Ultrasound-Assisted Hydrothermal Fabrication of AgI/MFeO3/g-C3N4 (M = Y, Gd, La) Nano Sheet–Sphere–Sheet Photocatalysts with Enhanced Photodegradation Activities for Norfloxacin
Abstract
:1. Introduction
2. Results and Discussion
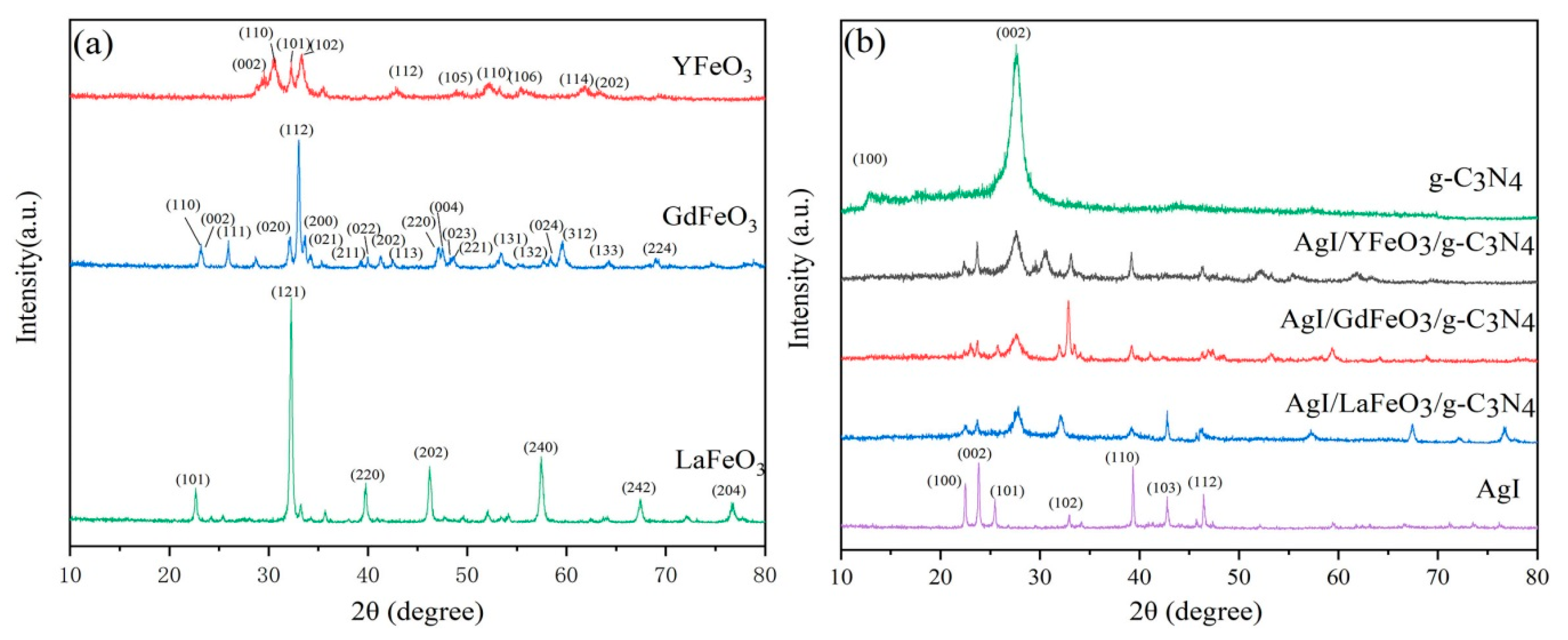
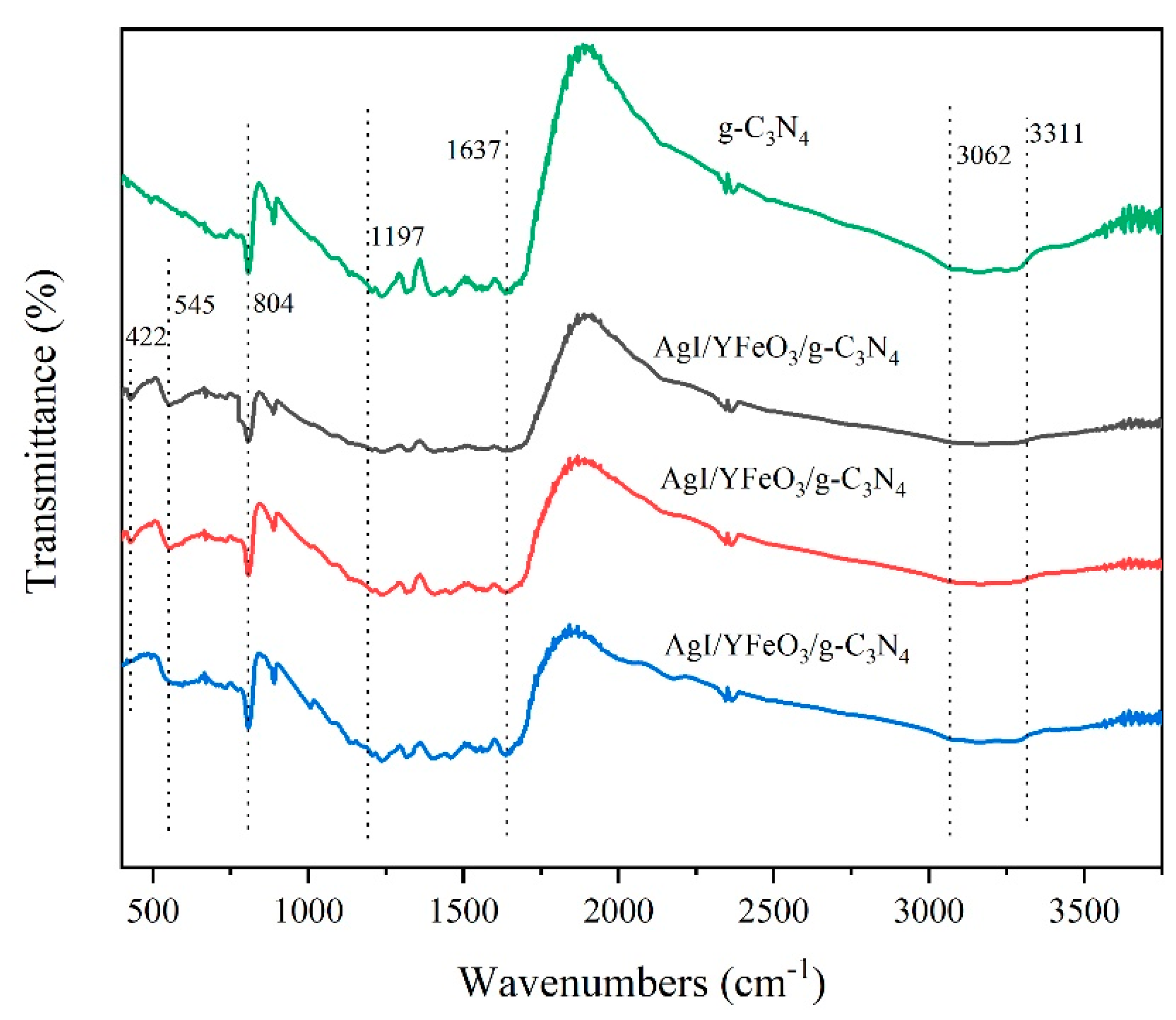
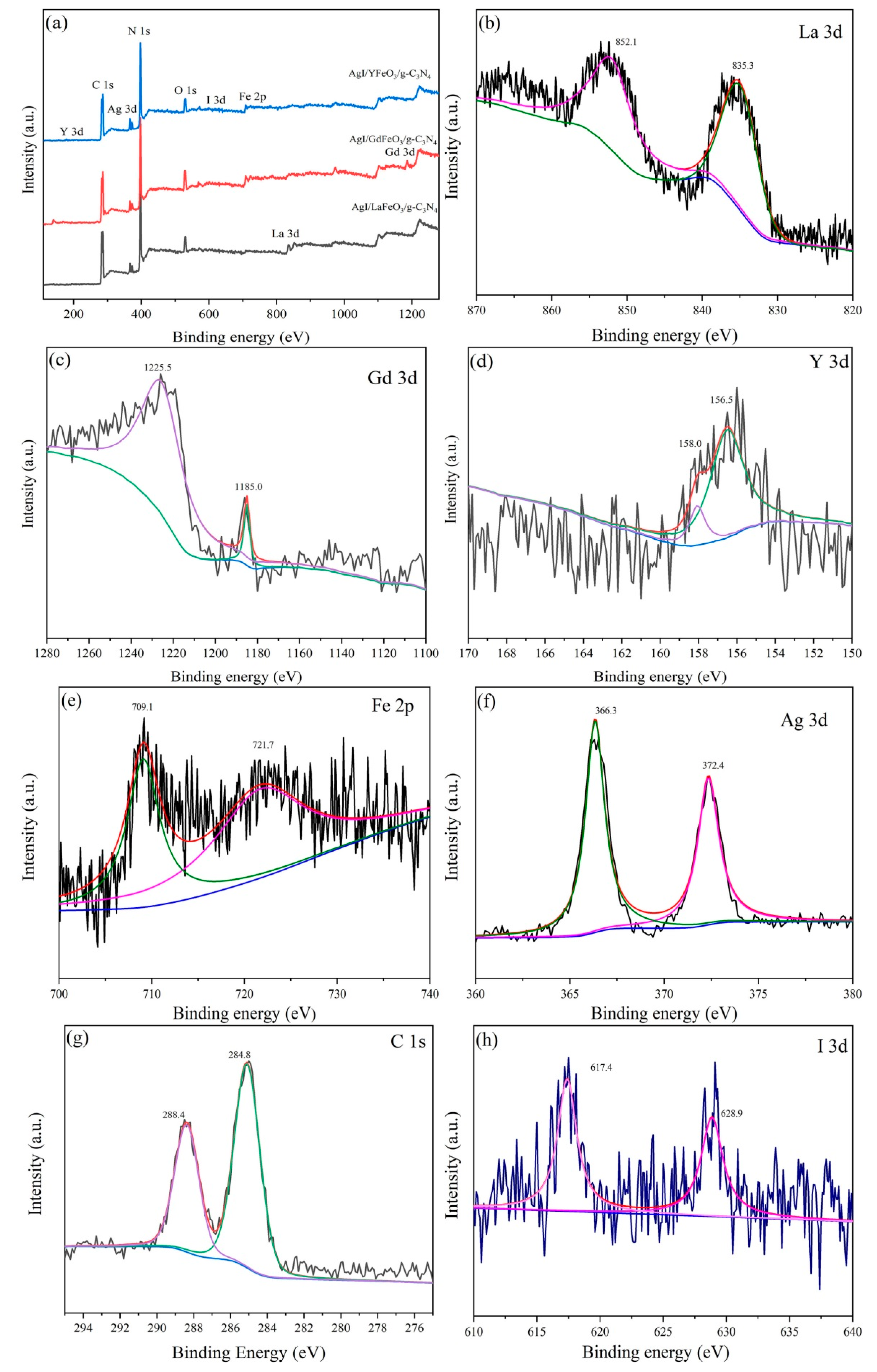
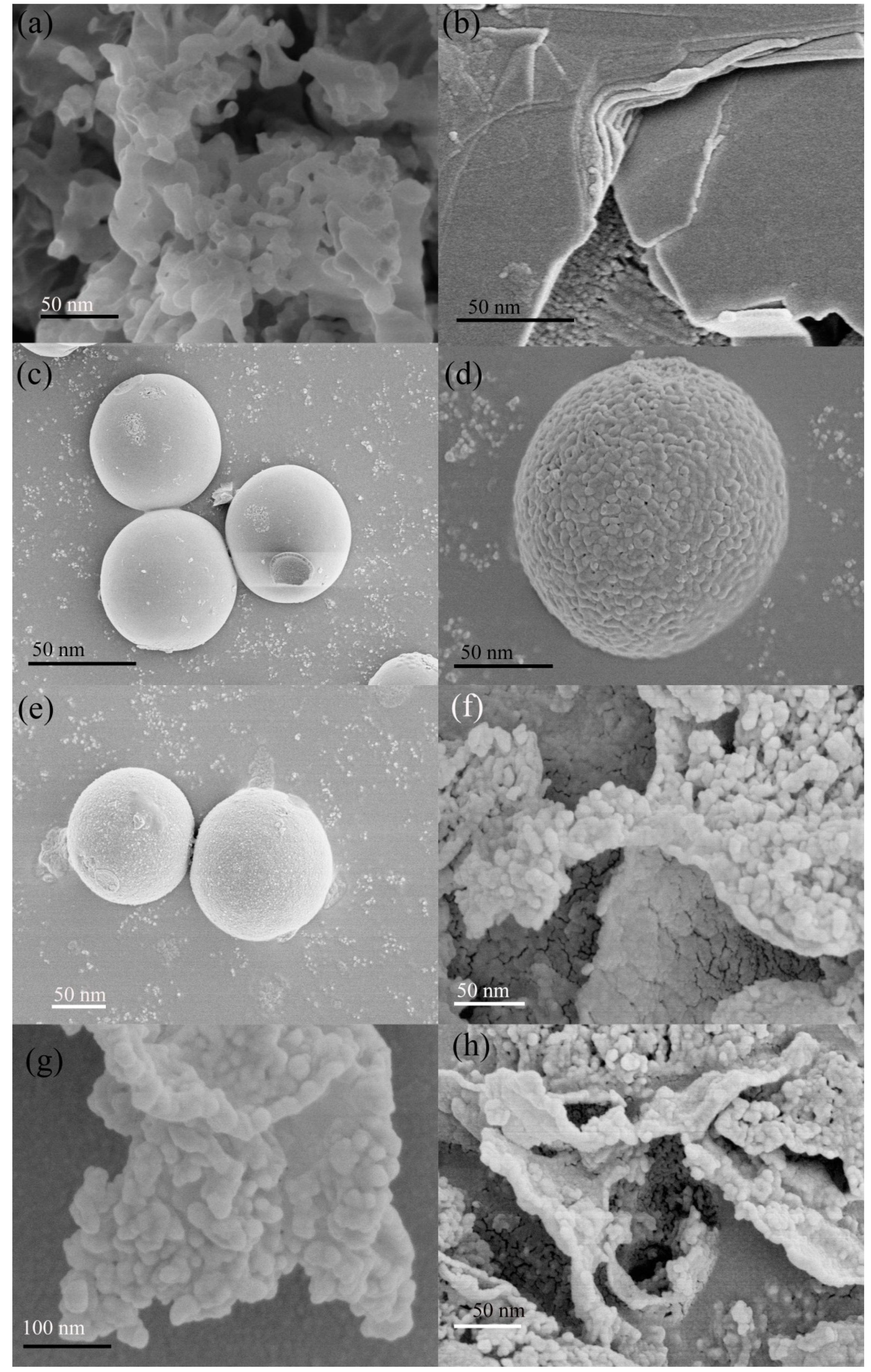
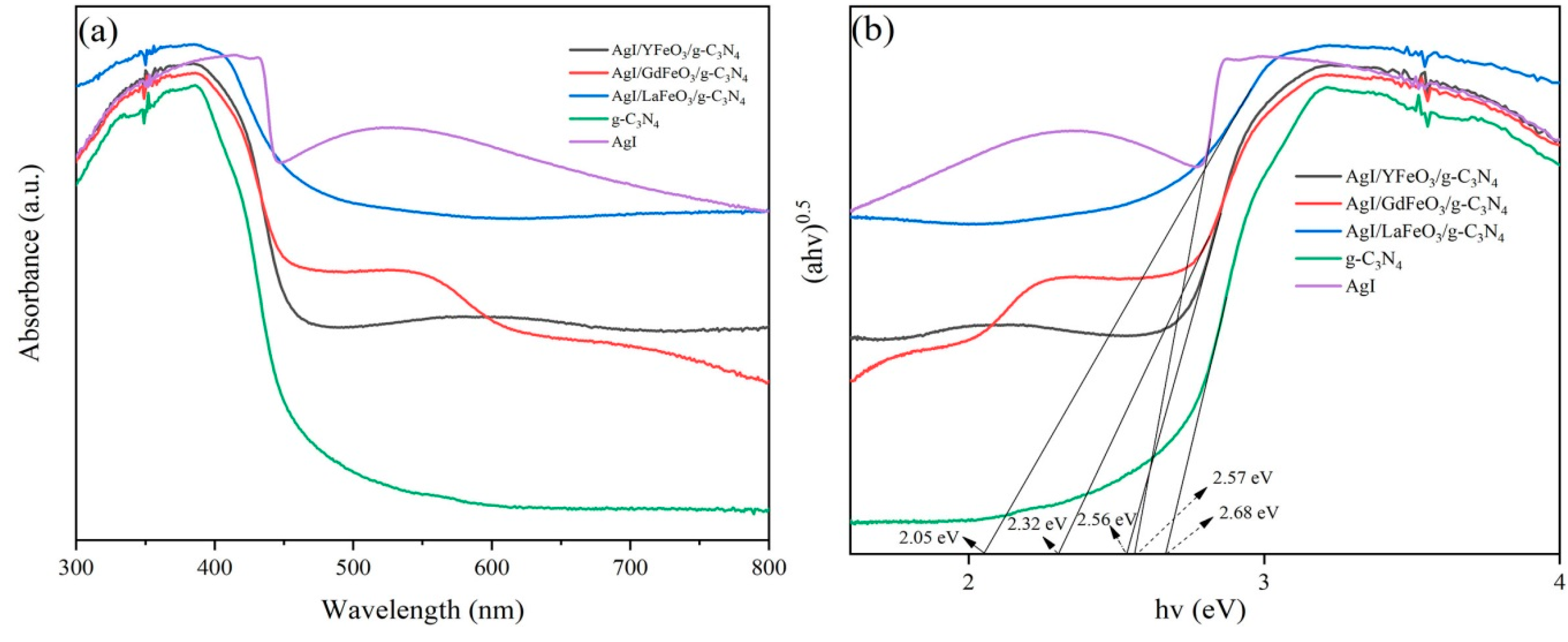
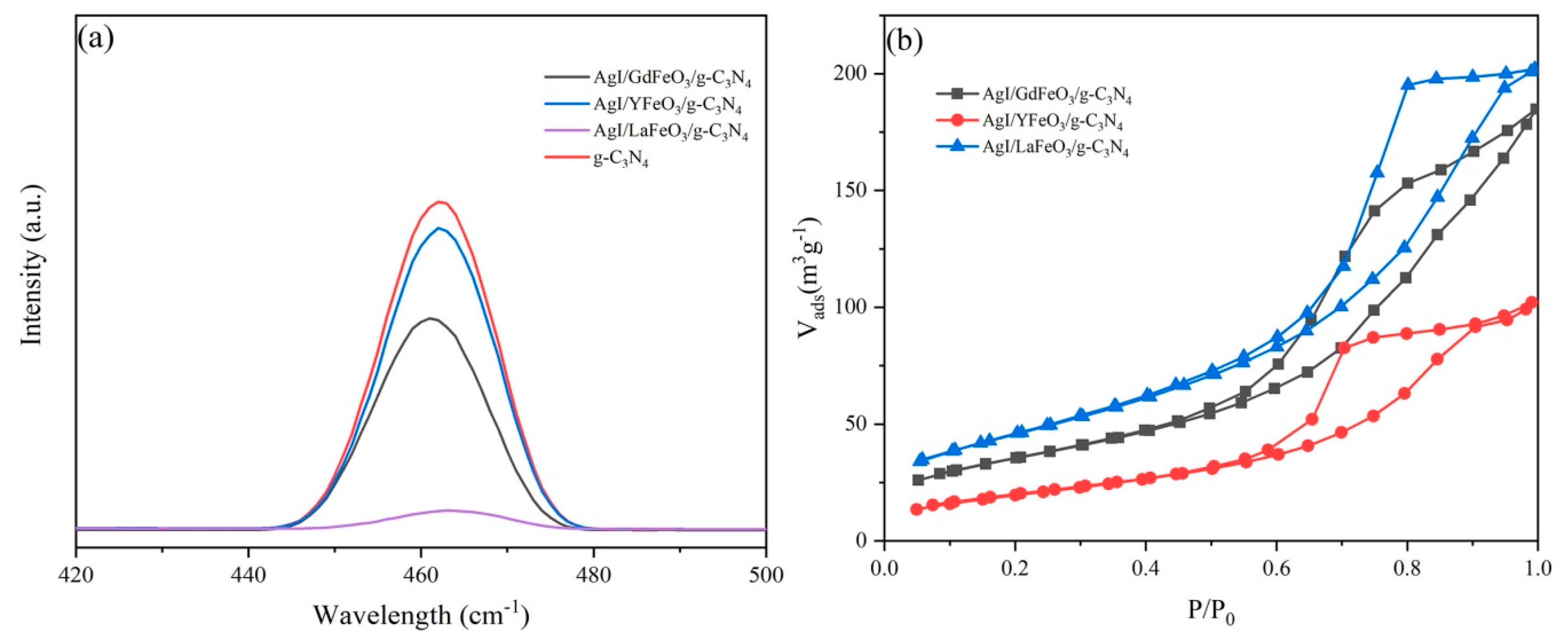
2.1. Composition and Structure of Materials
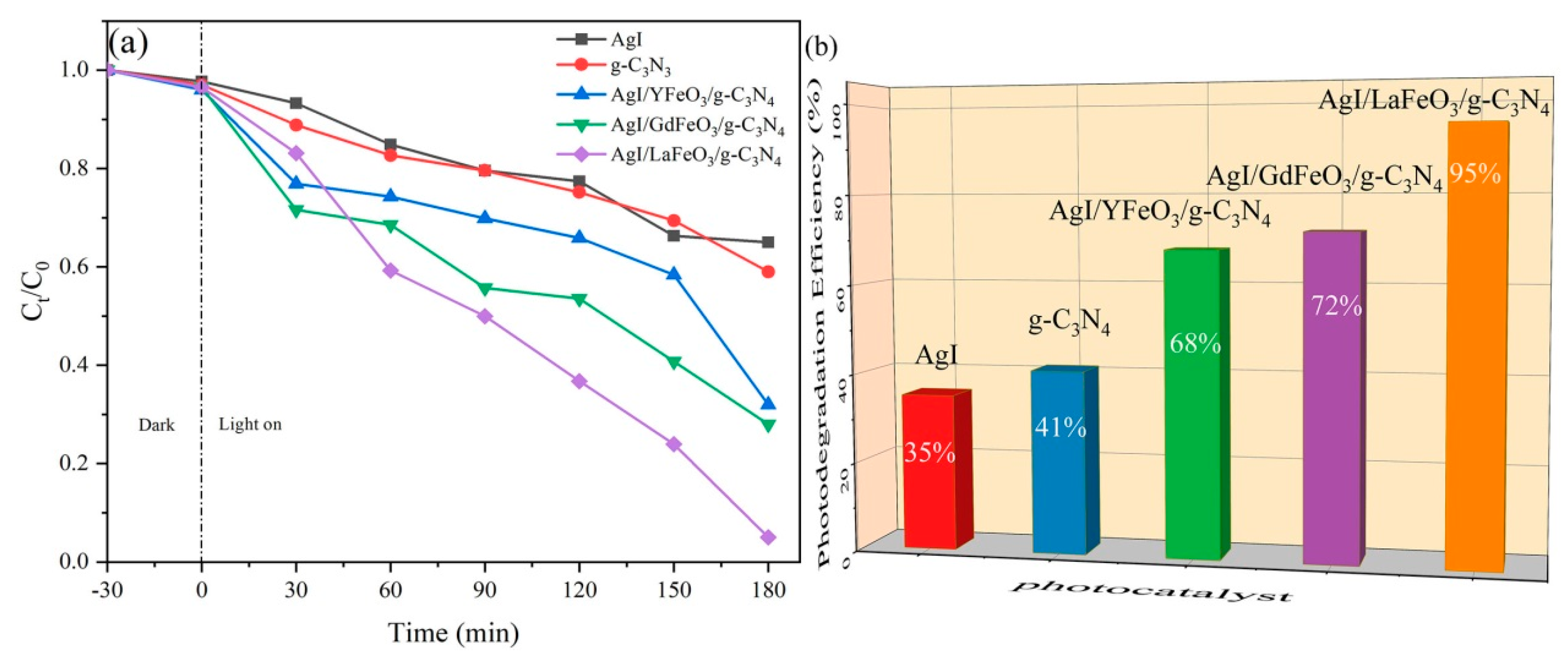
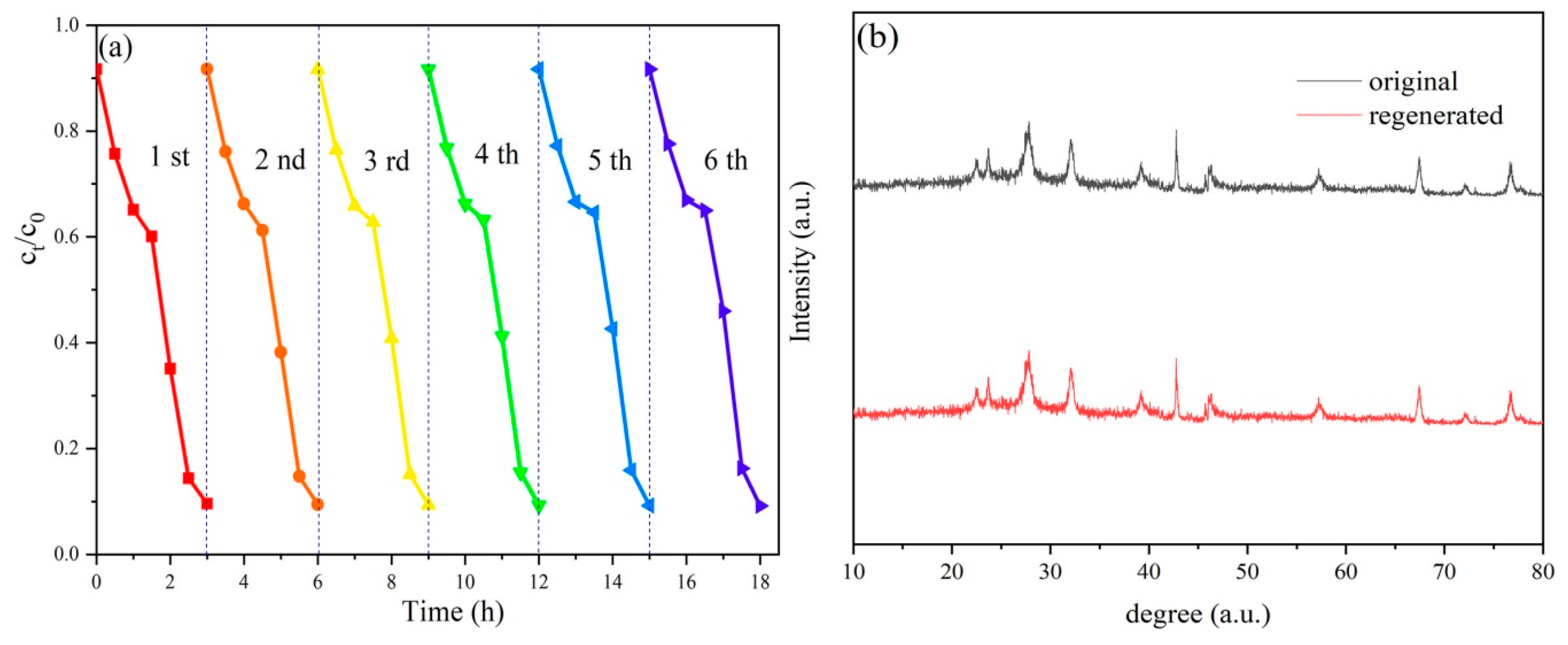
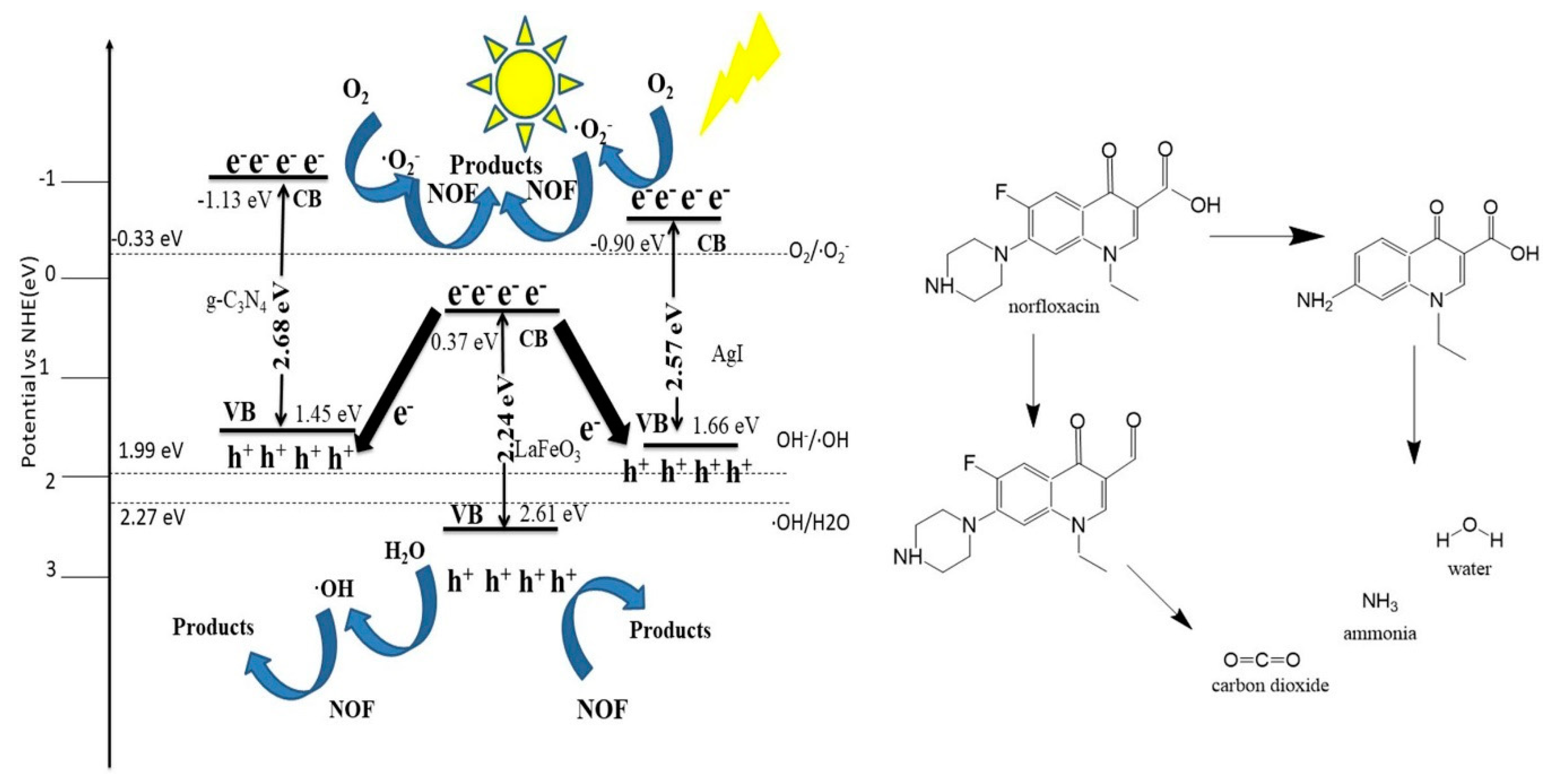
2.2. Photocatalytic Performance and Photodegradation Mechanism
3. Materials and Methods
3.1. Materials
3.2. Preparations of g-C3N4 Nanosheets
3.3. Preparations of MFeO3 Nanospheres
3.4. Preparations of AgI Nanosheets
3.5. Preparations of AgI/MFeO3/g-C3N4 Composites
3.6. Characterization
3.7. Photodegradation Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, M.; Chu, W. Photocatalytic degradation and decomposition mechanism of fluoroquinolones norfloxacin over bismuth tungstate: Experiment and mathematic model. Appl. Catal. B Environ. 2015, 168–169, 175–182. [Google Scholar] [CrossRef]
- Jiang, J.; Mu, Z.; Xing, H.; Wu, Q.; Yue, X.; Lin, Y. Insights into the synergetic effect for enhanced UV/visible-light activated photodegradation activity via Cu-ZnO photocatalyst. Appl. Surf. Sci. 2019, 478, 1037–1045. [Google Scholar] [CrossRef]
- Deng, Y.; Tang, L.; Zeng, G.; Wang, J.; Zhou, Y.; Wang, J.; Tang, J.; Liu, Y.; Peng, B.; Chen, F. Facile fabrication of a direct Z-scheme Ag2CrO4/g-C3N4 photocatalyst with enhanced visible light photocatalytic activity. J. Mol. Catal. A Chem. 2016, 421, 209–221. [Google Scholar] [CrossRef]
- Guo, Y.; Ao, Y.; Wang, P.; Wang, C. Mediator-free direct dual-Z-scheme Bi2S3/BiVO4/MgIn2S4 composite photocatalysts with enhanced visible-light-driven performance towards carbamazepine degradation. Appl. Catal. B Environ. 2019, 254, 479–490. [Google Scholar] [CrossRef]
- Akhundi, A.; Habibi-Yangjeh, A. Ternary g-C3N4 /ZnO/AgCl nanocomposites: Synergistic collaboration on visible-light-driven activity in photodegradation of an organic pollutant. Appl. Surf. Sci. 2015, 358, 261–269. [Google Scholar] [CrossRef]
- Ye, L.; Wu, D.; Chu, K.H.; Wang, B.; Xie, H.; Yip, H.Y.; Wong, P.K. Phosphorylation of g-C3N4 for enhanced photocatalytic CO2 reduction. Chem. Eng. J. 2016, 304, 376–383. [Google Scholar] [CrossRef]
- Jiang, D.; Zhu, J.; Chen, M.; Xie, J. Highly efficient heterojunction photocatalyst based on nanoporous g-C3N4 sheets modified by Ag3PO4 nanoparticles: Synthesis and enhanced photocatalytic activity. J. Colloid Interface Sci. 2014, 417, 115–120. [Google Scholar] [CrossRef]
- Liu, L.; Qi, Y.; Yang, J.; Cui, W.; Li, X.; Zhang, Z. An AgI@g-C3N4 hybrid core@shell structure: Stable and enhanced photocatalytic degradation. Appl. Surf. Sci. 2015, 358, 319–327. [Google Scholar] [CrossRef]
- Wei, X.; Shao, C.; Li, X.; Lu, N.; Wang, K.; Zhang, Z. Facile in situ synthesis of plasmonic nanoparticles-decorated gC3N4/TiO2 heterojunction nanofibers and comparison study of their photosynergistic effects for efficient photocatalytic H 2 evolution. Nanoscale 2016, 8, 11034–11043. [Google Scholar] [CrossRef]
- Chen, W.; Duan, G.-R.; Liu, T.-Y.; Chen, S.-M. Fabrication of Bi2MoO6 nanoplates hybridized with g-C3N4 nanosheets as highly efficient visible light responsive heterojunction photocatalysts for Rhodamine B degradation. Mater. Sci. Semicond. Process. 2015, 35, 45–54. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, J.; Zhang, M.; Yuan, Q.; Dong, B. Ultrathin hexagonal SnS2 nanosheets coupled with g-C3N4 nanosheets as 2D/2D heterojunction photocatalysts toward high photocatalytic activity. Appl. Catal. B Environ. 2015, 163, 298–305. [Google Scholar] [CrossRef]
- Jo, W.K.; Natarajan, T.S. Fabrication and efficient visible light photocatalytic properties of novel zinc indium sulfide (ZnIn2S4)—Graphitic carbon nitride (g-C3N4)/bismuth vanadate (BiVO4) nanorod-based ternary nanocomposites with enhanced charge separation via Z-scheme transfe. J. Colloid Interface Sci. 2016, 482, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, X. Self-propagating combustion synthesis and synergistic photocatalytic activity of GdFeO3 nanoparticles. J. Sol-Gel Sci. Technol. 2016, 79, 107–113. [Google Scholar] [CrossRef]
- Wang, M.; Wang, T. Structural, magnetic and optical properties of Gd and Co Co-doped YFeO3 nanopowders. Materials 2019, 12, 2423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosales-González, O.; Sánchez-De Jesús, F.; Cortés-Escobedo, C.A.; Bolarín-Miró, A.M. Crystal structure and multiferroic behavior of perovskite YFeO3. Ceram. Int. 2018, 44, 15298–15303. [Google Scholar] [CrossRef]
- Li, S.; Zhao, Y.; Wang, C.; Li, D.; Gao, K. Fabrication and characterization unique ribbon-like porous Ag/LaFeO3 nanobelts photocatalyst via electrospinning. Mater. Lett. 2016, 170, 122–125. [Google Scholar] [CrossRef]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation Performance of g-C3N4 Fabricated by Directly Heating Melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [CrossRef]
- Choi, J.; Reddy, D.A.; Kim, T.K. Enhanced photocatalytic activity and anti-photocorrosion of AgI nanostructures by coupling with graphene-analogue boron nitride nanosheets. Ceram. Int. 2015, 41, 13793–13803. [Google Scholar] [CrossRef]
- Lakshmana Rao, T.; Pradhan, M.K.; Chandrasekhar, M.; Ramakrishna, P.V.; Dash, S. Structural, magnetic, grain and grain boundary mediated conduction features of low dimensional LaFeO3 nanoparticles. J. Phys. Condens. Matter 2019, 31, 345803. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, T.; Wang, Z.; Dawson, G.; Chen, W. Simple pyrolysis of urea into graphitic carbon nitride with recyclable adsorption and photocatalytic activity. J. Mater. Chem. 2011, 21, 14398–14401. [Google Scholar] [CrossRef]
- Akhundi, A.; Habibi-Yangjeh, A. Ternary magnetic g-C3N4/Fe3O4/AgI nanocomposites: Novel recyclable photocatalysts with enhanced activity in degradation of different pollutants under visible light. Mater. Chem. Phys. 2016, 174, 59–69. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, L.; Shi, J.; Deng, H. Fabrication of novel visible-light-driven AgI/g-C3N4 composites with enhanced visible-light photocatalytic activity for diclofenac degradation. J. Colloid Interface Sci. 2017, 496, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Xue, S.; Wang, G.; Jin, J.; Liang, Q.; Li, Z.; Xu, S. Enhanced photocatalytic decomposition of an organic dye under visible light with a stable LaFeO3/AgBr heterostructured photocatalyst. J. Phys. Chem. Solids 2018, 121, 329–338. [Google Scholar] [CrossRef]
- Uppara, H.P.; Dasari, H.; Singh, S.K.; Labhsetwar, N.K.; Murari, M.S. Effect of Copper Doping Over GdFeO3 Perovskite on Soot Oxidation Activity. Catal. Lett. 2019, 149, 3097–3110. [Google Scholar] [CrossRef]
- Ismael, M.; Elhaddad, E.; Taffa, D.H.; Wark, M. Solid state route for synthesis of YFeO3/g-C3N4 composites and its visible light activity for degradation of organic pollutants. Catal. Today 2018, 313, 47–54. [Google Scholar] [CrossRef]
- Lei, C.; Pi, M.; Zhu, X.; Xia, P.; Guo, Y.; Zhang, F. Highly efficient visible-light photocatalytic performance based on novel AgI/g-C3N4 composite photocatalysts. Chem. Phys. Lett. 2016, 664, 167–172. [Google Scholar] [CrossRef]
- Gao, X.; Shang, Y.; Gao, K.; Fu, F. Plasmon sensitized heterojunction 2d ultrathin Ag/AgI-δ-Bi2O3 for enhanced photocatalytic nitrogen fixation. Nanomaterials 2019, 9, 781. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Hu, H.; Lu, W.; Lu, Y.; Wang, S. Novel composite BiFeO3/ZrO2 and its high photocatalytic performance under white LED visible-light irradiation. Mater. Res. Bull. 2019, 120, 110605. [Google Scholar] [CrossRef]
- Jiao, Z.; Tang, Y.; Zhao, P.; Li, S.; Sun, T.; Cui, S.; Cheng, L. Synthesis of Z-scheme g-C3N4/PPy/Bi2WO6 composite with enhanced visible-light photocatalytic performance. Mater. Res. Bull. 2019, 113, 241–249. [Google Scholar] [CrossRef]
- Xue, W.; Huang, D.; Li, J.; Zeng, G.; Deng, R.; Yang, Y.; Chen, S.; Li, Z.; Gong, X.; Li, B. Assembly of AgI nanoparticles and ultrathin g-C3N4 nanosheets codecorated Bi2WO6 direct dual Z-scheme photocatalyst: An efficient, sustainable and heterogeneous catalyst with enhanced photocatalytic performance. Chem. Eng. J. 2019, 373, 1144–1157. [Google Scholar] [CrossRef]
- Wang, G.; Liu, S.; He, T.; Liu, X.; Deng, Q.; Mao, Y.; Wang, S. Enhanced visible-light-driven photocatalytic activities of Bi2Fe4O9/g-C3N4 composite photocatalysts. Mater. Res. Bull. 2018, 104, 104–111. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhu, Z.; Jiang, J.; Li, H. Ultrasound-Assisted Hydrothermal Fabrication of AgI/MFeO3/g-C3N4 (M = Y, Gd, La) Nano Sheet–Sphere–Sheet Photocatalysts with Enhanced Photodegradation Activities for Norfloxacin. Catalysts 2020, 10, 373. https://doi.org/10.3390/catal10040373
Zhang J, Zhu Z, Jiang J, Li H. Ultrasound-Assisted Hydrothermal Fabrication of AgI/MFeO3/g-C3N4 (M = Y, Gd, La) Nano Sheet–Sphere–Sheet Photocatalysts with Enhanced Photodegradation Activities for Norfloxacin. Catalysts. 2020; 10(4):373. https://doi.org/10.3390/catal10040373
Chicago/Turabian StyleZhang, Junjiao, Zhengru Zhu, Junchao Jiang, and Hong Li. 2020. "Ultrasound-Assisted Hydrothermal Fabrication of AgI/MFeO3/g-C3N4 (M = Y, Gd, La) Nano Sheet–Sphere–Sheet Photocatalysts with Enhanced Photodegradation Activities for Norfloxacin" Catalysts 10, no. 4: 373. https://doi.org/10.3390/catal10040373



