szostak, M. Twisted Amides: From Obscurity to Broadly Useful Transition-Metal-Catalyzed Reactions by N–C Amide Bond Activation. Chem. Eur. J. 2017, 23, 7157–7173. [Google Scholar] [CrossRef]
- Lei, P.; Meng, G.; Szostak, M. General Method for the Suzuki-Miyaura Cross-Coupling of Amides Using Commercially Available, Air- and Moisture-Stable Palladium/NHC (NHC = N-Heterocyclic Carbene) Complexes. ACS Catal. 2017, 7, 1960–1965. [Google Scholar] [CrossRef]
- Lei, P.; Meng, G.; Ling, Y.; An, J.; Szostak, M. Pd-PEPPSI: Pd-NHC Precatalyst for Suzuki-Miyaura Cross-Coupling Reactions of Amides. J. Org. Chem. 2017, 82, 6638–6646. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Li, G.; Nolan, S.P.; Szostak, M. [Pd(NHC)(acac)Cl]: Well-Defined, Air-Stable, and Readily Available Precatalysts for Suzuki and Buchwald-Hartwig Cross-coupling (Transamidation) of Amides and Esters by N-C/O-C Activation. Org. Lett. 2019, 21, 3304–3309. [Google Scholar] [CrossRef]
- Wang, T.; Guo, J.; Wang, H.; Guo, H.; Jia, D.; Zhang, W.; Liu, L. N-heterocyclic carbene palladium(II)-catalyzed Suzuki-Miyaura cross coupling of N-acylsuccinimides by C-N cleavage. J. Organomet. Chem. 2018, 877, 80–84. [Google Scholar] [CrossRef]
- Buchspies, J.; Szostak, M. Recent Advances in Acyl Suzuki Cross-Coupling. Catalysts 2019, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Weires, N.A.; Baker, E.L.; Garg, N.K. Nickel-catalysed Suzuki−Miyaura coupling of Amides. Nat. Chem. 2016, 8, 76–80. [Google Scholar] [CrossRef]
- Dander, J.E.; Weires, N.A.; Garg, N.K. Benchtop Delivery of Ni(cod)2 using Paraffin Capsules. Org. Lett. 2016, 18, 3934–3936. [Google Scholar] [CrossRef]
- Ritleng, V.; Oertel, A.M.; Chetcuti, M.J. Half-sandwich NHC-nickel(II) complexes as pre-catalysts for the fast Suzuki coupling of aryl halides: A comparative study. Dalton Trans. 2010, 39, 8153–8160. [Google Scholar] [CrossRef]
- Oertel, A.M.; Ritleng, V.; Burr, L.; Chetcuti, M.J. Synthesis and structural characterization of half-sandwich nickel complexes bearing two different N-heterocyclic carbene ligands. Organometallics 2011, 30, 6685–6691. [Google Scholar] [CrossRef]
- Oertel, A.M.; Ritleng, V.; Chetcuti, M.J. Synthesis and Catalytic Activity in Suzuki Coupling of Nickel Complexes Bearing n-Butyl- and Triethoxysilylpropyl-Substituted NHC Ligands: Toward the Heterogenization of Molecular Catalysts. Organometallics 2012, 31, 2829–2840. [Google Scholar] [CrossRef]
- Henrion, M.; Chetcuti, M.J.; Ritleng, V. From acetone metalation to the catalytic α-arylation of acyclic ketones with NHC–nickel(ii) complexes. Chem. Commun. 2014, 50, 4624–4627. [Google Scholar] [CrossRef] [PubMed]
- Bheeter, L.P.; Henrion, M.; Brelot, L.; Darcel, C.; Chetcuti, M.J.; Sortais, J.B.; Ritleng, V. Hydrosilylation of Aldehydes and Ketones Catalyzed by an N-Heterocyclic Carbene-Nickel Hydride Complex under Mild Conditions. Adv. Synth. Catal. 2012, 354, 2619–2624. [Google Scholar] [CrossRef]
- Bheeter, L.P.; Henrion, M.; Chetcuti, M.J.; Darcel, C.; Ritleng, V.; Sortais, J.B. Cyclopentadienyl N-heterocyclic carbene–nickel complexes as efficient pre-catalysts for the hydrosilylation of imines. Catal. Sci. Technol. 2013, 3, 3111–3116. [Google Scholar] [CrossRef]
- Oertel, A.M.; Freudenreich, J.; Gein, J.; Ritleng, V.; Veiros, L.F.; Chetcuti, M.J. Intramolecular Nitrile C–H Bond Activation in Nickel NHC Complexes: A Route to New Nickelacycles. Organometallics 2011, 30, 3400–3411. [Google Scholar] [CrossRef]
- Danopoulos, A.A.; Simler, T.; Braunstein, P. N-Heterocyclic Carbene Complexes of Copper, Nickel, and Cobalt. Chem. Rev. 2019, 119, 3730–3961. [Google Scholar] [CrossRef]
- Henrion, M.; Ritleng, V.; Chetcuti, M.J. Nickel N-Heterocyclic Carbene-Catalyzed C–C Bond Formation: Reactions and Mechanistic Aspects. ACS Catal. 2015, 5, 1283–1302. [Google Scholar] [CrossRef]
- Ritleng, V.; Henrion, M.; Chetcuti, M.J. Nickel N-Heterocyclic Carbene-Catalyzed C–Heteroatom Bond Formation, Reduction, and Oxidation: Reactions and Mechanistic Aspects. ACS Catal. 2016, 6, 890–906. [Google Scholar] [CrossRef]
- Banach, L.; Guńka, P.A.; Zachara, J.; Buchowicz, W. Half-sandwich Ni(II) complexes [Ni(Cp)(X)(NHC)]: From an underestimated discovery to a new chapter in organonickel chemistry. Coord. Chem. Rev. 2019, 389, 19–58. [Google Scholar] [CrossRef]
- Zhao, Q.; Meng, G.; Nolan, S.P.; Szostak, M. N-Heterocyclic Carbene Complexes in C–H Activation Reactions. Chem. Rev. 2020, 120, 1981–2048. [Google Scholar] [CrossRef]
- Kelly, R.A.; Scott, N.M.; Díez-González, S.; Stevens, E.D.; Nolan, S.P. Simple Synthesis of CpNi(NHC)Cl Complexes (Cp = Cyclopentadienyl; NHC = N-Heterocyclic Carbene). Organometallics 2005, 24, 3442–3447. [Google Scholar] [CrossRef]
- Iglesias, M.J.; Prieto, A.; Nicasio, M.C. Kumada–Tamao–Corriu Coupling of Heteroaromatic Chlorides and Aryl Ethers Catalyzed by (IPr)Ni(allyl)Cl. Org. Lett. 2012, 14, 4318–4321. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Ke, W.C.; Chan, K.T.; Lai, C.L.; Hu, C.H.; Lee, H.M. Nickel(II) Complexes of Bidentate N-Heterocyclic Carbene/Phosphine Ligands: Efficient Catalysts for Suzuki Coupling of Aryl Chlorides. Chem. Eur. J. 2007, 13, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Leadbeater, N.E. Bis-cyclopentadienyl nickel (nickelocene): A convenient starting material for reactions catalyzed by Ni(0) phosphine complexes. J. Org. Chem. 2001, 66, 7539–7541. [Google Scholar] [CrossRef] [PubMed]
- Beromi, M.M.; Nova, A.; Balcells, D.; Brasacchio, A.M.; Brudvig, G.W.; Guard, L.M.; Hazari, N.; Vinyard, D.J. Mechanistic Study of an Improved Ni Precatalyst for Suzuki–Miyaura Reactions of Aryl Sulfamates: Understanding the Role of Ni(I) Species. J. Am. Chem. Soc. 2017, 139, 922–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsubara, K.; Ueno, K.; Shibata, Y. Synthesis and Structures of Nickel Halide Complexes Bearing Mono- and Bis-coordinated N-Heterocyclic Carbene Ligands, Catalyzing Grignard Cross-Coupling Reactions. Organometallics 2006, 25, 3422–3427. [Google Scholar] [CrossRef]
- Izquierdo, F.; Manzini, S.; Nolan, S.P. The use of the sterically demanding IPr* and related ligands in catalysis. Chem. Commun. 2014, 50, 14926–14937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Suárez, A.; Nelson, D.J.; Nolan, S.P. Quantifying and understanding the steric properties of N-heterocyclic carbenes. Chem. Commun. 2017, 53, 2650–2660. [Google Scholar] [CrossRef] [Green Version]
- Shaw, P.; Kennedy, A.R.; Nelson, D.J. Synthesis and characterisation of an N-heterocyclic carbene with spatially-defined steric impact. Dalton Trans. 2016, 45, 11772–11780. [Google Scholar] [CrossRef] [Green Version]
- Martin, A.R.; Makida, Y.; Meiries, S.; Slawin, A.M.Z.; Nolan, S.P. Enhanced Activity of [Ni(NHC)CpCl] Complexes in Arylamination Catalysis. Organometallics 2013, 32, 6265–6270. [Google Scholar] [CrossRef]
- Shi, S.; Meng, G.; Szostak, M. Synthesis of Biaryls via Nickel Catalyzed Suzuki–Miyaura Coupling of Amides by Carbon–Nitrogen Cleavage. Angew. Chem. Int. Ed. 2016, 55, 6959–6963. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.Q.; Hong, X. Computational studies on Ni-catalyzed amide C–N bond activation. Chem. Commun. 2019, 55, 11330–11341. [Google Scholar] [CrossRef] [PubMed]
- Buchspies, J.; Pyle, D.J.; He, H.; Szostak, M. Pd-Catalyzed Suzuki-Miyaura Cross-Coupling of Pentafluorophenyl Esters. Molecules 2018, 23, 3144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, P.; Meng, G.; Shi, S.; Ling, Y.; An, J.; Szostak, R.; Szostak, M. Suzuki–Miyaura cross-coupling of amides and esters at room temperature: Correlation with barriers to rotation around C–N and C–O bonds. Chem. Sci. 2017, 8, 6525–6530. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Entry | Catalyst | [Ni] (mol%) | Base | Solvent | T (°C) | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | [CpNi(IPr)Cl] | 10 | K2CO3 | toluene | 80 | 85 |
| 2 | [CpNi(IPr)Cl] | 5 | K2CO3 | toluene | 80 | 42 |
| 3 | [CpNi(IPr)Cl] | 10 | K2CO3 | toluene | 120 | 80 |
| 4 | [CpNi(IPr)Cl] | 5 | K2CO3 | toluene | 120 | 39 |
| 5 2 | [CpNi(IPr)Cl] | 10 | K2CO3 | toluene | 120 | 40 |
| 6 3 | [CpNi(IPr)Cl] | 10 | K2CO3 | toluene | 120 | 54 |
| 7 3 | [CpNi(IPr)Cl] | 10 | K2CO3 | toluene | 80 | 27 |
| 8 | [CpNi(IPr)Cl] | 5 | K2CO3 | dioxane | 120 | 34 |
| 9 | [CpNi(IPr)Cl] | 10 | K2CO3 | dioxane | 120 | 48 |
| 10 | [CpNi(IPr)Cl] | 10 | K2CO3 | THF | 80 | <10 |
| 11 | [CpNi(IPr)Cl] | 10 | Na2CO3 | THF | 80 | 20 |
| 12 | [CpNi(IPr)Cl] | 10 | Na2CO3 | THF | 120 | <5 |
| 13 | [CpNi(IPr)Cl] | 10 | Na2CO3 | dioxane | 80 | <5 |
| 14 | [CpNi(IPr)Cl] | 10 | Na2CO3 | dioxane | 120 | <5 |
| 15 | [CpNi(IPr)Cl] | 10 | K3PO4 | toluene | 80 | 38 |
| 16 | [Ni(PCy3)2Cl2] | 10 | Na2CO3 | dioxane | 80 | 31 |
| 17 | [Ni(PCy3)2Cl2] | 10 | Na2CO3 | dioxane | 120 | 16 |
| 18 | [Ni(PPh3)2Cl2] | 10 | K2CO3 | toluene | 120 | <5 |
| 19 | [Ni(PPh3)2Cl2] | 10 | Na2CO3 | dioxane | 80 | <5 |
| 20 | [NiCp2] | 10 | K2CO3 | toluene | 120 | <5 |
| 21 | [Ni(dppf)(o-tol)Cl] | 10 | K2CO3 | toluene | 120 | <5 |
| 22 | [Ni(IPr)(PPh3)Cl2] | 10 | K2CO3 | toluene | 120 | 64 |
| 23 | [CpNi(IPr)(NCMe)](PF6) | 10 | K2CO3 | toluene | 80 | 44 |
| 24 | [CpNi(IPr)(NCMe)](PF6) | 5 | K2CO3 | toluene | 80 | 28 |
| 25 | [CpNi(IMes)Cl] | 10 | K2CO3 | toluene | 80 | 77 |
| 26 | [CpNi(IMes)Cl] | 5 | K2CO3 | toluene | 80 | 40 |
| 27 | [CpNi(IPaul)Cl] | 10 | K2CO3 | toluene | 80 | 68 |
| 28 | [CpNi(IPaul)Cl] | 5 | K2CO3 | toluene | 80 | 39 |
| 29 | [CpNi(IPr*)Cl] | 10 | K2CO3 | toluene | 80 | 63 |
| 30 | [CpNi(IPr*)Cl] | 5 | K2CO3 | toluene | 80 | 42 |

| Entry | Amide | Ar-B(OH)2 | 3 | Yield (%) |
|---|---|---|---|---|
| 1 | C6H5 | 4-Me-C6H4 | 3a | 85 |
| 2 | C6H5 | 4-t-Bu-C6H4 | 3b | 87 |
| 3 | C6H5 | 4-MeO-C6H4 | 3c | 79 |
| 4 | C6H5 | 2-Me-C6H4 | 3d | 85 |
| 5 | C6H5 | 2-MeO-C6H4 | 3e | 58 |
| 6 | C6H5 | 3-F-C6H4 | 3f | 48 |
| 7 | C6H5 | 3-CF3-C6H4 | 3g | 56 |
| 8 | C6H5 | 2-Np | 3h | 71 |
| 9 | C6H5 | 4-Ph-C6H4 | 3i | 67 |
| 102 | C6H5 | Cyclopentyl | - | <5 |

| Entry | Amide | Ar-B(OH)2 | 3 | Yield (%) |
|---|---|---|---|---|
| 1 | 4-Me-C6H4 | C6H5 | 3a | 70 |
| 2 | 4-MeO-C6H4 | C6H5 | 3c | 67 |
| 3 | 4-CF3-C6H4 | C6H5 | 3j | 96 |
| 4 | 2-Me-C6H4 | C6H5 | 3d | 39 |
| 5 | 3,4-F2-C6H3 | C6H5 | 3k | 70 |
| 6 | 2-thienyl | C6H5 | 3l | 55 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buchspies, J.; Rahman, M.M.; Szostak, M. Suzuki–Miyaura Cross-Coupling of Amides Using Well-Defined, Air- and Moisture-Stable Nickel/NHC (NHC = N-Heterocyclic Carbene) Complexes. Catalysts 2020, 10, 372. https://doi.org/10.3390/catal10040372
Buchspies J, Rahman MM, Szostak M. Suzuki–Miyaura Cross-Coupling of Amides Using Well-Defined, Air- and Moisture-Stable Nickel/NHC (NHC = N-Heterocyclic Carbene) Complexes. Catalysts. 2020; 10(4):372. https://doi.org/10.3390/catal10040372
Chicago/Turabian StyleBuchspies, Jonathan, Md. Mahbubur Rahman, and Michal Szostak. 2020. "Suzuki–Miyaura Cross-Coupling of Amides Using Well-Defined, Air- and Moisture-Stable Nickel/NHC (NHC = N-Heterocyclic Carbene) Complexes" Catalysts 10, no. 4: 372. https://doi.org/10.3390/catal10040372
APA StyleBuchspies, J., Rahman, M. M., & Szostak, M. (2020). Suzuki–Miyaura Cross-Coupling of Amides Using Well-Defined, Air- and Moisture-Stable Nickel/NHC (NHC = N-Heterocyclic Carbene) Complexes. Catalysts, 10(4), 372. https://doi.org/10.3390/catal10040372







