Photoreforming of Glucose over CuO/TiO2
Abstract
1. Introduction
2. Results and Discussion
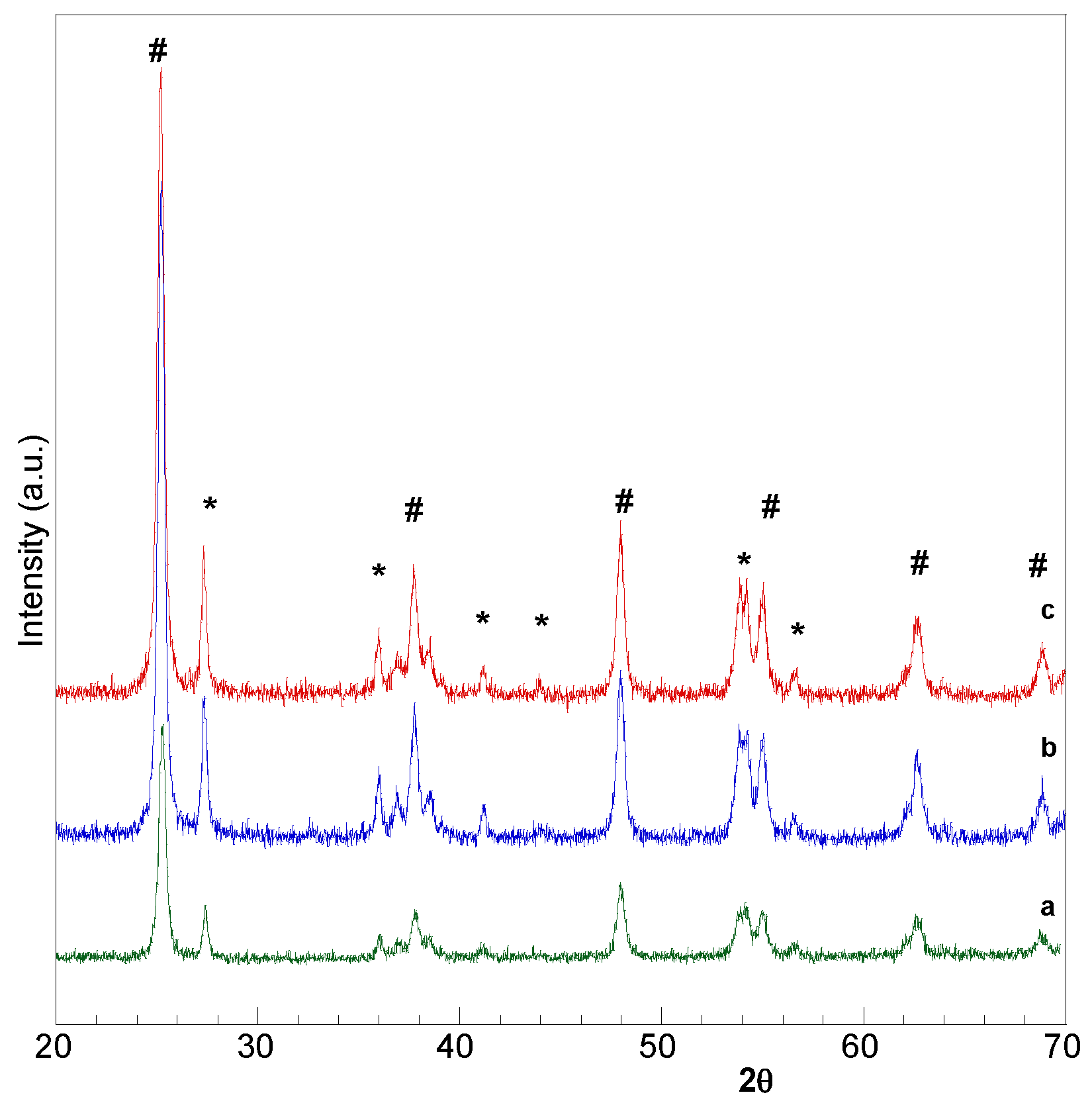
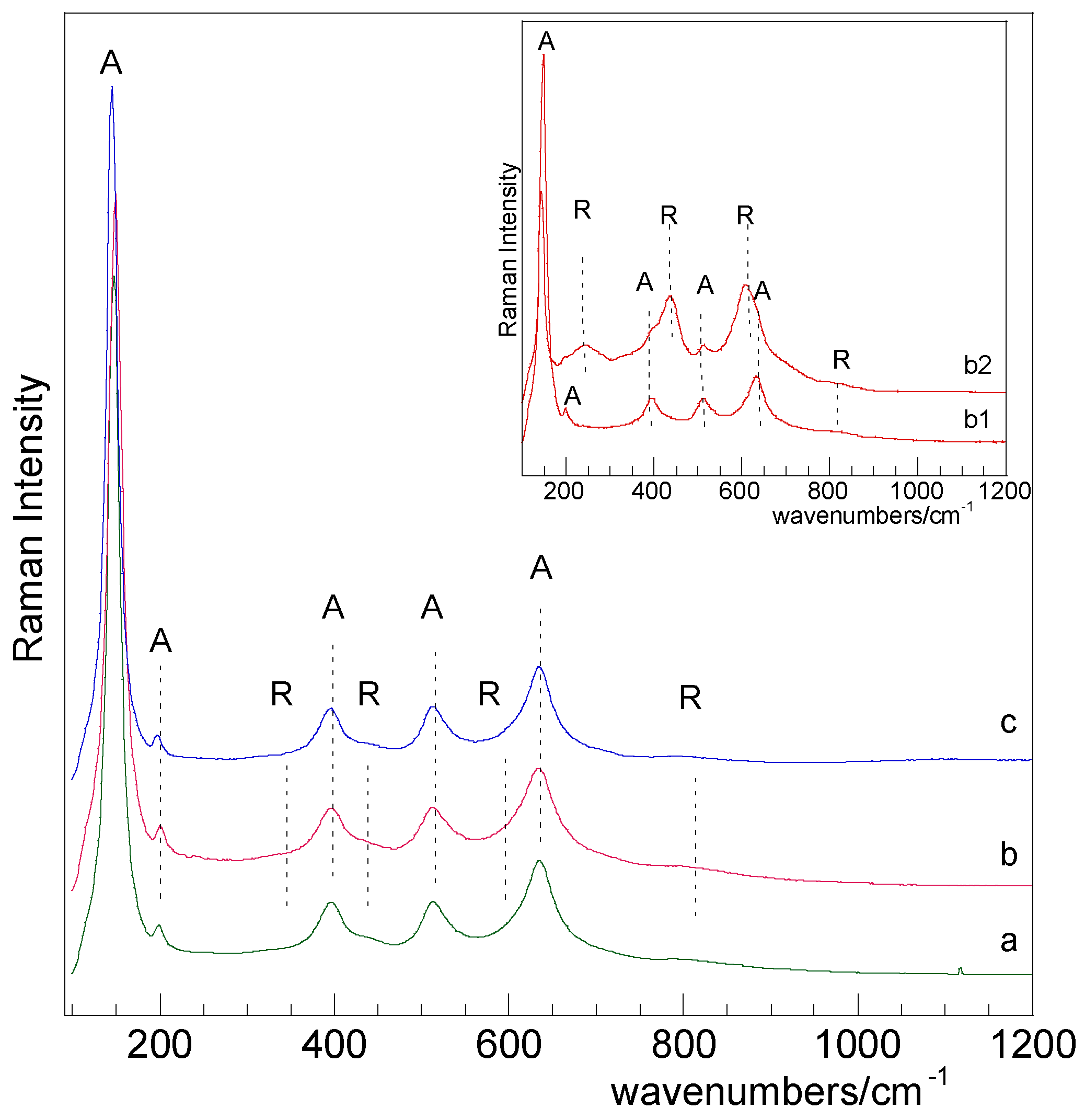
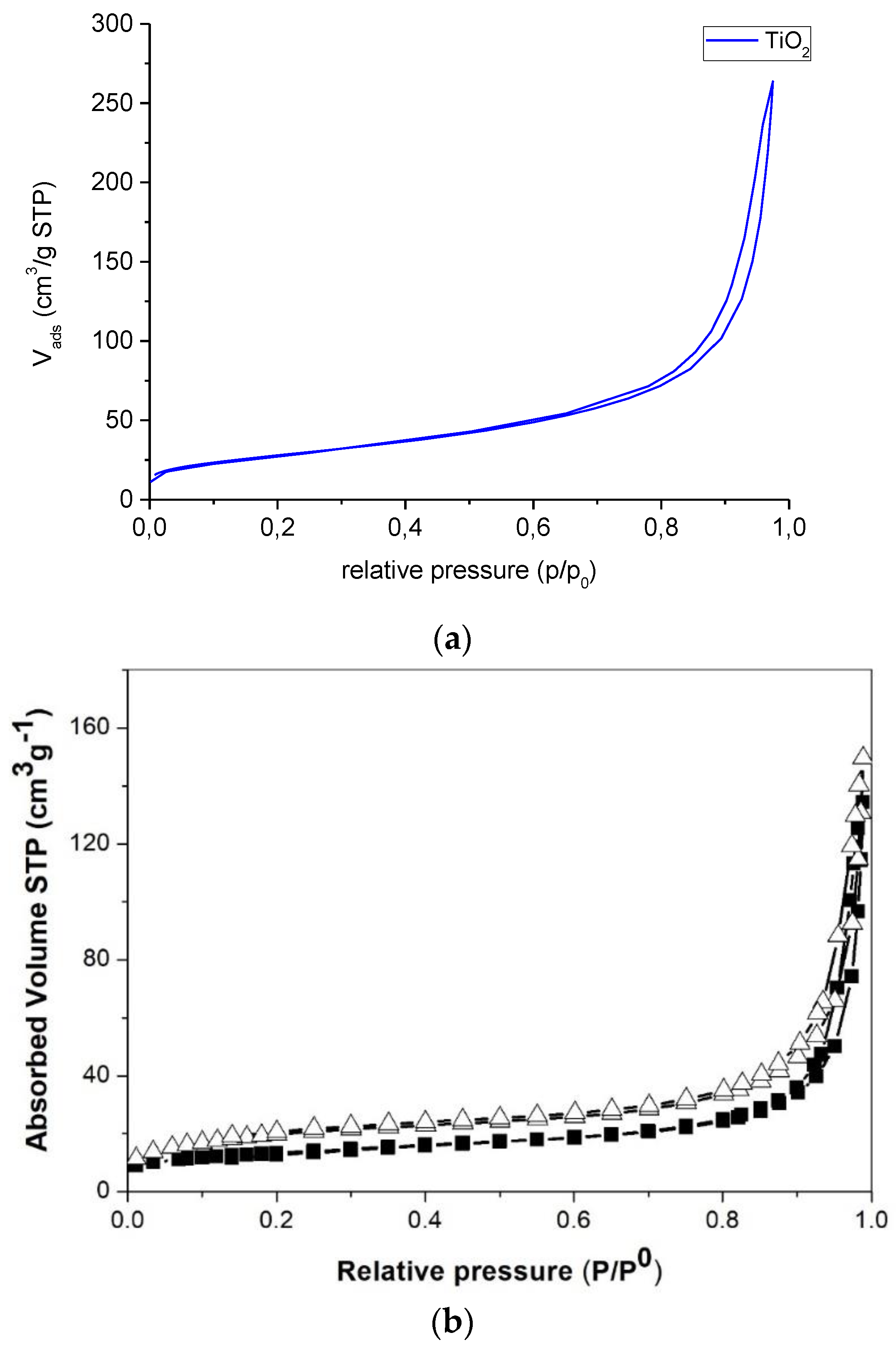
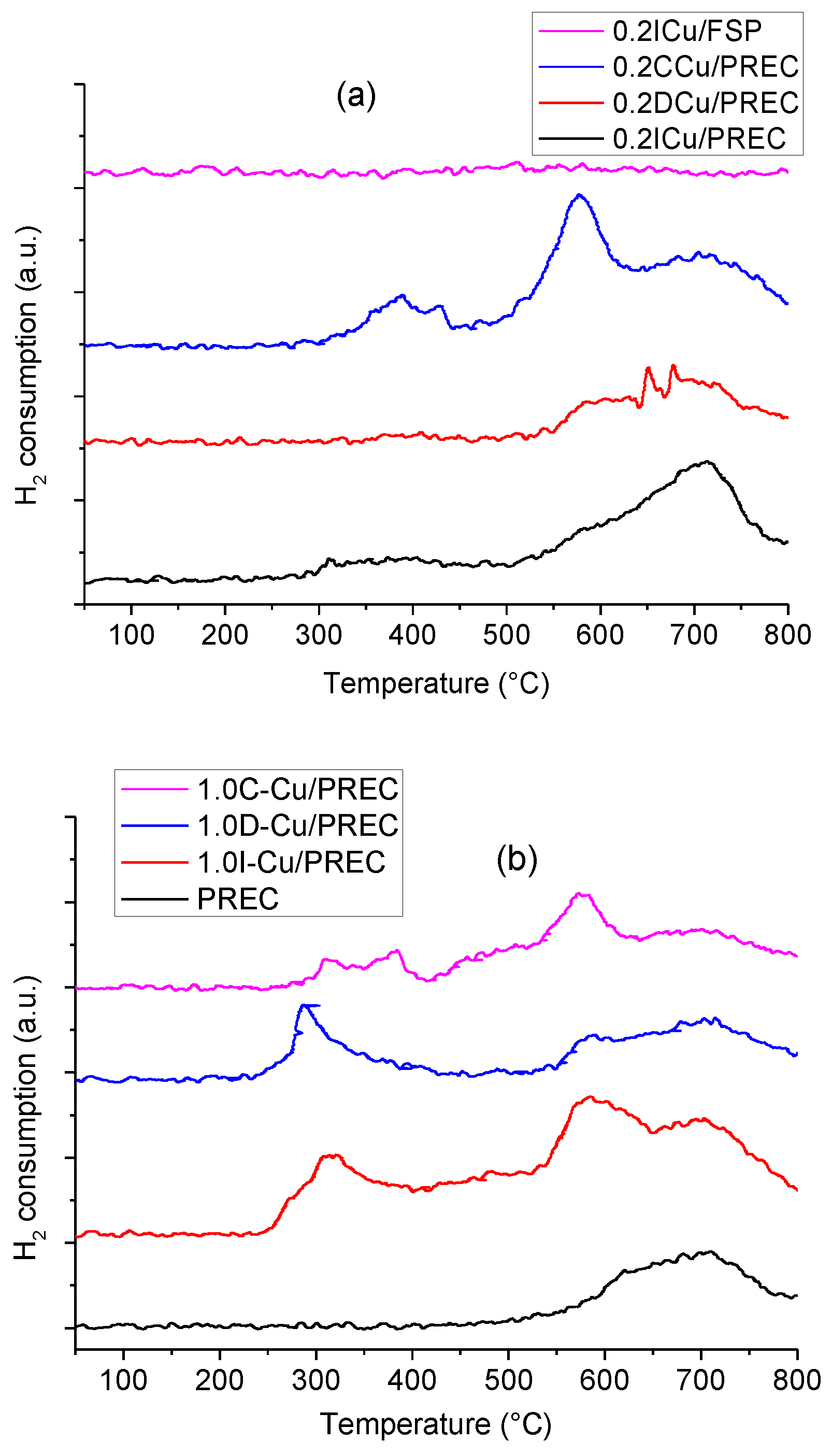
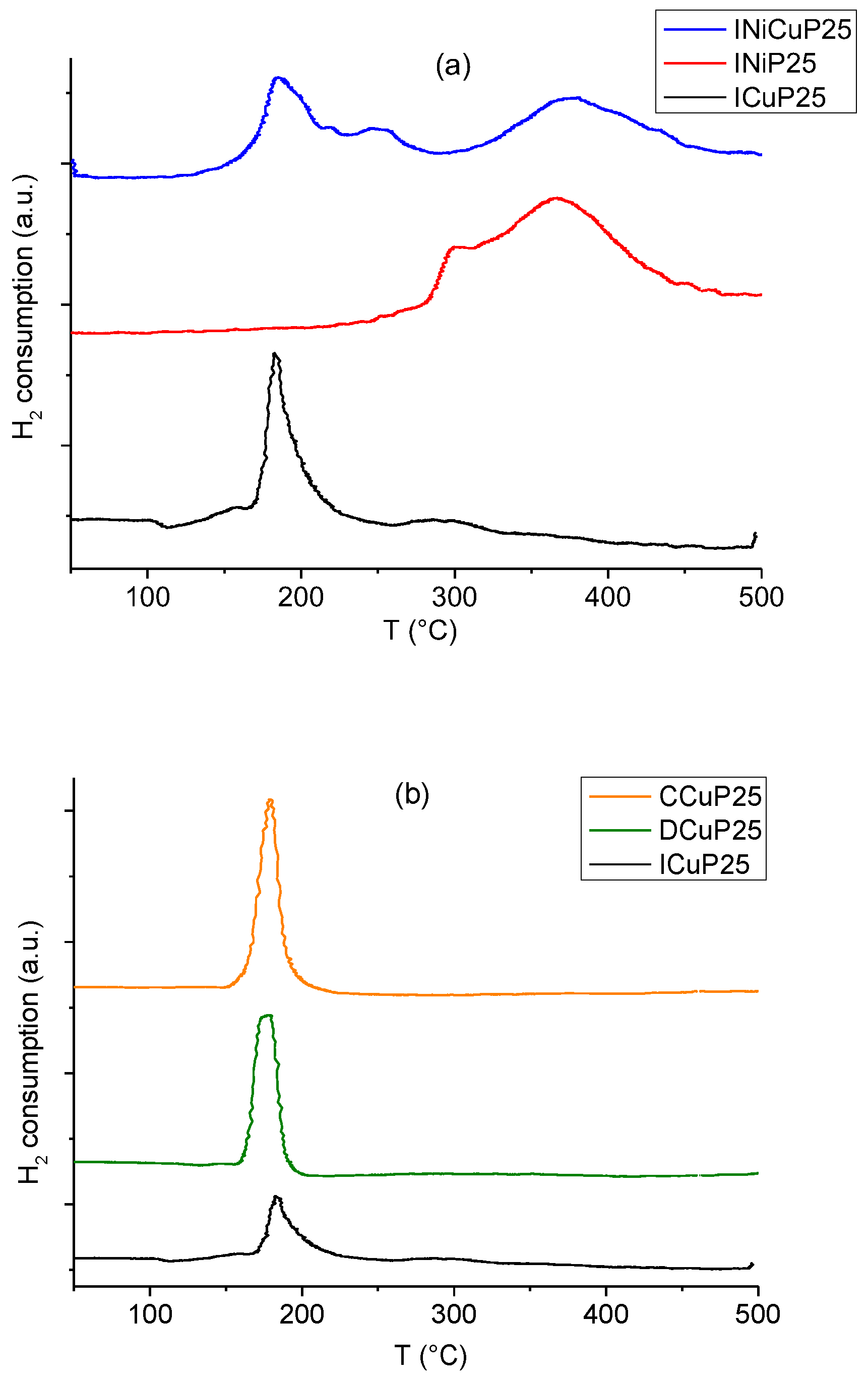
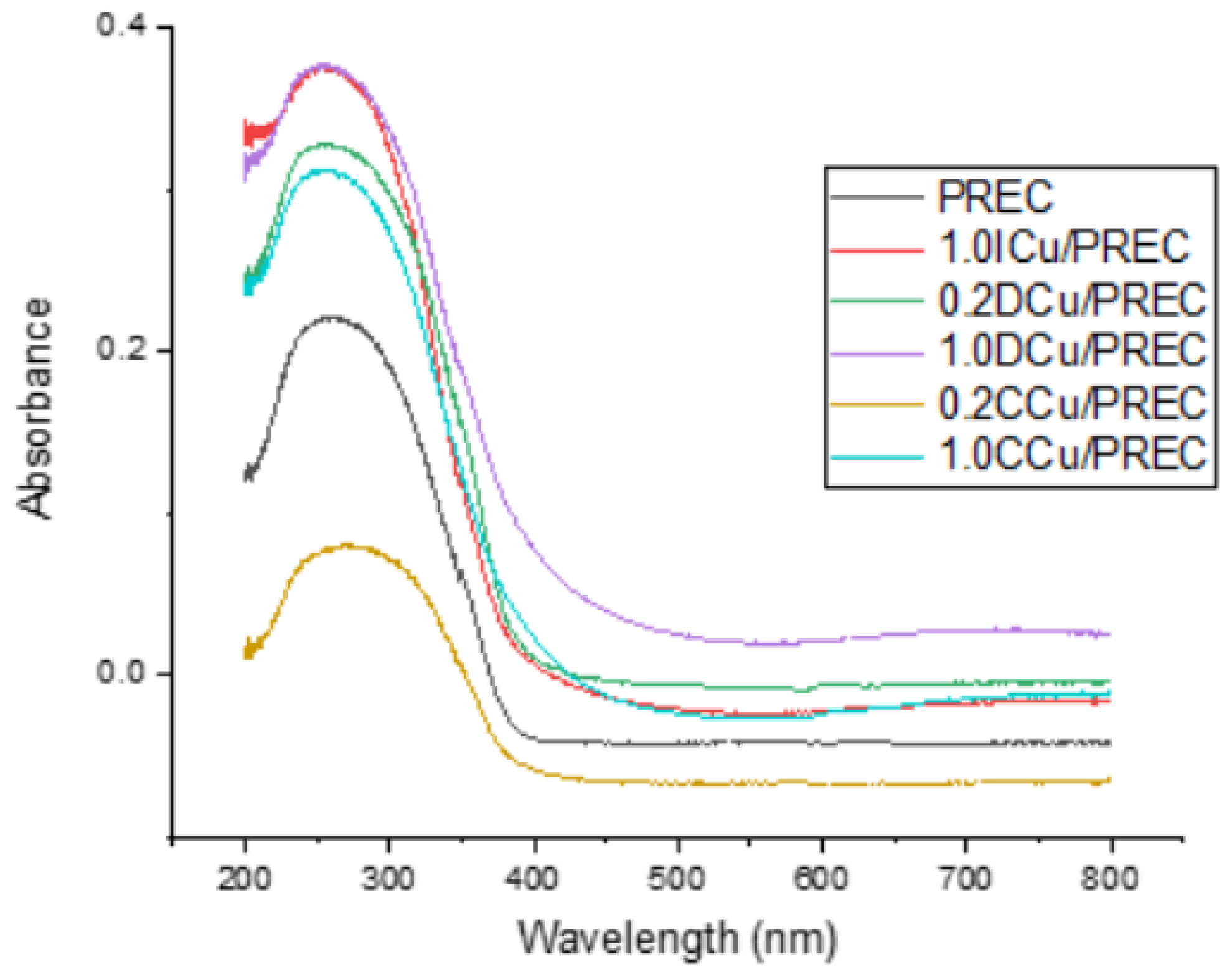
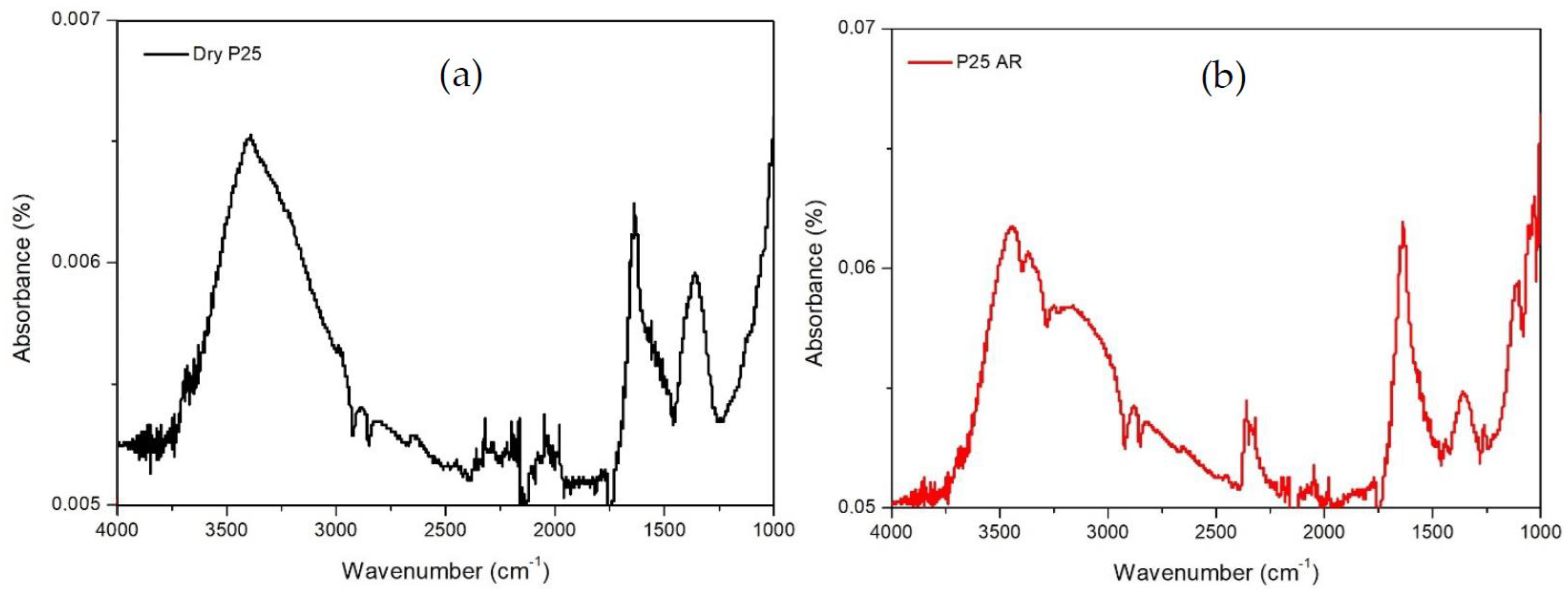
2.1. Catalysts Characterization
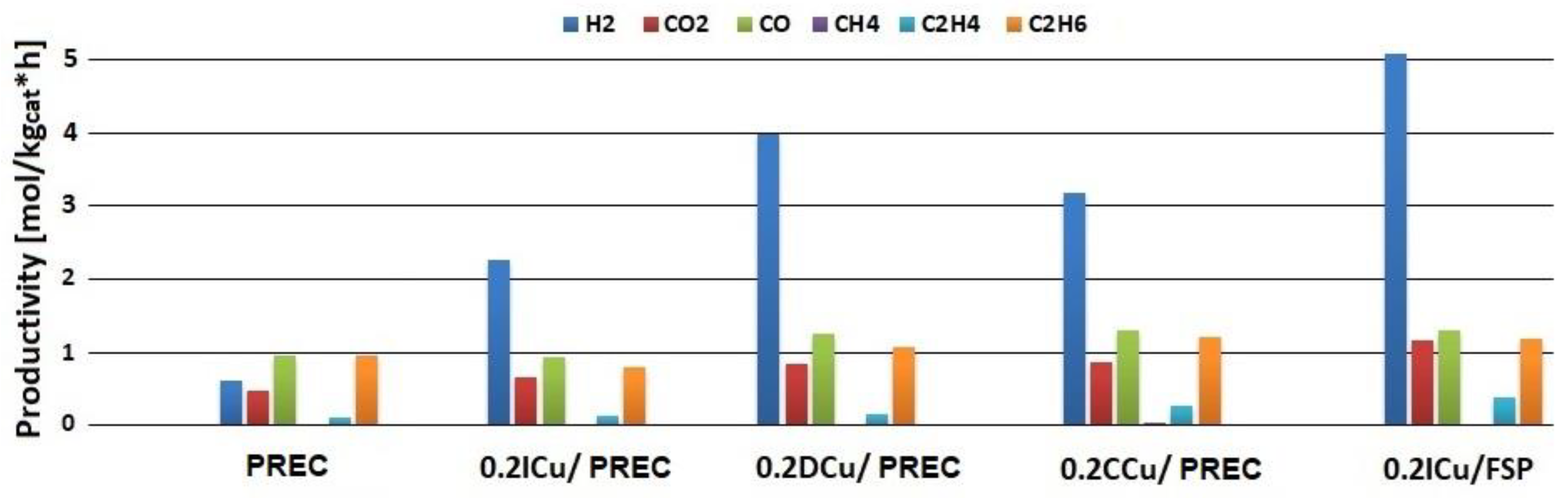
2.2. Catalytic Activity
2.3. Bare Titania Samples
2.4. Metal-loaded Titania Samples
2.5. Basic Cost Assessment
3. Materials and Methods
3.1. Photocatalysts
3.2. Characterization
3.3. Activity Testing
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kawai, T.; Sakata, T. Conversion of carbohydrate into hydrogen fuel by a photocatalytic process. Nature 1980, 286, 474–476. [Google Scholar] [CrossRef]
- Daskalaki, V.M.; Kondarides, D.I. Efficient production of hydrogen by photo-induced reforming of glycerol at ambient conditions. Catal. Today 2009, 144, 75–80. [Google Scholar] [CrossRef]
- Luo, Z.J.N.; Shi, H.; Cao, F.; Xiao, T.; Edwards, P.P. Photo-catalytic conversion of oxygenated hydrocarbons to hydrogen over heteroatom-doped TiO2catalysts. Int. J. Hydrog. Energy 2009, 34, 125–129. [Google Scholar] [CrossRef]
- Kida, T.; Guan, G.; Yamada, N.; Ma, T.; Kimura, K.; Yoshida, A. Hydrogen production from sewage sludge solubilized in hot-compressed water using photocatalyst under light irradiation. Int. J. Hydrog. Energy 2004, 29, 269–274. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Magdziarz, A.; Aramendia, M.A.; Marinas, A.; Marinas, J.M.; Urbano, F.J.; Navio, J.A. Influence of the strong metal support interaction effect (SMSI) of Pt/TiO2 and Pd/TiO2 systems in the photocatalytic biohydrogen production from glucose solution. Catal. Commun. 2011, 16, 1–6. [Google Scholar] [CrossRef]
- Rossetti, I. Hydrogen production by photoreforming of renewable substrates. ISRN Chem. Eng. 2012, 2012, 1–21. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Fornasiero, P. Photocatalytic Hydrogen Production: A Rift into the Future Energy Supply. ChemCatChem 2017, 9, 1523–1544. [Google Scholar] [CrossRef]
- Caravaca, A.; Jones, W.; Hardacre, C.; Bowker, M. H2 production by the photocatalytic reforming of cellulose and raw biomass using Ni, Pd, Pt and Au on titania. Proc. R. Soc. A 2016, 472, 20160054. [Google Scholar] [CrossRef]
- Huang, Y.-B.; Fu, Y. Hydrolysis of cellulose to glucose by solid acid catalysts. Green Chem. 2013, 15, 1095–1111. [Google Scholar] [CrossRef]
- Fu, X.; Long, J.; Wang, X.; Leung, D.Y.C.; Ding, Z.; Wu, L.; Zhang, Z.; Li, Z.; Fu, X. Photocatalytic reforming of biomass: A systematic study of hydrogen evolution from glucose solution. Int. J. Hydrog. Energy 2008, 33, 6484–6491. [Google Scholar] [CrossRef]
- Gomathisankar, P.; Kawamura, T.; Katsumata, H.; Suzuki, T.; Kaneco, S. Photocatalytic hydrogen production from aqueous methanol solution using titanium dioxide with the aid of simultaneous metal deposition. Energy Sources Part A Recover. Util. Environ. Eff. 2016, 38, 110–116. [Google Scholar] [CrossRef]
- Bellardita, M.; García-López, E.I.; Marcì, G.; Palmisano, L. Photocatalytic formation of H2 and value-added chemicals in aqueous glucose (Pt)-TiO2suspension. Int. J. Hydrog. Energy 2016, 41, 5934–5947. [Google Scholar] [CrossRef]
- Zhou, B.; Song, J.; Zhou, H.; Wu, T.; Han, B. Using the hydrogen and oxygen in water directly for hydrogenation reactions and glucose oxidation by photocatalysis. Chem. Sci. 2016, 7, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, X.; Jia, Y.; Chen, X.; Han, H.; Li, C. Titanium Dioxide-Based Nanomaterials for Photocatalytic Fuel Generations. Chem. Rev. 2014, 114, 9987–10043. [Google Scholar] [CrossRef] [PubMed]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Subramanian, V.; Wolf, E.E.; Kamat, P.V. Catalysis with TiO2/Gold Nanocomposites. Effect of Metal Particle Size on the Fermi Level Equilibration. J. Am. Chem. Soc. 2004, 126, 4943–4950. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Keiji, H.; Kominami, H. Visible-Light-Induced Hydrogen and Oxygen Formation over Pt/Au/WO3 Photocatalyst Utilizing Two Types of Photoabsorption Due to Surface Plasmon Resonance and Band-Gap Excitation. J. Am. Chem. Soc. 2014, 136, 586−589. [Google Scholar] [CrossRef]
- Clarizia, L.; Spasiano, D.; di Somma, I.; Marotta, R.; Andreozzi, R.; Dionysiou, D.D. Copper modified-TiO2catalysts for hydrogen generation through photoreforming of organics. A short review. Int. J. Hydrog. Energy 2014, 39, 16812–16831. [Google Scholar] [CrossRef]
- Sun, S.; Wang, W.; Zhang, L.; Shang, M.; Wang, L. Ag@C core/shell nanocomposite as a highly efficient plasmonic photocatalyst. Catal. Commun. 2009, 11, 290–293. [Google Scholar] [CrossRef]
- Chen, W.-T.; Jovic, V.; Sun-Waterhouse, D.; Idriss, H.; Waterhouse, G.I.N. The role of CuO in promoting photocatalytic hydrogen production over TiO2. Int. J. Hydrog. Energy 2013, 38, 15036–15048. [Google Scholar] [CrossRef]
- Ampelli, C.; Genovese, C.; Passalacqua, R.; Perathoner, S.; Centi, G. A gas-phase reactor powered by solar energy and ethanol for H2 production. Appl. Therm. Eng. 2014, 70, 1270–1275. [Google Scholar] [CrossRef]
- Jung, M.; Hart, J.N.; Boensch, D.; Scott, J.; Ng, Y.H.; Amal, R. Hydrogen evolution via glycerol photoreforming over Cu–Pt nanoalloys on TiO2. Appl. Catal. A 2016, 518, 221–230. [Google Scholar] [CrossRef]
- Puga, A.V. Review-Photocatalytic production of hydrogen from biomass-derived feedstocks. Coord. Chem. 2016, 315, 1–66. [Google Scholar] [CrossRef]
- Kwon, J.-D.; Kwon, S.H.; Jung, T.-H.; Nam, K.-S.; Chung, K.-B.; Kim, D.-H.; Park, J.S. Controlled growth and properties of p-type cuprous oxide films by plasma-enhanced atomic layer deposition at low temperature. Appl. Surf. Sci. 2013, 285, 373–379. [Google Scholar] [CrossRef]
- Montini, T.; Gombac, V.; Sordelli, L.; Delgado, J.J.; Chen, X.; Adami, G.; Fornasiero, P. Nanostructured Cu/TiO2 Photocatalysts for H2 Production from Ethanol and Glycerol Aqueous Solutions. ChemCatChem 2011, 3, 574–577. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Forni, L.; Selli, E. Photocatalytic hydrogen production by liquid- and gas-phase reforming of CH3OH over flame-made TiO2 and Au/TiO2. Catal. Today 2009, 144, 69–74. [Google Scholar] [CrossRef]
- Yu, J.; Hai, Y.; Jaroniec, M. Photocatalytic hydrogen production over CuO-modified titania. J. Colloid Interface Sci. 2011, 375, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Bandara, J.; Udawatta, C.P.K.; Rajapakse, C.S.K. Highly stable CuO incorporated TiO2 catalyst for photocatalytic hydrogen production from H2O. Photobiol. Sci. 2005, 4, 857–861. [Google Scholar] [CrossRef]
- Davda, R.R.; Shabaker, J.W.; Huber, G.W.; Cortright, R.D.; Dumesic, J.A. A review of catalytic issues and process conditions for renewable hydrogen and alkanes by aqueous-phase reforming of oxygenated hydrocarbons over supported metal catalysts. Appl. Catal. B Environ. 2005, 56, 171–186. [Google Scholar] [CrossRef]
- Olivo, A.; Zanardo, D.; Ghedini, E.; Menegazzo, F.; Signoretto, M. Solar Fuels by Heterogeneous Photocatalysis: From Understanding Chemical Bases to Process Development. Chem. Eng. 2018, 2, 42. [Google Scholar] [CrossRef]
- Santo, V.D.; Gallo, A.; Naldoni, A.; Guidotti, M.; Psaro, R. Bimetallic heterogeneous catalysts for hydrogen production. Catal. Today 2012, 197, 190. [Google Scholar] [CrossRef]
- Ramis, G.; Bahadori, E.; Rossetti, I. Photoreactors design for hydrogen production. Chem. Eng. Trans. 2019, 74, 481–486. [Google Scholar] [CrossRef]
- Rossetti, I.; Bahadori, E.; Tripodi, A.; Villa, A.; Prati, L.; Ramis, G. Conceptual design and feasibility assessment of photoreactors for solar energy storage. Sol. Energy 2018, 172, 225–231. [Google Scholar] [CrossRef]
- Zanardo, D.; Ghedini, E.; Menegazzo, F.; Cattaruzza, E.; Manzoli, M.; Cruciani, G.; Signoretto, M. Titanium dioxide-based nanocomposites for enhanced gas-phase photodehydrogenation. Materials (Basel) 2019, 12, 3093. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.G.; Low, J.X.; Xiao, W.; Zhou, P.; Jaroniec, M. Enhanced photocatalytic CO₂-reduction activity of anatase TiO₂ by coexposed {001} and {101} facets. J. Am. Chem. Soc. Am. Chem. Soc. 2014, 136, 8839−8842. [Google Scholar]
- Zhang, J.; Li, M.; Feng, Z.; Chen, J.; Li, C. UV Raman Spectroscopic Study on TiO2. I. Phase Transformation at the Surface and in the Bulk. J. Phys. Chem. B 2006, 110, 927−935. [Google Scholar] [CrossRef]
- Bahadori, E.; Rapf, M.; di Michele, A.; Rossetti, I. Photochemical vs. photocatalytic azo-dye removal in a pilot free-surface reactor: Is the catalyst effective? Sep. Purif. Technol. 2020, 237, 116320. [Google Scholar] [CrossRef]
- Nair, J.; Nair, P.; Mizukami, F.; Oosawa, Y.; Okubo, T. Microstructure and phase transformation behavior of doped nanostructured titania. Mater. Res. Bull. 1999, 34, 1275–1290. [Google Scholar] [CrossRef]
- Yoong, L.S.; Chong, F.; Dutta, B. Development of copper-doped TiO2 photocatalyst for hydrogen production under visible light. Energy 2009, 34, 1652–1661. [Google Scholar] [CrossRef]
- Boccuzzi, F.; Chiorino, A.; Martra, G.; Gargano, M.; Ravasio, N.; Carrozzini, B. Preparation, Characterization, and Activity of Cu/TiO2 Catalysts I. Influence of the Preparation Method on the Dispersion of Copper in Cu/TiO2. J. Catal. 1997, 165, 129–139. [Google Scholar] [CrossRef]
- Bahadori, E.; Tripodi, A.; Ramis, G.; Rossetti, I. Semi-Batch Photocatalytic Reduction of Nitrates: Role of Process Conditions and Cocatalysts. ChemCatChem 2019, 11, 4642–4652. [Google Scholar] [CrossRef]
- Bahadori, E.; Conte, F.; Tripodi, A.; Ramis, G.; Rossetti, I. Semi-batch selective photocatalytic oxidation of ammonia: Unravelling the effect of reaction conditions and metal cocatalysts. Chem. Eng. J. submitted.
- Cai, X.; Wang, C.; Chen, Y.; Cheng, Z.; Shu, R.; Zhang, J.; Bu, E.; Liao, M.; Song, Q. A novel approach for enhancing hydrogen production from bio-glycerol photoreforming by improving colloidal dispersion stability. Sci. Total Envrion. 2018, 627, 1464–1472. [Google Scholar] [CrossRef] [PubMed]
- Hurum, D.C.; Agrios, A.G.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Explaining the Enhanced Photocatalytic Activity of Degussa P25 Mixed-Phase TiO2 Using EPR. J. Phys. Chem. B 2003, 107, 4545–4549. [Google Scholar] [CrossRef]
- Hurum, D.C.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Recombination pathways in the Degussa P25 formulation of TiO2: Surface versus lattice mechanisms. J. Phys. Chem. B 2005, 109, 977–980. [Google Scholar] [CrossRef]
- Xu, Q.; Ma, Y.; Zhang, J.; Wang, X.; Feng, Z.; Li, C. Enhancing hydrogen production activity and suppressing CO formation from photocatalytic biomass reforming on Pt/TiO2 by optimizing anatase-rutile phase structure. J. Catal. 2011, 278, 329–335. [Google Scholar] [CrossRef]
- Schwarz, J.A.; Contescu, C.; Contescu, A. Methods for Preparation of Catalytic Materials. Chem. Rev. 1995, 95, 477–510. [Google Scholar] [CrossRef]
- Wu, G.; Chen, T.; Zhou, G.; Zong, X.; Li, C. H2 production with low CO selectivity from photocatalytic reforming of glucose on metal/TiO2 catalysts. Sci. China Ser. B Chem. 2008, 51, 97–100. [Google Scholar] [CrossRef]
- Chen, W.-T.; Chan, A.; Sun-Waterhouse, D.; Moriga, T.; Idriss, H.; Waterhouse, G.I.N. Ni/TiO2: A promising low-cost photocatalytic system for solar H2 production from ethanol–water mixtures. J. Catal. 2015, 326, 43–53. [Google Scholar] [CrossRef]
- Ramis, G.; Bahadori, E.; Rossetti, I. Design of efficient photocatalytic processes for the production of hydrogen from biomass derived substrates. Int. J. Hydrog. Energy 2020, in press. [Google Scholar] [CrossRef]
- Glenk, G.; Reichelstein, S. Economics of converting renewable power to hydrogen. Nat. Energy 2019, 4, 216–222. [Google Scholar] [CrossRef]
- Compagnoni, M.; Mostafavi, E.; Tripodi, A.; Mahinpey, N.; Rossetti, I. Techno-economic analysis of a bioethanol to hydrogen centralized plant. Energy Fuels 2017, 31, 12988–12996. [Google Scholar] [CrossRef]
- Compagnoni, M.; Tripodi, A.; Mostafavi, E.; Mahinpey, N.; Rossetti, I. Hydrogen Production by Steam Reforming of Bio-ethanol: Process Design and Economic Assessment; Tagungsbericht; DGMK: Hamburg, Germany, 2017. [Google Scholar]
- Calise, F.; D’Accadia, M.D.; Santarelli, M.; Lanzini, A.; Ferrero, D. Solar Hydrogen Production. Processes, Systems and Technologies; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Olivo, A.; Trevisan, V.; Ghedini, E.; Pinna, F.; Bianchi, C.L.; Naldoni, A.; Cruciani, G.; Signoretto, M. CO2 photoreduction with water: Catalyst and process investigation. J. CO2 Util. 2015, 12, 86–94. [Google Scholar] [CrossRef]
- Compagnoni, M.; Lasso, J.; di Michele, A.I.; Rossetti, I. Catalysis Science & Technology Flame-pyrolysis-prepared catalysts for the steam reforming of ethanol. Catal. Sci. Technol. 2016, 6, 6247–6256. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Rossetti, I.; Forni, L. Flame-spray pyrolysis preparation of perovskites for methane catalytic combustion. J. Catal. 2005, 236, 251–261. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.S.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Bahadori, E.; Tripodi, A.; Villa, A.; Pirola, C.; Prati, L.; Ramis, G.; Rossetti, I. High Pressure Photoreduction of CO2: Effect of Catalyst Formulation, Hole Scavenger Addition and Operating Conditions. Catalysts 2018, 8, 430. [Google Scholar] [CrossRef]
- Rossetti, I.; Villa, A.; Pirola, C.; Prati, L.; Ramis, G. A novel high-pressure photoreactor for CO2 photoconversion to fuels. RSC Adv. 2014, 4, 28883–28885. [Google Scholar] [CrossRef]
- Compagnoni, M.; Ramis, G.; Freyria, F.S.; Armandi, M.; Bonelli, B.; Rossetti, I. Innovative photoreactors for unconventional photocatalytic processes: The photoreduction of CO2 and the photo-oxidation of ammonia. Rend. Lincei 2017, 28, 151–158. [Google Scholar] [CrossRef]
- Bahdori, E.; Tripodi, A.; Villa, A.; Pirola, C.; Prati, L.; Ramis, G.; Dimitratos, N.; Wang, D.; Rossetti, I. High pressure CO2 photoreduction using Au/TiO2: Unravelling the effect of the cocatalyst and of the titania polymorph. Catal. Sci. Technol. 2019, 9, 2253–2265. [Google Scholar] [CrossRef]
- Olivo, A.; Ghedini, E.; Signoretto, M.; Compagnoni, M.; Rossetti, I. Liquid vs. gas phase CO2 photoreduction process: Which is the effect of the reaction medium? Energies 2017, 10, 1394. [Google Scholar] [CrossRef]
- Galli, F.; Compagnoni, M.; Vitali, D.; Pirola, C.; Bianchi, C.L.; Villa, A.; Prati, L.; Rossetti, I. CO2 photoreduction at high pressure to both gas and liquid products over titanium dioxide. Appl. Catal. B Environ. 2017, 200, 386–391. [Google Scholar] [CrossRef]
- Rossetti, I.; Villa, A.; Compagnoni, M.; Prati, L.; Ramis, G.; Pirola, C.; Bianchi, C.L.; Wang, W.; Wang, D. CO2 photoconversion to fuels under high pressure: Effect of TiO2 phase and of unconventional reaction conditions. Catal. Sci. Technol. 2015, 5, 4481–4487. [Google Scholar] [CrossRef]
- Rossetti, I.; Bahadori, E.; Tripodi, A.; Ramis, G. Modelling of Photoreactors for Water Treatment. Chem. Eng. Trans. 2019, 74, 289–294. [Google Scholar] [CrossRef]


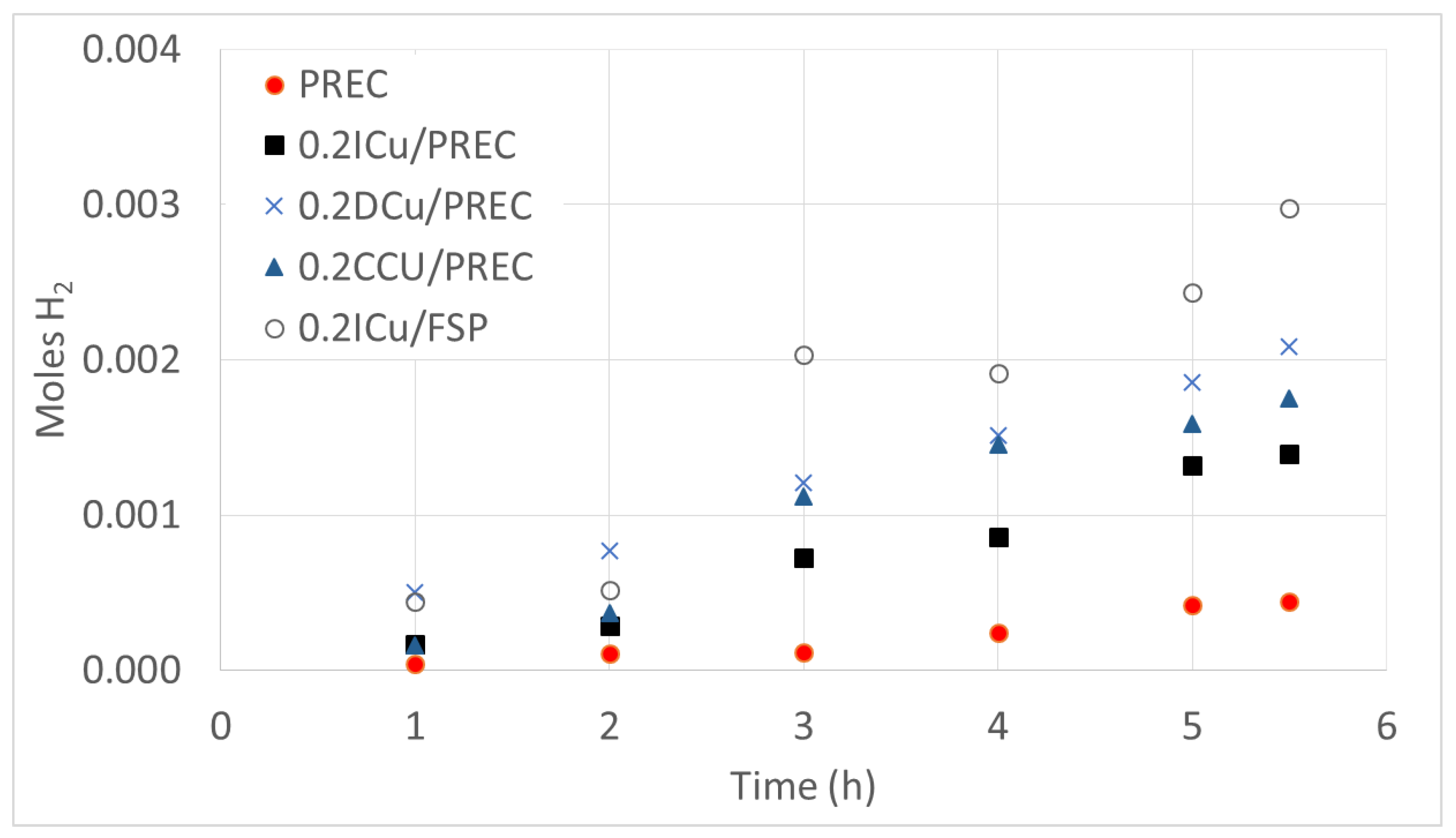
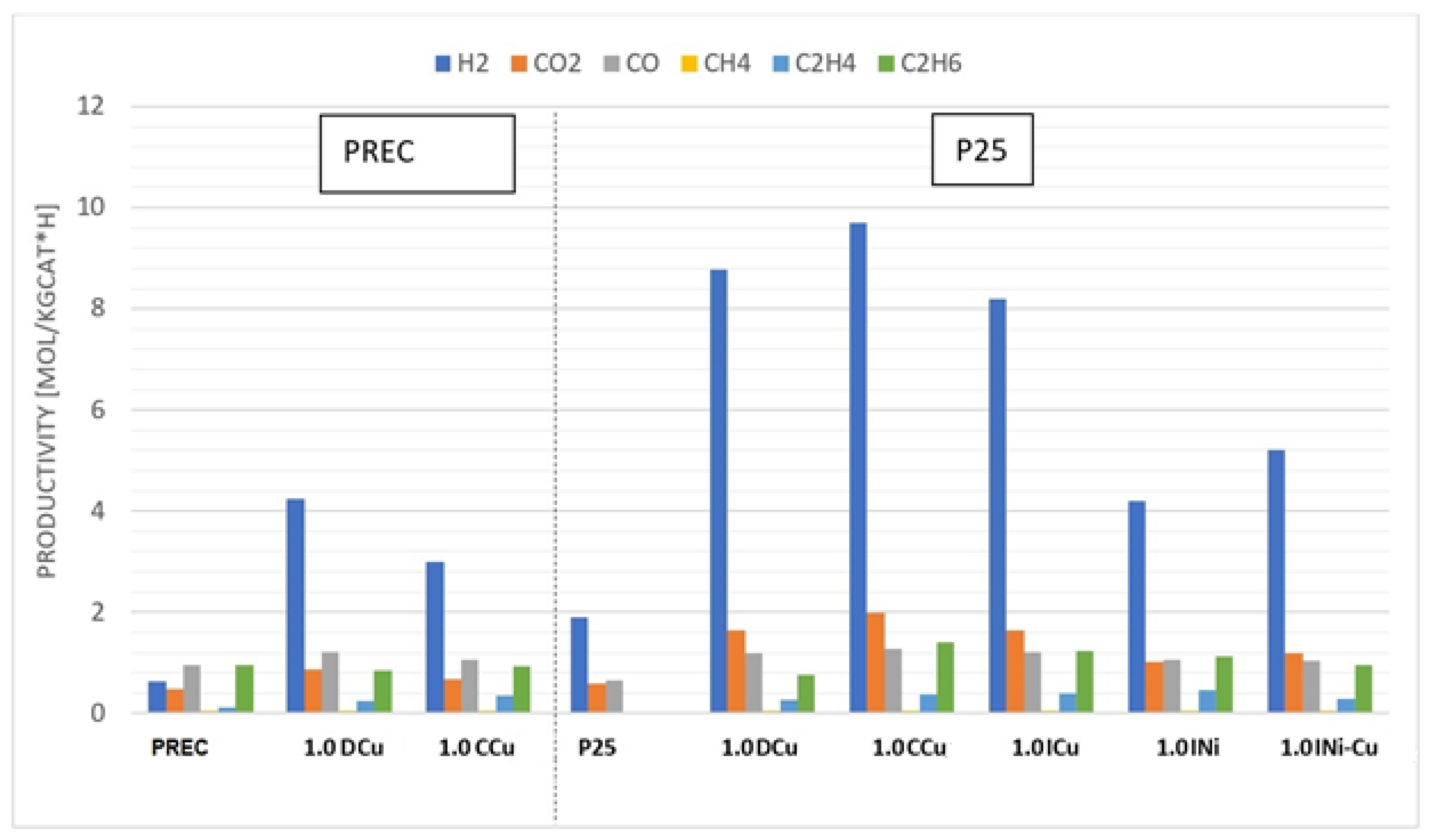
| Sample | Metal Loading (wt %) | Anatase/Rutile | SBET (m2/g) | Total Pore Volume (cm3/g) |
|---|---|---|---|---|
| P25 | - | 78/22 | 45 | 0.11 |
| FSP | - | 65/35 | 67 | 0.14 |
| PREC | - | 100/0 | 114 | 0.45 |
| 0.2ICu/PREC | 0.15 | 100/0 | 151 | 0.27 |
| 1.0ICu/PREC | 0.49 | 100/0 | 89 | 0.17 |
| 0.2DCu/PREC | 0.15 | 100/0 | 118 | 0.23 |
| 1.0DCu/PREC | 0.86 | 100/0 | 118 | 0.23 |
| 0.2CCu/PREC | 0.16 | 100/0 | 108 | 0.27 |
| 1.0CCu/PREC | 0.85 | 100/0 | 116 | 0.22 |
| 1.0DCu/P25 | 0.98 | 85.2/14.8 | 42 a | - |
| 1.0CCu/P25 | 0.99 | 85/15 | 43 a | - |
| 1.0ICu/P25 | 0.93 | 85/15 | 42 a | - |
| 1.0INi/P25 | 0.71 | - | - | - |
| 0.5ICu0.5Ni/P25 | 0.50 (Cu) 0.38 (Ni) | - | - | - |
| 0.2ICu/FSP | - | 65/35 | 72 | 0.15 |
| Catalyst | H2 Productivity (mol/h kgcat) | COD% 1 | C Bal. % 2 | C bal. (TGA) % 3 |
|---|---|---|---|---|
| P25 | 1.91 | 20.3 | 81 | 85 |
| 1.0DCu/P25 | 8.78 | 19.5 | 84 | 88 |
| 1.0CCu/P25 | 9.71 | 30.6 | 75 | 79 |
| 1.0ICu/P25 | 8.20 | 30.8 | 74 | 78 |
| 1.0INi/P25 | 4.20 | 26.1 | 78 | 82 |
| 0.5ICu0.5Ni/P25 | 5.21 | 26.1 | 77 | 82 |
| PREC | 0.62 | 5.7 | 96 | 100 |
| 0.2ICu/PREC | 2.26 | 15.5 | 87 | 91 |
| 1.0ICu/PREC | 3.26 | 65.4 | 38 | 42 |
| 0.2DCu/PREC | 3.98 | 13.2 | 90 | 95 |
| 1.0DCu/PREC | 4.24 | - | 106 | 110 |
| 0.2CCu/PREC | 3.18 | 0.3 | 104 | 108 |
| 1.0CCu/PREC | 2.99 | 2.5 | 101 | 105 |
| FSP | 1.20 | 8.5 | 94 | 99 |
| 0.2ICu/FSP | 5.07 | 0.8 | 103 | 108 |
| Catalyst | Cost for 5 h of Test (euro) | Cost of H2 Production for 5 h Test (euro/kg) | Cost of H2 Production for 30 d, 24 h (euro/kg) |
|---|---|---|---|
| P25 | 0.0028 | 486 | 388 |
| Cu/P25 | 0.0118 | 402 | 78 |
| Pt/P25 | 0.0388 | 873 | 56 |
| Catalyst | Method |
|---|---|
| P25 | Commercial nanostructured TiO2 (Evonik) |
| PREC | TiO2 by precipitation with NaOH (pH = 7, constant) |
| FSP | TiO2 by flame spray pyrolysis |
| ICu/PREC | CuO added by impregnation on PREC |
| DCu/PREC | CuO added by PC on PREC with 1,3-propandiol |
| CCu/PREC | CuO added by PC on PREC with citric acid |
| DCu/P25 | CuO added by PC on P25 with 1,3-propandiol |
| CCu/P25 | CuO added by PC on P25 with citric acid |
| ICu/P25 | CuO added by impregnation on P25 |
| INi/P25 | NiO added by impregnation on P25 |
| INi-Cu/P25 | CuO and NiO added by impregnation on P25 |
| ICu/FSP | CuO added by impregnation on FSP |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahadori, E.; Ramis, G.; Zanardo, D.; Menegazzo, F.; Signoretto, M.; Gazzoli, D.; Pietrogiacomi, D.; Michele, A.D.; Rossetti, I. Photoreforming of Glucose over CuO/TiO2. Catalysts 2020, 10, 477. https://doi.org/10.3390/catal10050477
Bahadori E, Ramis G, Zanardo D, Menegazzo F, Signoretto M, Gazzoli D, Pietrogiacomi D, Michele AD, Rossetti I. Photoreforming of Glucose over CuO/TiO2. Catalysts. 2020; 10(5):477. https://doi.org/10.3390/catal10050477
Chicago/Turabian StyleBahadori, Elnaz, Gianguido Ramis, Danny Zanardo, Federica Menegazzo, Michela Signoretto, Delia Gazzoli, Daniela Pietrogiacomi, Alessandro Di Michele, and Ilenia Rossetti. 2020. "Photoreforming of Glucose over CuO/TiO2" Catalysts 10, no. 5: 477. https://doi.org/10.3390/catal10050477
APA StyleBahadori, E., Ramis, G., Zanardo, D., Menegazzo, F., Signoretto, M., Gazzoli, D., Pietrogiacomi, D., Michele, A. D., & Rossetti, I. (2020). Photoreforming of Glucose over CuO/TiO2. Catalysts, 10(5), 477. https://doi.org/10.3390/catal10050477











