Multitarget Evaluation of the Photocatalytic Activity of P25-SiO2 Prepared by Atomic Layer Deposition
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
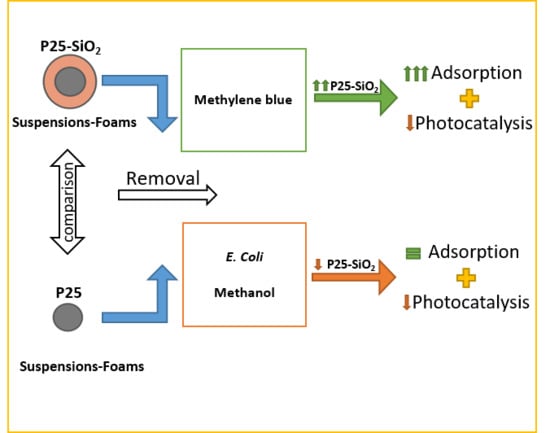
2.2. Photocatalytic Experiments
3. Materials and Methods
3.1. Atomic Layer Deposition
3.2. Photocatalytic Experiments
3.3. Photocatalytic Reactors
3.4. Foams Coating
3.5. Characterization Techniques
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ohno, T.; Sarukawa, K.; Tokieda, K.; Matsumura, M. Morphology of a TiO2 photocatalyst (Degussa, P25) consisting of anatase and rutile crystalline phases. J. Catal. 2001, 203, 82–86. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 for environmental photocatalytic applications: A review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Marschall, R.; Wang, L. Non-metal doping of transition metal oxides for visible-light photocatalysis. Catal. Today 2014, 225, 111–135. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Rehman, S.; Ullah, R.; Butt, A.M.; Gohar, N.D. Strategies of making TiO2 and ZnO visible light active. J. Hazard. Mater. 2009, 170, 560–569. [Google Scholar] [CrossRef]
- Osman, Y.; Jamal, R.; Rahman, A.; Xu, F.; Ali, A.; Abdiryim, T. Comparative study on poly(3,4-propylenedioxythiophene)/TiO2 nanocomposites synthesized by mechanochemical and chemical solution methods. Synth. Met. 2013, 179, 54–59. [Google Scholar] [CrossRef]
- Cheng, L.; Qiu, S.; Chen, J.; Shao, J.; Cao, S. A practical pathway for the preparation of Fe2O3 decorated TiO2 photocatalyst with enhanced visible-light photoactivity. Mater. Chem. Phys. 2017, 190, 53–61. [Google Scholar] [CrossRef]
- Li, X.; Lin, H.; Chen, X.; Niu, H.; Liu, J.; Zhang, T.; Qu, F. Dendritic α-Fe2O3//TiO2 nanocomposites with improved visible light photocatalytic activity. Phys. Chem. Chem. Phys. 2016, 18, 9176–9185. [Google Scholar] [CrossRef]
- Cheng, G.; Xu, F.; Xiong, J.; Wei, Y.; Stadler, F.J.; Chen, R. A novel protocol to design TiO2-Fe2O3 hybrids with effective charge separation efficiency for improved photocatalysis. Adv. Powder Technol. 2017, 28, 665–670. [Google Scholar] [CrossRef]
- Michalchuk, A.A.L.; Tumanov, I.A.; Konar, S.; Kimber, S.A.J.; Pulham, C.R.; Boldyreva, E.V. Challenges of mechanochemistry: Is in situ real-time quantitative phase analysis always reliable? A case study of organic salt formation. Adv. Sci. 2017, 4, 1700132. [Google Scholar] [CrossRef] [Green Version]
- Ritala, M.; Leskelä, M. Atomic layer deposition. Handb. Thin Film. 2002, 409, 103–159. [Google Scholar]
- Grillo, F.; Kreutzer, M.T.; van Ommen, J.R. Modeling the precursor utilization in atomic layer deposition on nanostructured materials in fluidized bed reactors. Chem. Eng. J. 2015, 268, 384–398. [Google Scholar] [CrossRef]
- van Ommen, J.R.; Goulas, A. Atomic layer deposition on particulate materials. Mater. Today Chem. 2019, 14, 100183. [Google Scholar] [CrossRef]
- Valencia, S.; Marín, J.M.; Restrepo, G. Study of the bandgap of synthesized titanium dioxide nanoparticules using the sol-gel method and a hydrothermal treatment. Open Mater. Sci. J. 2010, 4, 9–14. [Google Scholar] [CrossRef]
- Kalpaklı, Y.K.; Akgun, M.; Köneçoğlu, G.; Toygun, Ş.; Kalpaklı, Y.; Akgün, M. Photocatalytic degradation of textile dye CI Basic Yellow 28 wastewater by Degussa P25 based TiO2. Adv. Environ. Res. 2015, 4, 25–38. [Google Scholar]
- Martín, A.; Morales, V.; Ortiz-Bustos, J.; Pérez-Garnes, M.; Bautista, L.F.; García-Muñoz, R.A.; Sanz, R. Modelling the adsorption and controlled release of drugs from the pure and amino surface-functionalized mesoporous silica hosts. Microporous Mesoporous Mater. 2018, 262, 23–34. [Google Scholar] [CrossRef]
- Martín-Sómer, M.; Vega, B.; Pablos, C.; van Grieken, R.; Marugán, J. Wavelength dependence of the efficiency of photocatalytic processes for water treatment. Appl. Catal. B Environ. 2018, 221, 258–265. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Soltani, T.; Entezari, M.H. Photolysis and photocatalysis of methylene blue by ferrite bismuth nanoparticles under sunlight irradiation. J. Mol. Catal. A Chem. 2013, 377, 197–203. [Google Scholar] [CrossRef]
- McCaughan, B.; Rouanet, C.; Fowley, C.; Nomikou, N.; McHale, A.P.; McCarron, P.A.; Callan, J.F. Enhanced ROS production and cell death through combined photo- and sono-activation of conventional photosensitising drugs. Bioorg. Med. Chem. Lett. 2011, 21, 5750–5752. [Google Scholar] [CrossRef]
- May, J.M.; Qu, Z.C.; Whitesell, R.R. Generation of oxidant stress in cultured endothelial cells by methylene blue: Protective effects of glucose and ascorbic acid. Biochem. Pharmacol. 2003, 66, 777–784. [Google Scholar] [CrossRef]
- Marugán, J.; van Grieken, R.; Pablos, C.; Sordo, C. Analogies and differences between photocatalytic oxidation of chemicals and photocatalytic inactivation of microorganisms. Water Res. 2010, 44, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Jouali, A.; Salhi, A.; Aguedach, A.; Aarfane, A.; Ghazzaf, H.; Lhadi, E.K.; el krati, M.; Tahiri, S. Photo-catalytic degradation of methylene blue and reactive blue 21 dyes in dynamic mode using TiO2 particles immobilized on cellulosic fibers. J. Photochem. Photobiol. A Chem. 2019, 383, 112013. [Google Scholar] [CrossRef]
- Pablos, C.; Marugán, J.; van Grieken, R.; Adán, C.; Riquelme, A.; Palma, J. Correlation between photoelectrochemical behaviour and photoelectrocatalytic activity and scaling-up of P25-TiO2 electrodes. Electrochim. Acta 2014, 130, 261–270. [Google Scholar] [CrossRef]
- Marugán, J.; van Grieken, R.; Sordo, C.; Cruz, C. Kinetics of the photocatalytic disinfection of Escherichia coli suspensions. Appl. Catal. B Environ. 2008, 82, 27–36. [Google Scholar] [CrossRef]
- Xiong, J.; Li, G.; Hu, C. Treatment of methylene blue by mesoporous Fe/SiO2 prepared from rice husk pyrolytic residues. Catal. Today. 2019. [Google Scholar] [CrossRef]
- Nishikawa, H.; Takahara, Y. Adsorption and photocatalytic decomposition of odor compounds containing sulfur using TiO2/SiO2 bead. J. Mol. Catal. A Chem. 2001, 172, 247–251. [Google Scholar] [CrossRef]
- Yaparatne, S.; Tripp, C.P.A. Amirbahman, Photodegradation of taste and odor compounds in water in the presence of immobilized TiO2-SiO2 photocatalysts. J. Hazard. Mater. 2018, 346, 208–217. [Google Scholar] [CrossRef]
- Uddin, M.T.; Islam, M.A.; Mahmud, S.; Rukanuzzaman, M. Adsorptive removal of methylene blue by tea waste. J. Hazard. Mater. 2009, 164, 53–60. [Google Scholar] [CrossRef]
- Li, J.; Zhou, S.L.; Hong, G.B.; Chang, C.T. Hydrothermal preparation of P25-graphene composite with enhanced adsorption and photocatalytic degradation of dyes. Chem. Eng. J. 2013, 219, 486–491. [Google Scholar] [CrossRef]
- Nguyen, C.H.; Juang, R.S. Efficient removal of methylene blue dye by a hybrid adsorption–photocatalysis process using reduced graphene oxide/titanate nanotube composites for water reuse. J. Ind. Eng. Chem. 2019, 76, 296–309. [Google Scholar] [CrossRef]
- Ngoh, Y.S.; Nawi, M.A. Fabrication and properties of an immobilized P25 TiO2-montmorillonite bilayer system for the synergistic photocatalytic-adsorption removal of methylene blue. Mater. Res. Bull. 2016, 76, 8–21. [Google Scholar] [CrossRef]
- Jucker, B.A.; Harms, H.; Hug, S.J.; Zehnder, A.J.B. Adsorption of bacterial surface polysaccharides on mineral oxides is mediated by hydrogen bonds. Colloids Surf. B Biointerfaces 1997, 9, 331–343. [Google Scholar] [CrossRef]
- Huang, T.T.; Sturgis, J.; Gomez, R.; Geng, T.; Bashir, R.; Bhunia, A.K.; Robinson, J.P.; Ladisch, M.R. Composite surface for blocking bacterial adsorption on protein biochips. Biotechnol. Bioeng. 2003, 81, 618–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erdural, B.; Bolukbasi, U.; Karakas, G. Photocatalytic antibacterial activity of TiO2-SiO2 thin films: The effect of composition on cell adhesion and antibacterial activity. J. Photochem. Photobiol. A Chem. 2014, 283, 29–37. [Google Scholar] [CrossRef]
- Martín-Sómer, M.; Pablos, C.; de Diego, A.; van Grieken, R.; Encinas, Á.; Monsalvo, V.M.; Marugán, J. Novel macroporous 3D photocatalytic foams for simultaneous wastewater disinfection and removal of contaminants of emerging concern. Chem. Eng. J. 2019, 366, 449–459. [Google Scholar] [CrossRef]
- Grillo, F.; Moulijn, J.A.; Kreutzer, M.T.; van Ommen, J.R. Nanoparticle sintering in atomic layer deposition of supported catalysts: Kinetic modeling of the size distribution. Catal. Today 2018, 316, 51–61. [Google Scholar] [CrossRef]
- Guo, J.; van Bui, H.; Valdesueiro, D.; Yuan, S.; Liang, B.; van Ommen, J. Suppressing the photocatalytic activity of TiO2 nanoparticles by extremely thin Al2O3 films grown by gas-phase deposition at ambient conditions. Nanomaterials 2018, 8, 61. [Google Scholar] [CrossRef] [Green Version]
- Philippe, K.K.; Timmers, R.; van Grieken, R.; Marugan, J. Photocatalytic disinfection and removal of emerging pollutants from effluents of biological wastewater treatments, using a newly developed large-scale solar simulator. Ind. Eng. Chem. Res. 2016, 55, 2952–2958. [Google Scholar] [CrossRef]







| Feature | TiO2 | SiO2-TiO2 |
|---|---|---|
| SiO2 (%) | 0 | 2.78 |
| β365nm (cm2/g) | 48,700 | 36,300 |
| Bandgap (eV) | 3.05 | 3.04 |
| SBET (m2/g) | 53.3 | 48.7 |
| Zeta Potential (mV) | 18.1 | −3.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Sómer, M.; Benz, D.; van Ommen, J.R.; Marugán, J. Multitarget Evaluation of the Photocatalytic Activity of P25-SiO2 Prepared by Atomic Layer Deposition. Catalysts 2020, 10, 450. https://doi.org/10.3390/catal10040450
Martín-Sómer M, Benz D, van Ommen JR, Marugán J. Multitarget Evaluation of the Photocatalytic Activity of P25-SiO2 Prepared by Atomic Layer Deposition. Catalysts. 2020; 10(4):450. https://doi.org/10.3390/catal10040450
Chicago/Turabian StyleMartín-Sómer, Miguel, Dominik Benz, J. Ruud van Ommen, and Javier Marugán. 2020. "Multitarget Evaluation of the Photocatalytic Activity of P25-SiO2 Prepared by Atomic Layer Deposition" Catalysts 10, no. 4: 450. https://doi.org/10.3390/catal10040450