Abstract
A TMSCl-catalyzed tandem reaction of dihydroisobenzofuran acetals with indoles has been developped, which could provide an efficient and straightforward access to various tetrahydroisoquinolones in moderate to excellent yields. This process involved the first addition of the indoles to acetals, followed by skeletal rearrangement.
1. Introduction
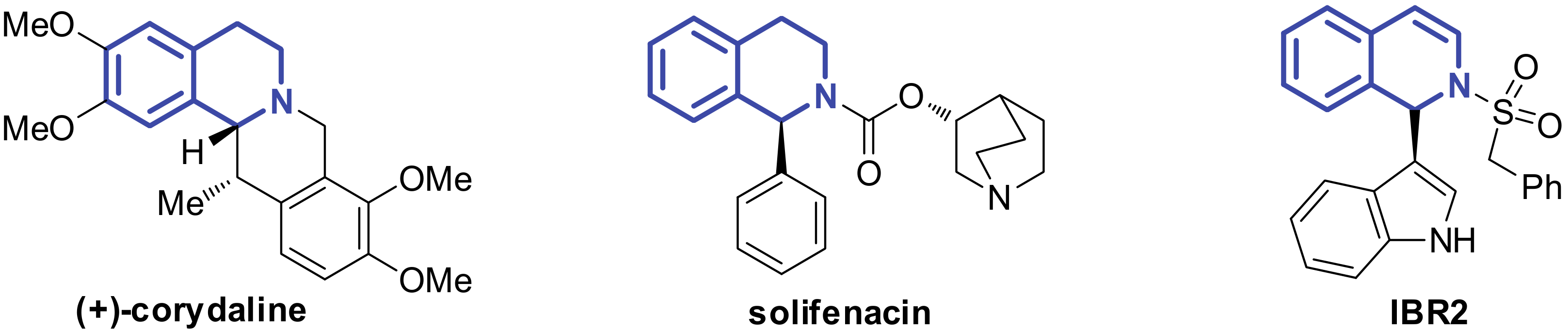
Isoquinoline frameworks widely exist in many natural bioactive products and synthetic pharmaceutical molecules (Figure 1) [1,2,3,4,5]. Among them, the C1-substituted isoquinolines constitute an important group. For example, the α-phenylisoquinoline solifenacin is an FDA-approved drug for urge incontinence [6]. α-Indolisoquinoline-like IBR2 has a significant inhibitory effect on triple-negative human breast cancer cells [7,8,9,10]. Such compounds are typically prepared by metal catalyzed cross-dehydrogenative coupling. [11,12,13]. Recently, we developed an acid catalyzed dearomative arylation strategy for the synthesis of α-indolisoquinolines [14]. We also found that the tandem reaction of dihydroisobenzofuran acetal with indoles could afford tetrahydroisoquinolones [15]. However, such a process was highly substrate dependant, and only 5-OMe dihydroisobenzofuran acetal was suitable for this reaction. Given the potential medicinal value of Cl-indole substituted isoquinoline structures, it is of great significance to synthetise α-indole isoquinolines with structural diversity in a mild, direct, and efficient manner.
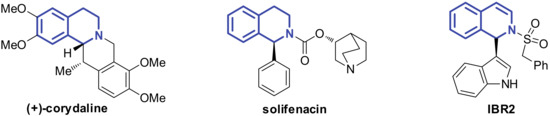
Figure 1.
Biologically Active Compounds.
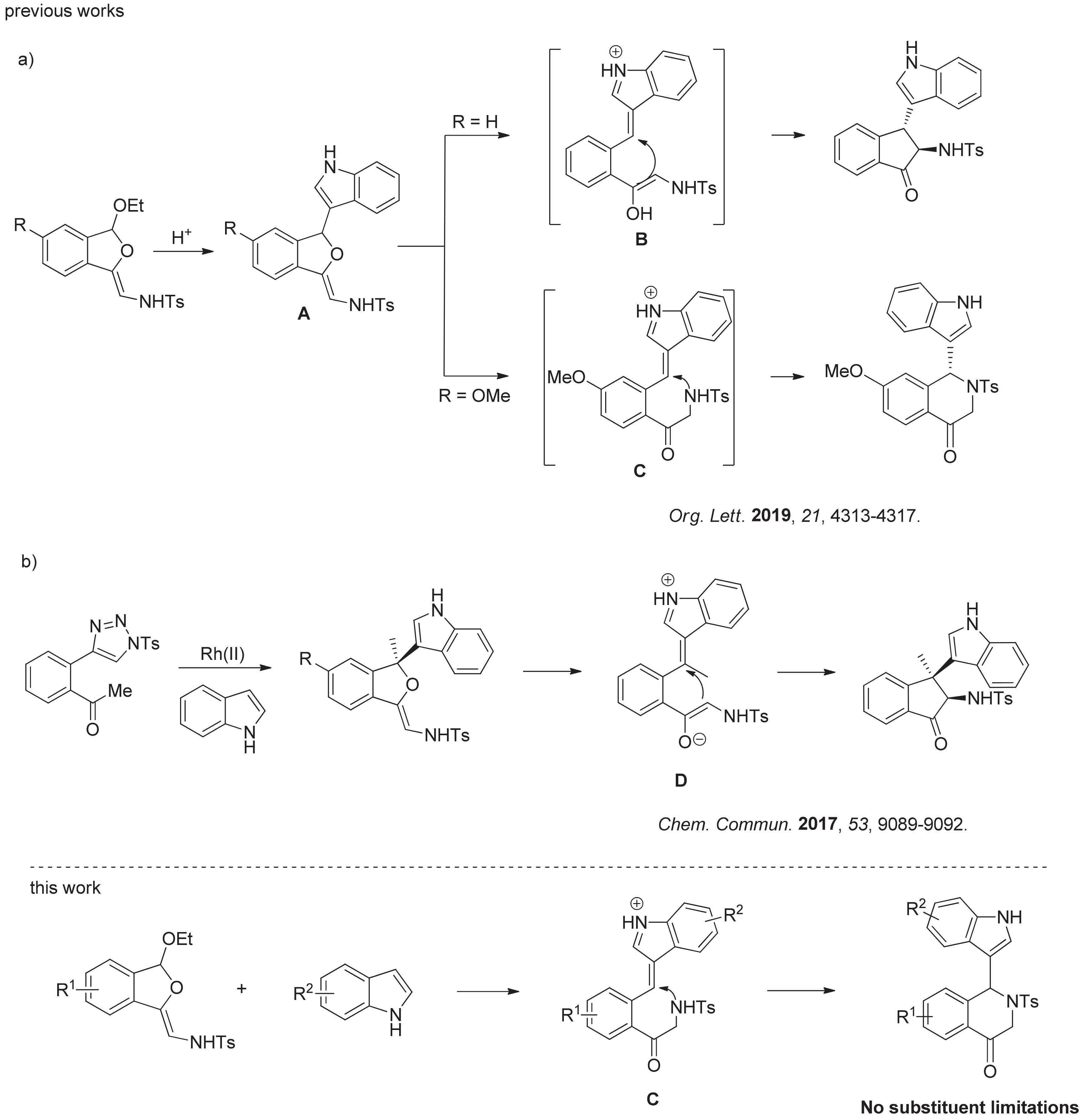
N-Sulfonyl-1,2,3-triazoles have received considerable attention, since they could serve as precursors of metal-bound imino carbenes or ketenimine intermediates [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36]. Recently, we found dihydroisobenzofuran acetals, prepared easily from N-sulfonyltriazoles [37], are good substrates for tandem reaction, because they could undergo nucleophilic addition and subsequent skeletal rearrangement (Figure 2a) [15]. By the catalysis of a phosphoric acid catalyst, the reaction firstly experiences an intermediate A, which can undergo intramolecular Michael or aza-Michael addition to selectively give amino indanones or tetrahydroisoquinolones frameworks. However, the process for the formation of tetrahydroisoquinolones can be challenging, because this type of reaction requires special substrate of 5-OMe dihydroisobenzofuran acetal and gives low to moderate yields, which greatly limit its use. In addition, Yang et al. found that rhodium could promote the reaction of N-sulfonyl triazoles with indoles in tandem manner (Figure 2b) [38]. In this case, the intramolecular Michael addition was realized to give amino indanones, which also demonstrated a challenge for the formation of tetrahydroisoquinolones. Intrigued by the formation of tetrahydroisoquinolones, we tried to optimize this particular process to expand the scope of dihydroisobenzofuran acetals.
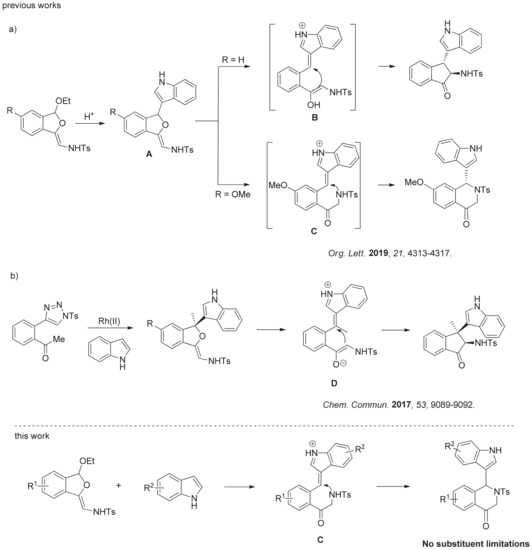
Figure 2.
Related Research and This Work. (a) Switchable Skeletal Rearrangement of Dihydroiso- benzofuran Acetals with Indoles. (b) Rhodium-catalyzed tandem reaction of N-sulfonyl triazoles with indoles.
2. Results and Discussion
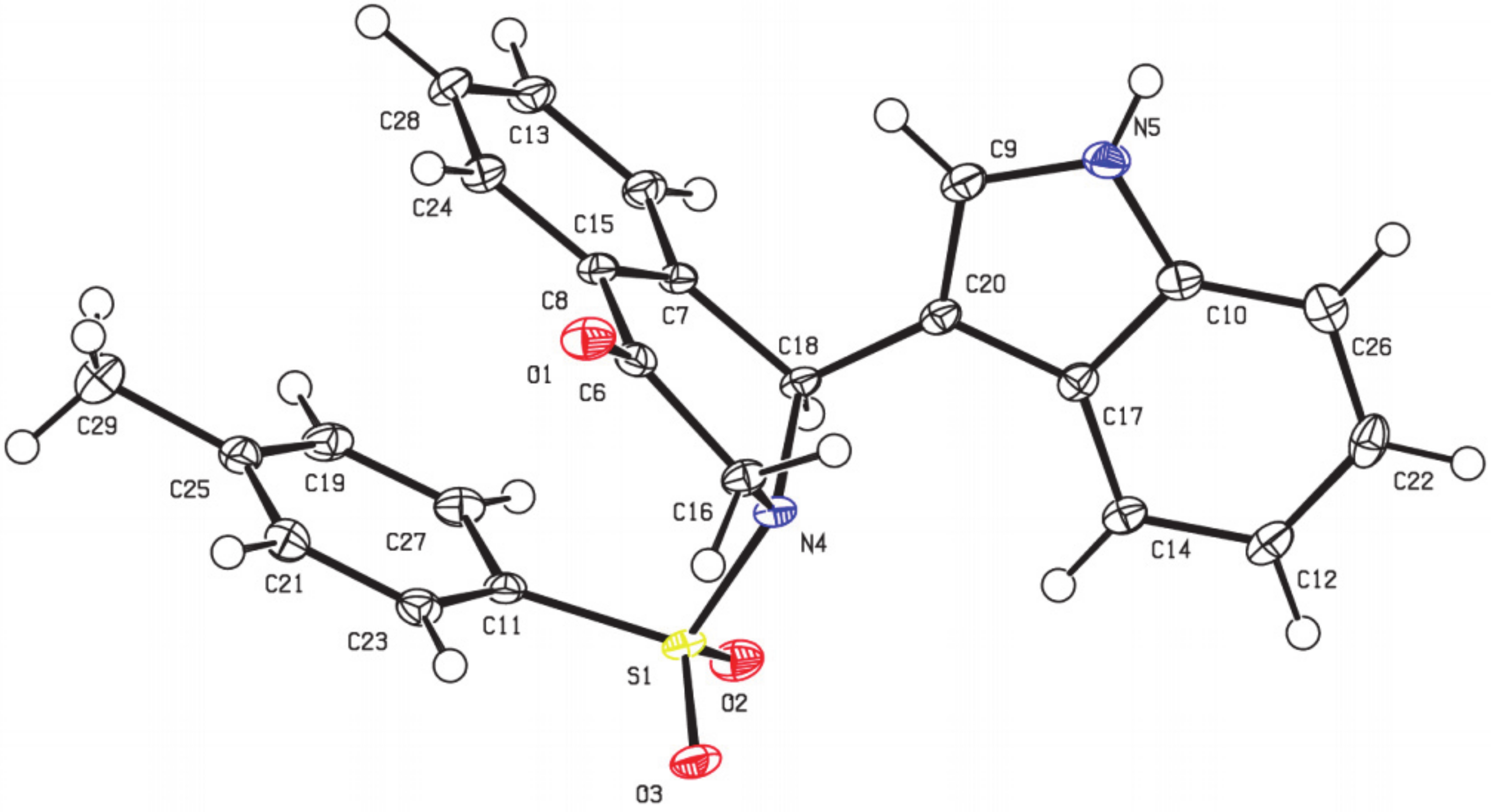
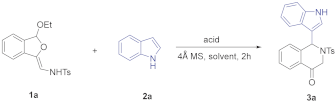
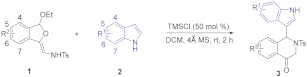
As part of our efforts to develop an efficient and straightforward access to various indole-substituted isoquinolines, we herein reported an efficient method to synthesize indole-substituted tetrahydroisoquinolones through a TMSCl-catalyzed tandem reaction of dihydroisobenzofuran acetals with indoles (see Supplementary Materials). According to our previous study, phosphoric acid could promote the reaction of 5-OMe dihydroisobenzofuran acetal with indoles to give indole-substituted tetrahydroisoquinolones. We wondered whether the use of an appropriate acid catalyst could promote this reaction with broader substrate scope. To explore this feasibility, we conducted the reaction of isobenzofuran acetal 1a and commercially available indole 2a by using several acids in CHCl3 at 0 °C for 2 h. When CF3CO2H was employed as an acid catalyst, the reaction could proceed successfully to give the desired dihydroisoquinolinone product 3a in 34% yield (Table 1, entry 1), while no reaction occurred using AcOH (Table 1, entry 2). The structure of 3a was characterized by X-ray diffraction (Figure 3) [39]. Metal Lewis acid such as AlCl3 could also catalyze this reaction, and the yield was improved to 58% (Table 1, entry 4). Further screening showed the use of trimethylchlorosilane could improve the yield to 60% (Table 1, entry 6). Meanwhile, the loading of indole 2a also played an important role on the yield. The results showed the yield could be increased from 60% to 76% by increasing the ratio of 2a:1a to 2.0 equiv (Table 1, entry 9). Further screening revealed that increasing the reaction temperature to room temperature resulted in an obvious increase in the yield to 84% (Table 1, entry 10). Next, we tested some other solvents with the indole loading of 1.2 equiv at room temperature. The best yield of 92% was obtained when dichloromethane was used (Table 1, entry 11), and no better results were obtained for other solvents such as dichloroethane, toluene, ethyl ether, methyl tert-butyl ether, and tetrahydrofuran (Table 1, entries 12–16). Finally, the reaction was conducted in the presence of 50 mol% TMSCl, with the 2.0 equiv of indole in dichloromethane at room temperature for 2 h.

Table 1.
Optimization of the Reaction for the Synthesis of Indole-substituted Dihydroisoquinolinone.
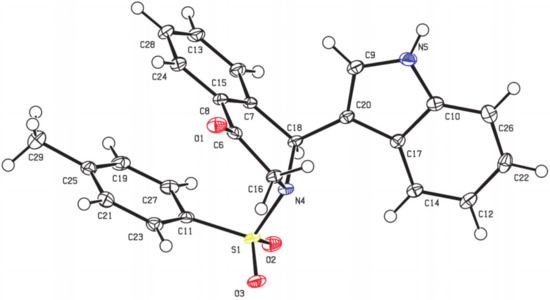
Figure 3.
X-ray structure of 3a.
Under the optimal reaction conditions, we next examined the generality and limitation of this tandem reaction (Table 2). A representative spread of indoles 2 worked well to afford the corresponding adducts 3. Both the electron-withdrawing group and electron-donating group on the different position at indoles could give the corresponding products in satisfactory yields (Table 2, entries 1–14). The 4-Methylindole could provide the desired product in 68% yield (Table 2, entry 2). Indoles with methyl, methoxy, benzyloxy, fluoro, chloro, and bromo substituents at C5 and C6 positions also provide satisfactory yields (71−86%) (Table 2, entries 3–11). C7-methyl, methoxy, and fluoro substituted indoles afford the products in 69–88% yields (Table 2, entries 12–14). The next was focused on different dihydroisobenzofuran acetals. Benzofuran acetals 1 with electron-donating substituents (-Me, -OMe) gave corresponding product in a 63–72% yield (Table 2, entries 18 and 19), while benzofuran acetals with electron-withdrawing substituents (-F, -Cl) led to lower yields (52–58%) (Table 2, entries 15–17 and 20).

Table 2.
Scope for the Synthesis of 3 a.
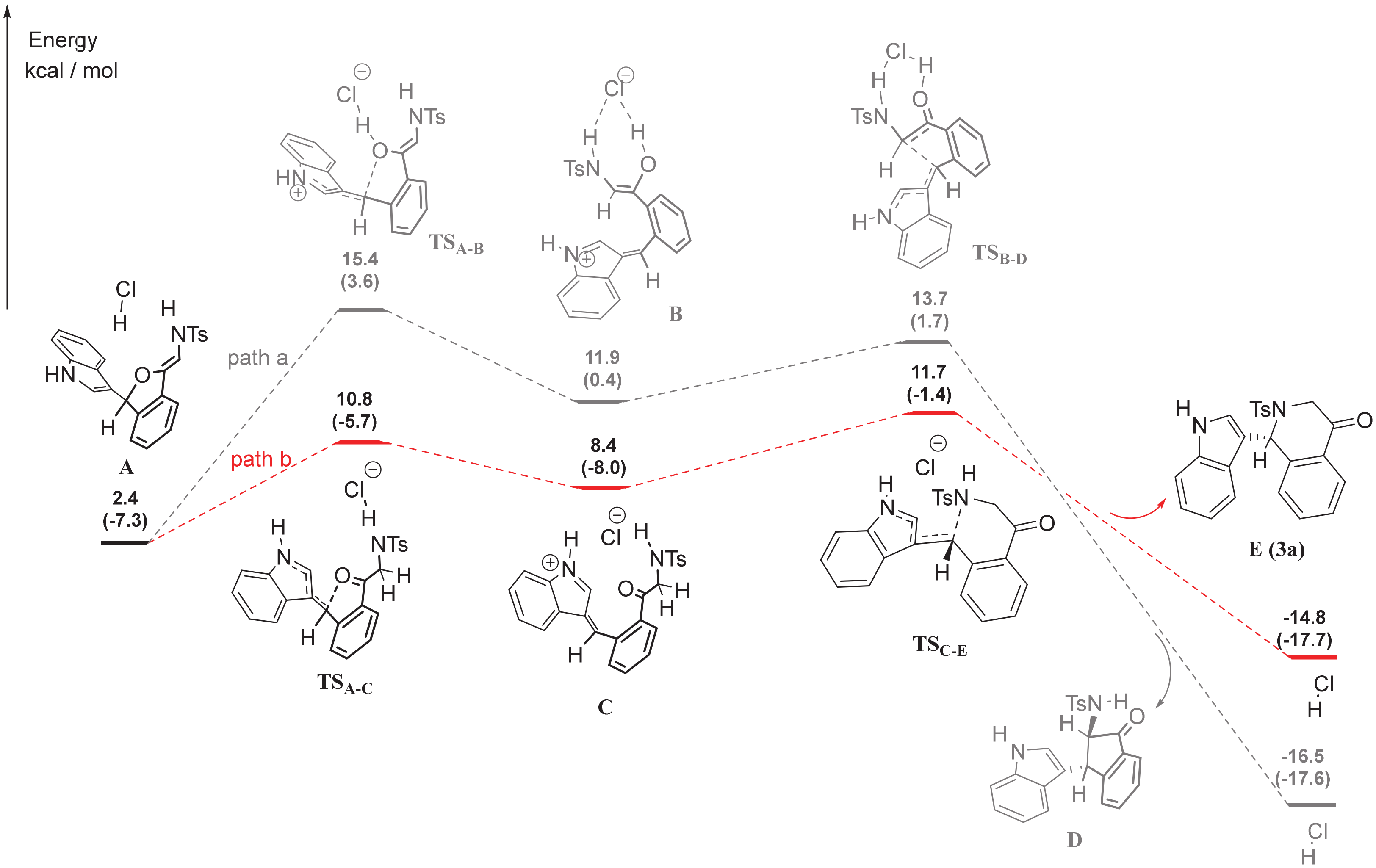
According to our previous work [15], indole could attack dihydroisobenzofuran acetals to form the intermediate indol-dihydroisobenzofuran, which subsequently underwent an intramolecular (aza)-Michael addition to give amino indanones or tetrahydroisoquinolones. To further understand the regioselectivity of intramolecular (aza)-Michael addition under TMSCl, we next performed the DFT calculations (Figure 4). To make the calculation easier, HCl was used as a catalyst instead of TMSCl. The detailed formation process of intermediate A could be found in SI. Starting from intermediate A, the reaction could proceed through two routes. By path a, the enol intermediate B could be obtained via TSA-B, while ketone intermediate C obtained via TSA-C by path b. Furthermore, intermediate B could undergo the Michael addition to form amino indanone D via TSB-D, while intermediate C could provide tetrahydroisoquinolones E via TSC-E. The energy for the formation of TSA-B (ΔG = 13.0 kcal/mol) is higher than the formation of TSA-C (ΔG = 8.4 kcal/mol). Therefore, the intermediate A was more likely to form tetrahydroisoquinolones E via path b.
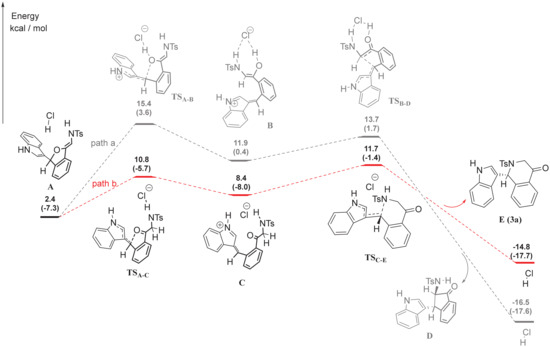
Figure 4.
Detailed Reaction Meahanism by DFT Calculations. A, B and C were the possible intermediates, D and E were the possible products.
3. Materials and Methods
In an ordinary vial, TMSCl (0.05 mmol, 50 mol%) was added to a solution of isobenzofuran acetal 1 (0.10 mmol, 1.0 equiv), indole 2 (0.20 mmol, 2.0 equiv), and 4 Å MS (100 mg) in dry CH2Cl2 (2 mL). After stirring for 2 h at room temperature, the solvent was removed under vacuum and residue was purified by flash column chromatography (petroleum ether/AcOEt 6:1) to give the pure desired products 3 as a whole solid.
4. Conclusions
In conclusion, we have developed a TMSCl-catalyzed tandem reaction of dihydroisobenzofuran acetals with indoles to easily access various indole-substituted tetrahydroisoquinolones in good yields. This strategy overcomes the limitation of substituents on the reaction under a simple and mild condition. A plausible reaction mechanistic was performed by DFT calculations, which indicated that it was easier to afford tetrahydroisoquinolones than amino indanone under TMSCl.
Supplementary Materials
The General Methods, Characterization Data, Computational Details, 1H and 13C NMR Spectra are available online at https://www.mdpi.com/2073-4344/10/4/392/s1.
Author Contributions
M.Z. and S.L. contributed the central idea and performed research. G.L. contributed to refining the ideas and doing the analyses of reaction mechanism. L.H. wrote the paper, carrying out additional analyses. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the NSFC (21871296, 21907111), the Guangdong Natural Science Founds for Distinguished Young Scholars (2017A030306017) and the Fundamental Research Funds for the Central Universities (19ykpy128).
Acknowledgments
We would like to dedicate to Albert S.C. Chan on the occasion of his 70th birthday.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Michael, J.P. Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep. 2002, 19, 742–746. [Google Scholar] [CrossRef]
- Hansch, C.; Sammes, P.G.; Taylor, J.B. Comprehensive Medicinal Chemistry, 1st ed.; Pergamon: Oxford, UK, 1990. [Google Scholar]
- Zhang, Q.; Tu, G.; Zhao, Y.; Cheng, T. Novel bioactive isoquinoline alkaloids from Carduus crispus. Tetrahedron 2002, 58, 6795–6798. [Google Scholar] [CrossRef]
- Reddy, N.S.S.; Reddy, B.J.M.; Reddy, B.S. A convergent and stereoselective total synthesis of (-)-crispine A, (-)-benzo[α]quinolizidine and (-)-salsolidine. Tetrahedron Lett. 2013, 54, 4228–4231. [Google Scholar] [CrossRef]
- Singh, H.; Singh, P.; Kumari, K.; Chandra, A.; K Dass, S.; Chandra, R. A review on noscapine, and its impact on heme metabolism. Curr. Drug Metab. 2013, 14, 351–360. [Google Scholar] [CrossRef]
- Ohtake, A.; Ukai, M.; Hatanaka, T.; Kobayashi, S.; Ikeda, K.; Sato, S.; Miyata, K.; Sasamata, M. In Vitro and In Vivo tissue selectivity profile of solifenacin succinate (YM905) for urinary bladder over salivary gland in rats. Eur. J. Pharmacol. 2004, 492, 243–250. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, L.; Wu, G.; Konig, H.; Lin, X.; Li, G.; Qiu, X.L.; Chen, C.F.; Hu, C.M.; Goldblatt, E. A novel small molecule RAD51 inactivator overcomes imatinib-resistance in chronic myeloid leukaemia. EMBO. Mol. Med. 2013, 5, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-H.; Chen, P.-L.; Zhu, J. Compositions and Methods for Disruption of BRCA2-RAD51 Interaction. PCT Int. Appl. WO 2006044933, 27 April 2006. Available online: https://worldwide.espacenet.com/patent/search/family/035695666/publication/WO2006044933A2?
- Chung, T.-W.; Hung, Y.-T.; Thikekar, T.; Paike, V.V.; Lo, F.Y.; Tsai, P.-H.; Liang, M.-C.; Sun, C.-M. Telescoped synthesis of 2-Acyl-1-aryl-1, 2-dihydroisoquinolines and their Inhibition of the transcription Factor NF-κB. ACS Comb. Sci. 2015, 17, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, H.; Guo, X.E.; Qiu, X.-L.; Hu, C.-M.; Chamberlin, A.R.; Lee, W.-H. Synthesis, molecular modeling, and biological evaluation of novel RAD51 inhibitors. Eur. J. Med. Chem. 2015, 96, 196–208. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.-J. CuBr-catalyzed direct indolation of tetrahydroisoquinolines via Cross-dehydrogenative coupling between sp3 C-H and sp2 C-H Bonds. J. Am. Chem. Soc. 2005, 127, 6968–6969. [Google Scholar] [CrossRef]
- Zhong, J.-J.; Meng, Q.-Y.; Liu, B.; Li, X.-B.; Gao, X.-W.; Lei, T.; Wu, C.-J.; Li, Z.-J.; Tung, C.-H.; Wu, L.-Z. Cross-Coupling hydrogen evolution reaction in homogeneous solution without noble metals. Org. Lett. 2014, 16, 1988–1991. [Google Scholar] [CrossRef]
- Patil, M.R.; Dedhia, N.P.; Kapdi, A.R.; Kumar, A.V. Cobalt (II)/N-Hydroxyphthalimide-catalyzed cross-dehydrogenative coupling reaction at room temperature under aerobic condition. J. Org. Chem. 2018, 83, 4477–4490. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, W.; Zhu, G.; Bao, G.; Zhang, B.; Hong, L.; Li, M.; Wang, R. Enantioselective dearomative arylation of Isoquinolines. ACS Catal. 2016, 6, 5290–5294. [Google Scholar] [CrossRef]
- Li, G.; Yao, Y.; Wang, Z.; Zhao, M.; Xu, J.; Huang, L.; Zhu, G.; Bao, G.; Sun, W.; Hong, L. Switchable skeletal rearrangement of dihydroisobenzofuran acetals with indoles. Org. Lett. 2019, 21, 4313–4317. [Google Scholar] [CrossRef]
- Davies, H.M.; Alford, J.S. Reactions of metallocarbenes derived from N-sulfonyl-1, 2, 3-triazoles. Chem. Soc. Rev. 2014, 43, 5151–5162. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, R.; Tang, X.Y.; Shi, M. Recent advances in the synthesis of heterocycles and related substances based on α-Imino Rhodium Carbene complexes derived from N-Sulfonyl-1, 2, 3-triazoles. Chem. Eur. J. 2016, 22, 17910–17924. [Google Scholar] [CrossRef]
- Li, Y.; Yang, H.; Zhai, H. The expanding utility of Rhodium-Iminocarbenes: Recent advances in the synthesis of natural products and related scaffolds. Chem. Eur. J. 2018, 24, 12757–12766. [Google Scholar] [CrossRef]
- Alford, J.S.; Davies, H.M. Mild aminoacylation of indoles and pyrroles through a three-component reaction with ynol ethers and sulfonyl azides. J. Am. Chem. Soc. 2014, 136, 10266–10269. [Google Scholar] [CrossRef]
- Chen, K.; Zhu, Z.Z.; Zhang, Y.S.; Tang, X.Y.; Shi, M. Rhodium (II)-Catalyzed Intramolecular Cycloisomerizations of Methylenecyclopropanes with N-Sulfonyl 1, 2, 3-Triazoles. Angew. Chem. Int. Ed. 2014, 53, 6645–6649. [Google Scholar] [CrossRef]
- Chuprakov, S.; Worrell, B.T.; Selander, N.; Sit, R.K.; Fokin, V.V. Stereoselective 1, 3-insertions of rhodium (II) azavinyl carbenes. J. Am. Chem. Soc. 2014, 136, 195–202. [Google Scholar] [CrossRef]
- He, J.; Shi, Y.; Cheng, W.; Man, Z.; Yang, D.; Li, C.Y. Rhodium-Catalyzed Synthesis of 4-Bromo-1, 2-dihydroisoquinolines: Access to Bromonium Ylides by the Intramolecular Reaction of a Benzyl Bromide and an α-Imino Carbene. Angew. Chem. Int. Ed. 2016, 55, 4557–4561. [Google Scholar] [CrossRef]
- Kwok, S.W.; Zhang, L.; Grimster, N.P.; Fokin, V.V. Catalytic Asymmetric Transannulation of NH-1, 2, 3-Triazoles with Olefins. Angew. Chem. Int. Ed. 2014, 53, 3452–3456. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, V.N.; Viart, H.M.-F.; Sarpong, R. Stereodivergent intramolecular C (sp3)-H functionalization of azavinyl carbenes: Synthesis of saturated heterocycles and fused N-heterotricycles. J. Am. Chem. Soc. 2015, 137, 8368–8371. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yao, W.; Liu, Y.; Wei, Q.; Chen, J.; Wu, X.; Xia, F.; Hu, W. A Rh (II)-catalyzed multicomponent reaction by trapping an α-amino enol intermediate in a traditional two-component reaction pathway. Sci. Adv. 2017, 3, e1602467. [Google Scholar] [CrossRef]
- Mi, P.; Kiran Kumar, R.; Liao, P.; Bi, X. Tandem O-H Insertion/[1,3]-Alkyl Shift of Rhodium Azavinyl Carbenoids with Benzylic Alcohols: A Route To Convert C–OH Bonds into C–C Bonds. Org. Lett. 2016, 18, 4998–5001. [Google Scholar] [CrossRef]
- Miura, T.; Tanaka, T.; Biyajima, T.; Yada, A.; Murakami, M. One-Pot Procedure for the Introduction of Three Different Bonds onto Terminal Alkynes through N-Sulfonyl-1, 2, 3-Triazole Intermediates. Angew. Chem. Int. Ed. 2013, 52, 3883–3886. [Google Scholar] [CrossRef]
- Schultz, E.E.; Lindsay, V.N.; Sarpong, R. Expedient Synthesis of Fused Azepine Derivatives Using a Sequential Rhodium (II)-Catalyzed Cyclopropanation/1-Aza-Cope Rearrangement of Dienyltriazoles. Angew. Chem. Int. Ed. 2014, 53, 9904–9908. [Google Scholar] [CrossRef]
- Xu, Z.-F.; Dai, H.; Shan, L.; Li, C.-Y. Metal-Free Synthesis of (E)-Monofluoroenamine from 1-Sulfonyl-1, 2, 3-triazole and Et2O· BF3 via Stereospecific Fluorination of α-Diazoimine. Org. Lett. 2018, 20, 1054–1057. [Google Scholar] [CrossRef]
- Yang, J.M.; Zhu, C.Z.; Tang, X.Y.; Shi, M. Rhodium (II)-Catalyzed Intramolecular Annulation of 1-Sulfonyl-1, 2, 3-Triazoles with Pyrrole and Indole Rings: Facile Synthesis of N-Bridgehead Azepine Skeletons. Angew. Chem. Int. Ed. 2014, 53, 5142–5146. [Google Scholar]
- Bae, I.; Han, H.; Chang, S. Highly efficient one-pot synthesis of N-sulfonylamidines by Cu-catalyzed three-component coupling of sulfonyl azide, alkyne, and amine. J. Am. Chem. Soc. 2005, 127, 2038–2039. [Google Scholar] [CrossRef]
- Cho, S.H.; Chang, S. Rate-Accelerated Nonconventional Amide Synthesis in Water: A Practical Catalytic Aldol-Surrogate Reaction. Angew. Chem. Int. Ed. 2007, 46, 1897–1900. [Google Scholar] [CrossRef]
- Cho, S.H.; Chang, S. Room Temperature Copper-Catalyzed 2-Functionalization of Pyrrole Rings by a Three-Component Coupling Reaction. Angew. Chem. Int. Ed. 2008, 47, 2836–2839. [Google Scholar] [CrossRef]
- Li, G.; Zhao, M.; Xie, J.; Yao, Y.; Mou, L.; Zhang, X.; Guo, X.; Sun, W.; Wang, Z.; Xu, J.; et al. Efficient synthesis of cyclic amidine-based fluorophores via 6p-electrocyclic ring closure. Chem. Sci. 2020. [Google Scholar] [CrossRef]
- Dodd, R.H.; Cariou, K. Ketenimines Generated from Ynamides: Versatile Building Blocks for Nitrogen-Containing Scaffolds. Chem. Eur. J. 2018, 24, 2297–2304. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, R.; Wang, Q.; Tang, X.-Y.; Shi, M. Cyclization of sulfide, ether or tertiary amine-tethered N-sulfonyl-1, 2, 3-triazoles: A facile synthetic protocol for 3-substituted isoquinolines or dihydroisoquinolines. Chem. Commun. 2015, 51, 16968–16971. [Google Scholar] [CrossRef]
- Shen, H.; Fu, J.; Gong, J.; Yang, Z. Tunable and Chemoselective Syntheses of Dihydroisobenzofurans and Indanones via Rhodium-Catalyzed Tandem Reactions of 2-Triazole-benzaldehydes and 2-Triazole-alkylaryl Ketones. Org. Lett. 2014, 16, 5588–5591. [Google Scholar] [CrossRef]
- Yuan, H.; Gong, J.; Yang, Z. A rhodium-catalyzed tandem reaction of N-sulfonyl triazoles with indoles: Access to indole-substituted indanones. Chem. Commun. 2017, 53, 9089–9092. [Google Scholar] [CrossRef]
- Li, G. CCDC 1832699: Experimental Crystal Structure Determination; Lanzhou University: Lanzhou, China, 2020. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).