MIL-101(Cr) for CO2 Conversion into Cyclic Carbonates, Under Solvent and Co-Catalyst Free Mild Reaction Conditions
Abstract
:1. Introduction
2. Results and Discussion
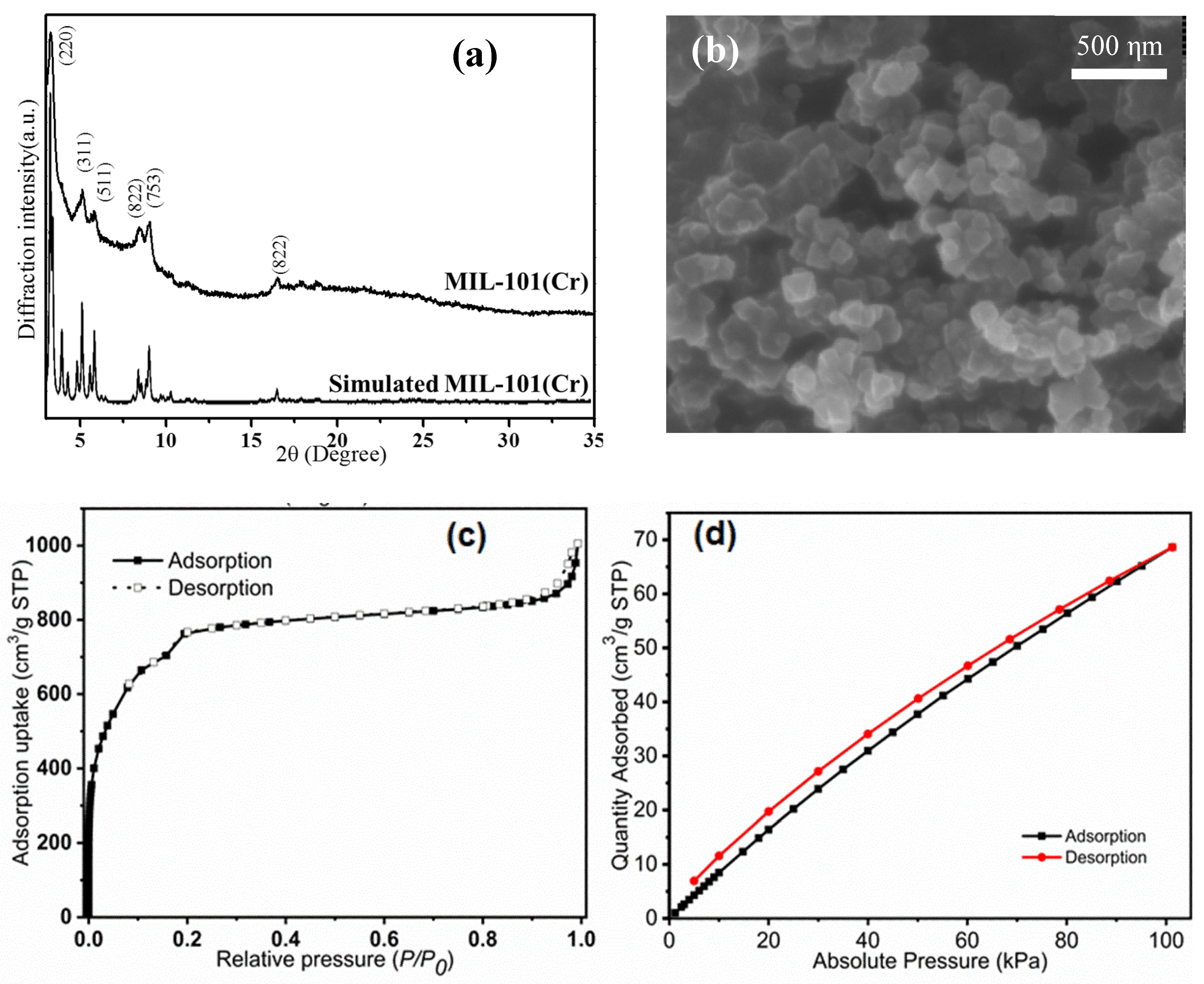
2.1. MIL-101(Cr) Characterization
2.2. Catalytic Reaction
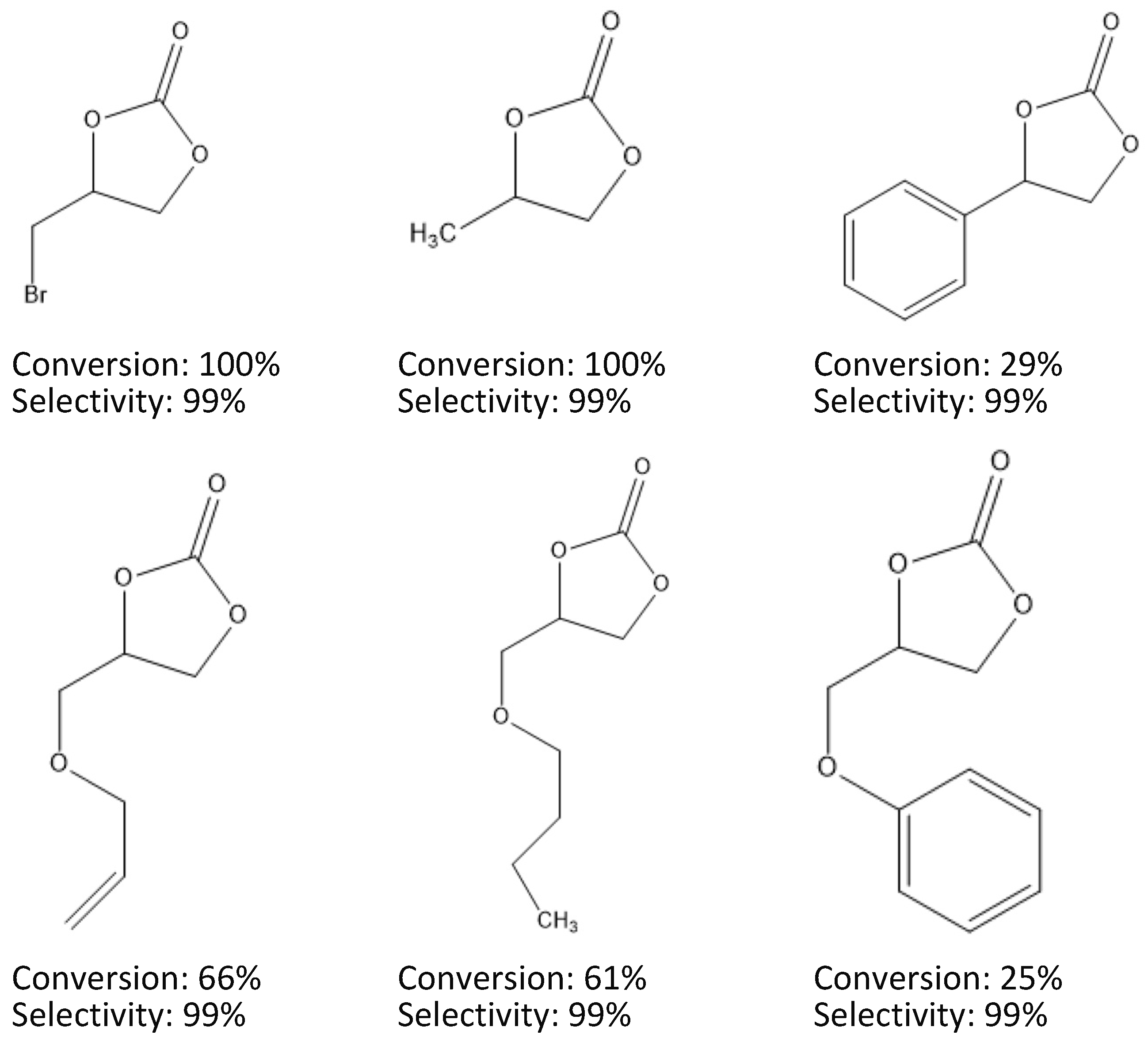
2.3. Substrate Scope
2.4. Catalytic Mechanism
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalytic Reaction
3.4. Catalyst Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tripati, A.K.; Roberts, C.D.; Eagle, R.A. Coupling of CO2 and ice sheet stability over major climate transitions of the last 20 million years. Science 2009, 326, 1394–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aresta, M.; Dibenedetto, A.; Angelini, A.; Catalysis for the valorization of exhaust carbon: from CO2 to chemicals, materials, and fuels. Technological use of CO2. Chem. Rev. 2013, 114, 1709–1742. [Google Scholar] [CrossRef]
- Alper, E.; Orhan, O.Y. CO2 utilization: Developments in conversion processes. Petroleum 2017, 3, 109–126. [Google Scholar] [CrossRef]
- Reithofer, M.R.; Sum, Y.N.; Zhang, Y. Synthesis of cyclic carbonates with carbon dioxide and cesium carbonate. Green Chem. 2013, 15, 2086–2090. [Google Scholar] [CrossRef]
- Phillips, C.E. Cobalt MOF-5: A Novel Catalyst for CO2 Conversion to Carbonates. Master Thesis, Chemical Engineering, University of Louisville, Louisville, KY, USA, 2012. [Google Scholar]
- North, M.; Pasquale, R. Mechanism of cyclic carbonate synthesis from epoxides and CO2. Angew. Chem. Int. Ed. 2009, 48, 2946–2948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousavi, B.; Chaemchuen, S.; Moosavi, B.; Zhou, K.; Yusubov, M.; Verpoort, F. CO2 Cycloaddition to Epoxides by using M-DABCO Metal–Organic Frameworks and the Influence of the Synthetic Method on Catalytic Reactivity. ChemistryOpen 2017, 6, 674–680. [Google Scholar] [CrossRef]
- Wang, T.; Song, X.; Luo, Q.; Yang, X.; Chong, S.; Zhang, J.; Ji, M. Acid-base bifunctional catalyst: Carboxyl ionic liquid immobilized on MIL-101-NH2 for rapid synthesis of propylene carbonate from CO2 and propylene oxide under facile solvent-free conditions. Microporous Mesoporous Mater. 2018, 267, 84–92. [Google Scholar] [CrossRef]
- Ullah, H.; Mousavi, B.; Younus, H.A.; Khattak, Z.A.; Suleman, S.; Jan, M.T.; Yu, B.; Chaemchuen, S.; Verpoort, F. ONO pincer type ligand complexes of Al(III) as efficient catalyst for chemical fixation of CO2 to epoxides at atmospheric pressure. J. Catal. 2019, 377, 190–198. [Google Scholar] [CrossRef]
- Suleman, S.; Younus, H.A.; Ahmad, N.; Khattak, Z.A.; Ullah, H.; Park, J.; Han, T.; Yu, B.; Verpoort, F. Triazole based cobalt catalyst for CO2 insertion into epoxide at ambient pressure. Appl. Catal.; A 2020, 591, 117384. [Google Scholar] [CrossRef]
- Ullah, H.; Mousavi, B.; Younus, H.A.; Khattak, Z.A.; Chaemchuen, S.; Suleman, S.; Verpoort, F. Chemical fixation of carbon dioxide catalyzed via cobalt(III) ONO pincer ligated complexes. Commun. Chem. 2019, 2, 1–9. [Google Scholar]
- Khattak, Z.A.; Younus, H.A.; Ahmad, N.; Ullah, H.; Suleman, S.; Hossain, M.S.; Elkadi, M.; Verpoort, F. Highly active dinuclear cobalt complexes for solvent-free cycloaddition of CO2 to epoxides at ambient pressure. Chem. Commun. 2019, 55, 8274–8277. [Google Scholar] [CrossRef] [PubMed]
- Pescarmona, P.P.; Taherimehr, M. Challenges in the catalytic synthesis of cyclic and polymeric carbonates from epoxides and CO2. Catal. Sci. Technol. 2012, 2, 2169–2187. [Google Scholar] [CrossRef]
- Herbst, A.; Janiak, C. MOF catalysts in biomass upgrading towards value-added fine chemicals. CrystEngComm 2017, 19, 4092–4117. [Google Scholar] [CrossRef] [Green Version]
- Darensbourg, D.J.; Mackiewicz, R.M.; Phelps, A.L.; Rodgers, J.L.; The coupling of epoxides and carbon dioxide in the presence of homogeneous transition-metal catalysts. Production of polycarbonates vs cyclic carbonates. Prepr. Pap-Am. Chem. Soc. Div. Fuel Chem 2004, 49, 5–6. [Google Scholar]
- Zhou, Y.X.; Chen, Y.Z.; Hu, Y.; Huang, G.; Yu, S.H.; Jiang, H.L. MIL-101-SO3H: A Highly Efficient Brønsted Acid Catalyst for Heterogeneous Alcoholysis of Epoxides under Ambient Conditions. Chem. Eur. J. 2014, 20, 14976–14980. [Google Scholar] [CrossRef]
- Kuruppathparambil, R.R.; Jose, T.; Babu, R.; Hwang, G.-Y.; Kathalikkattil, A.C.; Kim, D.-W.; Park, D.-W. A room temperature synthesizable and environmental friendly heterogeneous ZIF-67 catalyst for the solvent less and co-catalyst free synthesis of cyclic carbonates. Appl. Catal.; B 2016, 182, 562–569. [Google Scholar] [CrossRef]
- Mousavi, B.; Chaemchuen, S.; Moosavi, B.; Luo, Z.; Gholampour, N.; Verpoort, F. Zeolitic imidazole framework-67 as an efficient heterogeneous catalyst for the conversion of CO2 to cyclic carbonates. New J. Chem. 2016, 40, 5170–5176. [Google Scholar] [CrossRef]
- Miralda, C.M.; Macias, E.E.; Zhu, M.; Ratnasamy, P.; Carreon, M.A. Zeolitic imidazole framework-8 catalysts in the conversion of CO2 to chloropropene carbonate. ACS Catal. 2011, 2, 180–183. [Google Scholar] [CrossRef]
- Férey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surblé, S.; Margiolaki, I. A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef]
- Leng, K.; Sun, Y.; Li, X.; Sun, S.; Xu, W. Rapid synthesis of metal–organic frameworks MIL-101(Cr) without the addition of solvent and hydrofluoric acid. Cryst. Growth Des. 2016, 16, 1168–1171. [Google Scholar] [CrossRef]
- Lin, K.-S.; Adhikari, A.K.; Su, Y.-H.; Chiang, C.-L.; Dehvari, K. Structural characterization of chromium atoms in MIL-101 metal organic frameworks using XANES/EXAFS spectroscopy. Chin. J. Phys. 2012, 50, 322–331. [Google Scholar]
- Liu, C.; Liu, X.-H.; Li, B.; Zhang, L.; Ma, J.-G.; Cheng, P. Salen–Cu (II)@ MIL-101(Cr) as an efficient heterogeneous catalyst for cycloaddition of CO2 to epoxides under mild conditions. J. Energy Chem. 2017, 26, 821–824. [Google Scholar] [CrossRef]
- Sun, X.; Xia, Q.; Zhao, Z.; Li, Y.; Li, Z. Synthesis and adsorption performance of MIL-101(Cr)/graphite oxide composites with high capacities of n-hexane. Chem. Eng. J. 2014, 239, 226–232. [Google Scholar] [CrossRef]
- Santiago-Portillo, A.; Navalón, S.; Cirujano, F.G.; Xamena, F.X.L.s.i.; Alvaro, M.; Garcia, H. MIL-101 as reusable solid catalyst for autoxidation of benzylic hydrocarbons in the absence of additional oxidizing reagents. ACS Catal. 2015, 5, 3216–3224. [Google Scholar] [CrossRef]
- Bahadori, M.; Tangestaninejad, S.; Bertmer, M.; Moghadam, M.; Mirkhani, V.; Mohammadpoor−Baltork, I.; Kardanpour, R.; Zadehahmadi, F. Task-Specific Ionic Liquid Functionalized–MIL–101(Cr) as a Heterogeneous and Efficient Catalyst for the Cycloaddition of CO2 with Epoxides Under Solvent Free Conditions. ACS Sustainable Chem. Eng. 2019, 7, 3962–3973. [Google Scholar] [CrossRef]
- Marandi, A.; Bahadori, M.; Tangestaninejad, S.; Moghadam, M.; Mirkhani, V.; Mohammadpoor-Baltork, I.; Frohnhoven, R.; Mathur, S.; Sandleben, A.; Klein, A. Cycloaddition of CO2 with epoxides and esterification reactions using the porous redox catalyst Co-POM@MIL-101(Cr). New J. Chem. 2019, 43, 15585–15595. [Google Scholar] [CrossRef]
- James, B.R.; Boissonnault, J.A.; Wong-Foy, A.G.; Matzger, A.J.; Sanford, M.S. Structure activity relationships in metal–organic framework catalysts for the continuous flow synthesis of propylene carbonate from CO2 and propylene oxide. RSC Adv. 2018, 8, 2132–2137. [Google Scholar] [CrossRef] [Green Version]
- Zalomaeva, O.V.; Chibiryaev, A.M.; Kovalenko, K.A.; Kholdeeva, O.A.; Balzhinimaev, B.S.; Fedin, V.P. Cyclic carbonates synthesis from epoxides and CO2 over metal–organic framework Cr-MIL-101. J. Catal. 2013, 298, 179–185. [Google Scholar] [CrossRef]
- Dai, W.; Mao, P.; Liu, Y.; Zhang, S.; Li, B.; Yang, L.; Luo, X.; Zou, J. Quaternary phosphonium salt-functionalized Cr-MIL-101: A bifunctional and efficient catalyst for CO2 cycloaddition with epoxides. J. CO2 Util. 2020, 36, 295–305. [Google Scholar] [CrossRef]
- Beyzavi, M.H.; Stephenson, C.J.; Liu, Y.; Karagiaridi, O.; Hupp, J.T.; Farha, O.K. Metal–organic framework-based catalysts: chemical fixation of CO2 with epoxides leading to cyclic organic carbonates. Front. Energy Res. 2015, 2, 63. [Google Scholar] [CrossRef] [Green Version]
- Nobar, S.N. Cu-BTC synthesis, characterization and preparation for adsorption studies. Mater. Chem. Phys. 2018, 213, 343–351. [Google Scholar] [CrossRef]
- He, H.; Perman, J.A.; Zhu, G.; Ma, S. Metal-Organic Frameworks for CO2 Chemical Transformations. Small 2016, 12, 6309–6324. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhang, Z.; Hu, S.; Wu, T.; Jiang, T.; Han, B. MOF-5/n-Bu4NBr: an efficient catalyst system for the synthesis of cyclic carbonates from epoxides and CO2 under mild conditions. Green Chem. 2009, 11, 1031–1036. [Google Scholar] [CrossRef]
- Vismara, R.; Tuci, G.; Mosca, N.; Domasevitch, K.V.; Di Nicola, C.; Pettinari, C.; Giambastiani, G.; Galli, S.; Rossin, A. Amino-decorated bis (pyrazolate) metal–organic frameworks for carbon dioxide capture and green conversion into cyclic carbonates. Inorganic Chemistry Frontiers 2019, 6, 533–545. [Google Scholar] [CrossRef]
- Kurisingal, J.F.; Rachuri, Y.; Gu, Y.; Kim, G.-H.; Park, D.-W. Binary metal-organic frameworks: Catalysts for the efficient solvent-free CO2 fixation reaction via cyclic carbonates synthesis. Appl. Catal.; A 2019, 571, 1–11. [Google Scholar] [CrossRef]
- Chaemchuen, S.; Xiao, X.; Ghadamyari, M.; Mousavi, B.; Klomkliang, N.; Yuan, Y.; Verpoort, F. Robust and efficient catalyst derived from bimetallic Zn/Co zeolitic imidazolate frameworks for CO2 conversion. J. Catal. 2019, 370, 38–45. [Google Scholar] [CrossRef]
- Ciprian, M.; Ruiz, K.H.; Kassymova, M.; Wang, T.; Zhuiykov, S.; Chaemchuen, S.; Tu, R.; Verpoort, F. 3D derived N-doped carbon matrix from 2D ZIF-L as an enhanced stable catalyst for chemical fixation. Microporous Mesoporous Mater. 2019, 285, 80–88. [Google Scholar] [CrossRef]
- Tharun, J.; Bhin, K.-M.; Roshan, R.; Kim, D.W.; Kathalikkattil, A.C.; Babu, R.; Ahn, H.Y.; Won, Y.S.; Park, D.-W. Ionic liquid tethered post functionalized ZIF-90 framework for the cycloaddition of propylene oxide and CO2. Green Chem. 2016, 18, 2479–2487. [Google Scholar] [CrossRef]
- Han, Y.-H.; Zhou, Z.-Y.; Tian, C.-B.; Du, S.-W. A dual-walled cage MOF as an efficient heterogeneous catalyst for the conversion of CO2 under mild and co-catalyst free conditions. Green Chem. 2016, 18, 4086–4091. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Zhang, Y.; Yang, X.; Zhao, L.; Wang, G. Functionalized IRMOF-3 as efficient heterogeneous catalyst for the synthesis of cyclic carbonates. J. Mol. Catal. A: Chem. 2012, 361–362, 12–16. [Google Scholar] [CrossRef]
- Huang, X.; Chen, Y.; Lin, Z.; Ren, X.; Song, Y.; Xu, Z.; Dong, X.; Li, X.; Hu, C.; Wang, B. Zn-BTC MOFs with active metal sites synthesized via a structure-directing approach for highly efficient carbon conversion. Chem. Commun. 2014, 50, 2624–2627. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Chen, R.-P.; Wang, X.-Y.; Liu, T.-T.; Wang, X.-S.; Huang, Y.-B.; Cao, R. Postsynthetic ionization of an imidazole-containing metal–organic framework for the cycloaddition of carbon dioxide and epoxides. Chem. Sci. 2017, 8, 1570–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demir, S.; Usta, S.; Tamar, H.; Ulusoy, M. Solvent free utilization and selective coupling of epichlorohydrin with carbon dioxide over zirconium metal-organic frameworks. Microporous Mesoporous Mater. 2017, 244, 251–257. [Google Scholar] [CrossRef]
- Ding, L.-G.; Yao, B.-J.; Jiang, W.-L.; Li, J.-T.; Fu, Q.-J.; Li, Y.-A.; Liu, Z.-H.; Ma, J.-P.; Dong, Y.-B. Bifunctional Imidazolium-Based Ionic Liquid Decorated UiO-67 Type MOF for Selective CO2 Adsorption and Catalytic Property for CO2 Cycloaddition with Epoxides. Inorg. Chem. 2017, 56, 2337–2344. [Google Scholar] [CrossRef]
- Zhu, J.; Usov, P.M.; Xu, W.; Celis-Salazar, P.J.; Lin, S.; Kessinger, M.C.; Landaverde-Alvarado, C.; Cai, M.; May, A.M.; Slebodnick, C.; et al. A New Class of Metal-Cyclam-Based Zirconium Metal–Organic Frameworks for CO2 Adsorption and Chemical Fixation. J. Am. Chem. Soc. 2018, 140, 993–1003. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, J.; Machain, Y.; Bonnett, B.; Lin, S.; Cai, M.; Kessinger, M.C.; Usov, P.M.; Xu, W.; Senanayake, S.D.; et al. Insights into CO2 adsorption and chemical fixation properties of VPI-100 metal–organic frameworks. J. Mater. Chem. A 2018, 6, 22195–22203. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.-N.; Jang, H.-G.; Seo, G.; Ahn, W.-S. CO2 cycloaddition of styrene oxide over MOF catalysts. Appl. Catal.; A 2013, 453, 175–180. [Google Scholar] [CrossRef]
- Meng, X.; Ju, Z.; Zhang, S.; Liang, X.; von Solms, N.; Zhang, X.; Zhang, X. Efficient transformation of CO2 to cyclic carbonates using bifunctional protic ionic liquids under mild conditions. Green Chem. 2019, 21, 3456–3463. [Google Scholar] [CrossRef]
- Sun, X.; Gu, J.; Yuan, Y.; Yu, C.; Li, J.; Shan, H.; Li, G.; Liu, Y. A Stable Mesoporous Zr-Based Metal Organic Framework for Highly Efficient CO2 Conversion. Inorg. Chem. 2019, 58, 7480–7487. [Google Scholar] [CrossRef]
- Chaemchuen, S.; Luo, Z.; Zhou, K.; Mousavi, B.; Phatanasri, S.; Jaroniec, M.; Verpoort, F. Defect formation in metal–organic frameworks initiated by the crystal growth-rate and effect on catalytic performance. J. Catal. 2017, 354, 84–91. [Google Scholar] [CrossRef]
- Taherimehr, M.; Decortes, A.; Al-Amsyar, S.M.; Lueangchaichaweng, W.; Whiteoak, C.J.; Escudero-Adán, E.C.; Kleij, A.W.; Pescarmona, P.P. A highly active Zn (salphen) catalyst for production of organic carbonates in a green CO2 medium. Catal. Sci. Technol. 2012, 2, 2231–2237. [Google Scholar] [CrossRef]
- Zanon, A.; Chaemchuen, S.; Mousavi, B.; Verpoort, F. 1 Zn-doped ZIF-67 as catalyst for the CO2 fixation into cyclic carbonates. J. CO2 Util. 2017, 20, 282–291. [Google Scholar] [CrossRef]


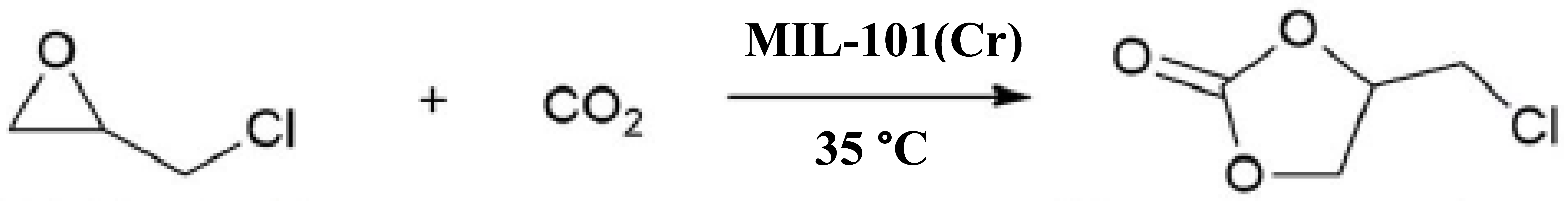 | ||||||
|---|---|---|---|---|---|---|
| Entry. | Catalyst (mg, mmol) | Time (h) | Pressure (bar) | Conversion (%) | Selectivity (%) | Yield 9 (%) |
| 1 | 50, 0.08 | 48 | 8 | 100 | 99 | 65 |
| 2 | 50, 0.08 | 48 | 4 | 100 | 99 | 62 |
| 3 | 50, 0.08 | 24 | 4 | 99 | 99 | 68 |
| 4 | 50, 0.08 | 6 | 4 | 53 | 99 | 43 |
| 5 | 50, 0.08 | 7 | 8 | 63 | 99 | 49 |
| 6 | 50, 0.08 | 48 | 1.5 | 100 | 99 | 70 |
| 7 | 50, 0.08 | 24 | 1.5 | 99 | 99 | 90 |
| 8 | 35, 0.06 | 24 | 1.5 | 65 | 99 | 52 |
| 9 | 25, 0.04 | 24 | 1.5 | 61 | 99 | 50 |
| 10 | 50, 0.08 | 12 | 1.5 | 52 | 99 | 42 |
| 11 | 50, 0.08 | 18 | 1.5 | 78 | 99 | 67 |
| 12 | 50, 0.08 | 22 | 1.5 | 88 | 99 | 70 |
| 13 2 | Blank | 24 | 1.5 | - | - | 0 |
| 14 3 | Cr(NO3)2·9H2O | 24 | 1.5 | - | - | 0 |
| 15 4 | BDC | 24 | 1.5 | - | - | 0 |
| 16 5 | 50, 0.08 | 24 | 1.5 | 99 | 99 | 90 |
| 17 6 | 50, 0.08 | 24 | 1.5 | 99 | 99 | 89 |
| 18 7 | 50, 0.08 | 24 | 1.5 | 99 | 99 | 89 |
| 19 8 | 50, 0.08 | 24 | 1.5 | 99 | 99 | 88 |
| Catalyst | Substrate | Temp. (°C) | Time (h) | Pressure (bar) | Conversion (%) | Selectivity (%) | Ref. |
|---|---|---|---|---|---|---|---|
| MIL-101 | EPH | 35 | 24 | 1.5 | 99 | 99 | This work |
| ZIF-8 | EPH | 100 | 4 | 7 | 98 | 33.4 | [19] |
| Zn-DABCO | EPH | 100 | 12 | 8 | >99 | 99 | [7] |
| Zn-MOF | EPH | 100 | 16 | 1 | 100 | 99 | [40] |
| F-IRMOF(Zn) | EPH | 140 | 1.5 | 20 | 80 | - | [41] |
| BIT-3(Zn) | EPH | 160 | 8 | 30 | 100 | 91 | [42] |
| HKUST-1 | EPH | 60 | 8 | 12 | 78 | >99 | [36] |
| UiO-66 | EPH | 60 | 8 | 12 | 67 | >99 | [36] |
| UiO/CuBTC | EPH | 60 | 8 | 12 | 91 | >99 | [36] |
| (I-)Meim-UiO-66 | EPH | 120 | 24 | 1 | 100 | 93 | [43] |
| UiO-67(Zr/V) | EPH | 100 | 2 | 16 | 64 | 96 | [44] |
| UiO-67-IL a | EPH | 90 | 3 | 1 | 99 | - | [45] |
| VPI-100(Zr/Cu) a | EPH | 90 | 6 | 10 | 99 | - | [46] |
| VPI-100(Hf/Cu) a | EPH | 90 | 6 | 1.5 | 97 | - | [47] |
| MIL-101-lL | EPH | 120 | 2 | 20 | 98 | 99 | [30] |
| MIL-101 | SO | 35 | 24 | 1.5 | 29 | >99 | This work |
| SOb | 60 | 18 | 80 | 50 | 100 | [40] | |
| SO | 120 | 14 | 20 | 63 | - | [48] | |
| MIL−101(Cr)−TSIL a | SO | 110 | 6 | 20 | 95 | 98 | [26] |
| Co-POM @MIL-101 a | SO | 110 | 2 | 20 | 88 | 88 | [27] |
| MOF-5 | SO | 120 | 14 | 20 | Inactive | - | [48] |
| UiO-66-NH2 | SO | 120 | 14 | 20 | 95 | - | [48] |
| (I-)Meim-UiO-66 | SO | 120 | 24 | 1 | 46 | 71 | [43] |
| MOF-74(Mg) | SO | 120 | 14 | 20 | 94 | - | [48] |
| HKUST-1 | SO | 120 | 14 | 20 | 48 | - | [48] |
| IRMOF-3 | SO | 120 | 14 | 20 | 33 | - | [48] |
| MIL-101a | PO | 100 | 14 | 5 | 80 | - | [28] |
| MIL-101-IL | PO | 120 | 24 | 1 | 89 | 99 | [30] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akimana, E.; Wang, J.; Likhanova, N.V.; Chaemchuen, S.; Verpoort, F. MIL-101(Cr) for CO2 Conversion into Cyclic Carbonates, Under Solvent and Co-Catalyst Free Mild Reaction Conditions. Catalysts 2020, 10, 453. https://doi.org/10.3390/catal10040453
Akimana E, Wang J, Likhanova NV, Chaemchuen S, Verpoort F. MIL-101(Cr) for CO2 Conversion into Cyclic Carbonates, Under Solvent and Co-Catalyst Free Mild Reaction Conditions. Catalysts. 2020; 10(4):453. https://doi.org/10.3390/catal10040453
Chicago/Turabian StyleAkimana, Emmanuelia, Jichao Wang, Natalya V. Likhanova, Somboon Chaemchuen, and Francis Verpoort. 2020. "MIL-101(Cr) for CO2 Conversion into Cyclic Carbonates, Under Solvent and Co-Catalyst Free Mild Reaction Conditions" Catalysts 10, no. 4: 453. https://doi.org/10.3390/catal10040453