Acetylation of Eugenol on Functionalized Mesoporous Aluminosilicates Synthesized from Amazonian Flint Kaolin
Abstract
1. Introduction
2. Results and Discussion
2.1. X-ray Fluorescence (XRF) Studies
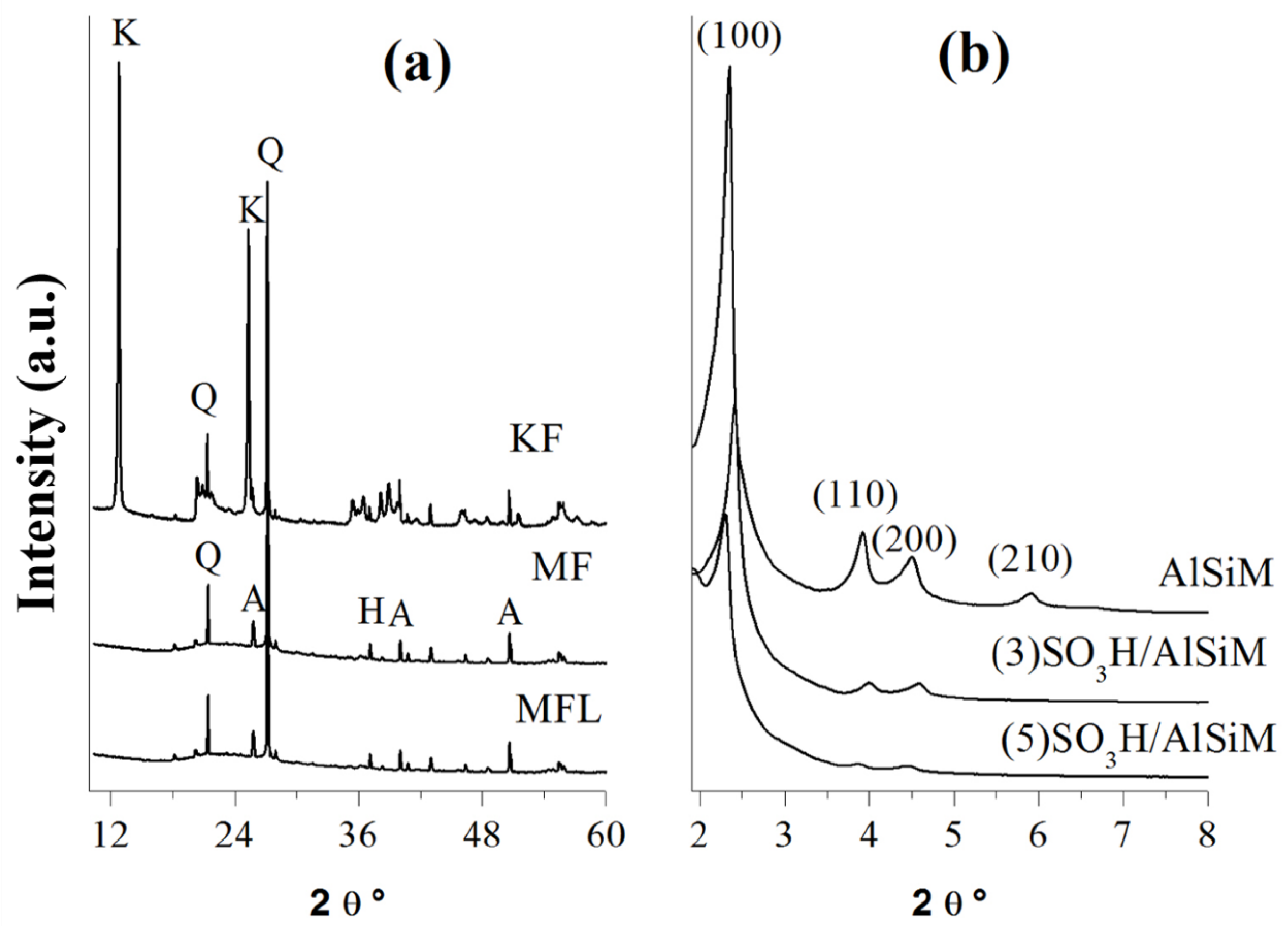
2.2. XRD Analysis
2.3. Nitrogen Physisorption Experiments
2.4. Fourier Transform Infrared Spectroscopy (FTIR) Experiments
2.5. Thermal Analysis (TGA/DTG) Results
2.6. Surface Acidity
2.7. Eugenol Acetylation Reaction Mechanism with Acetic Anhydride
2.8. Effect of Temperature
2.9. Amount of Catalyst
2.10. Molar Ratio between Reagents
2.11. Effect of Reaction Time
2.12. Kinetic Studies
2.13. Catalyst Reuse
2.14. Characterization of Reused Catalyst
3. Materials and Methods
3.1. Materials
3.2. Preparation of Catalysts
3.2.1. Thermal and Acid Treatments of Amazon Flint Kaolin
3.2.2. Synthesis of Mesoporous Aluminosilicate (AlSiM)
3.2.3. Functionalization of AlSiM with MTPS
3.3. Characterization
3.4. Catalytic Tests
Acetylation of Eugenol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, L.B.; Kasai, S.; Scott, J.G. Pyrethroid resistance in Aedes aegypti and Aedes albopictus: Important mosquito vectors of human diseases. Pestic. Biochem. Physiol. 2016, 133, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.D.F.; Silva, V.B.; Alves, B.; Gumina, G.; Santos, R.L.C.; Sousa, D.P.; Cavalcanti, S.C.H. Structure—Activity relationships of eugenol derivatives against Aedes aegypti (Diptera: Culicidae) larvae. Pest Manag. Sci. 2012, 68, 1478–1483. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, M.; Rajeswary, M.; Benelli, G. Chemical composition, toxicity and non-target effects of Pinus kesiya essential oil: An eco-friendly and novel larvicide against malaria, dengue and lymphatic fi lariasis mosquito vectors. Ecotoxicol. Environ. Saf. 2016, 129, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Machado, J.R.; Pereira, G.N.; de Oliveira, P.D.S.; Zenevicz, M.C.; Lerin, L.; de Oliveira, R.D.R.B.; de Holanda Cavalcanti, S.C.; Ninow, J.L.; de Oliveira, D. Synthesis of eugenyl acetate by immobilized lipase in a packed bed reactor and evaluation of its larvicidal activity. Process Biochem. 2017, 58, 114–119. [Google Scholar] [CrossRef]
- Silva, M.J.A.; Loss, R.A.; Laroque, D.A.; Lerin, L.A.; Pereira, G.N.; Thon, É.; Oliveira, J.V.; Ninow, J.L.; Hense, H.; Oliveira, D. Lipozyme TL IM as Catalyst for the Synthesis of Eugenyl Acetate in Solvent-Free Acetylation. Appl. Biochem. Biotechnol. 2015, 176, 782–795. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.K.; Tandon, S.; Ahmad, A.; Singh, A.K.; Tripathi, A.K. Structure—Activity relationships of monoterpenes and acetyl derivatives against Aedes aegypti (Diptera: Culicidae) larvae. Pest Manag. Sci. 2013, 69, 1235–1238. [Google Scholar] [CrossRef] [PubMed]
- Charan Raja, M.R.; Velappan, A.B.; Chellappan, D.; Debnath, J.; Mahapatra, S.K. Eugenol derived immunomodulatory molecules against visceral leishmaniasis. Eur. J. Med. Chem. 2017, 139, 503–518. [Google Scholar] [CrossRef]
- Giovannini, P.P.; Sacchetti, G.; Catani, M.; Massi, A.; Tacchini, M.; De Oliveira, D.; Lerin, L.A. Continuous production of eugenol esters using enzymatic bed microreactors and an evaluation of the products as antifungal agents. Flavour Fragr. J. 2019, 34, 201–210. [Google Scholar] [CrossRef]
- Da Siva, F.F.M.; Monte, F.J.Q.; de Lemos, T.L.G.; do Nascimento, P.G.G.; de Medeiros Costa, A.K.; Paiva, L.M.M. Eugenol derivatives: Synthesis, characterization, and evaluation of antibacterial and antioxidant activities. Chem. Cent. J. 2018, 12, 34. [Google Scholar] [CrossRef]
- Yadav, G.D.; Yadav, A.R. Insight into esterification of eugenol to eugenol benzoate using a solid super acidic modified zirconia catalyst UDCaT-5. Chem. Eng. J. 2012, 192, 146–155. [Google Scholar] [CrossRef]
- Manan, F.M.A.; Attan, N.; Zakaria, Z.; Keyon, A.S.A.; Wahab, R.A. Enzymatic esterification of eugenol and benzoic acid by a novel chitosan-chitin nanowhiskers supported Rhizomucor miehei lipase: Process optimization and kinetic assessments. Enzyme Microb. Technol. 2018, 108, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Manan, F.M.A.; Rahman, I.N.A.; Marzuki, N.H.C.; Mahat, N.A.; Huyop, F.; Wahab, R.A. Statistical modelling of eugenol benzoate synthesis using Rhizomucor miehei lipase reinforced nanobioconjugates. Process Biochem. 2016, 51, 249–262. [Google Scholar] [CrossRef]
- Cansian, R.L.; Vanin, A.B.; Orlando, T.; Piazza, S.P.; Puton, B.M.S.; Cardoso, R.I.; Gonçalves, I.L.; Honaiser, T.C.; Paroul, N.; Oliveira, D. Toxicity of clove essential oil and its ester eugenyl acetate against Artemia salina. Braz. J. Biol. 2017, 77, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.R.; Gazolla, P.A.R.; da Silva, A.M.; Borsodi, M.P.G.; Bergmann, B.R.; Ferreira, R.S.; Vaz, B.G.; Vasconcelos, G.A.; Lima, W.P. Synthesis and leishmanicidal activity of eugenol derivatives bearing. Eur. J. Med. Chem. 2018, 146, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Slamenová, D.; Horváthová, E.; Wsólová, L.; Sramková, M.; Navarová, J. Investigation of anti-oxidative, cytotoxic, DNA-damaging and DNA-protective effects of plant volatiles eugenol and borneol in human-derived HepG2, Caco-2 and VH10 cell lines. Mutat. Res. 2009, 677, 46–52. [Google Scholar] [CrossRef]
- Sadeghian, H.; Seyedi, S.M.; Saberi, M.R.; Arghiani, Z.; Riazi, M. Design and synthesis of eugenol derivatives, as potent 15-lipoxygenase inhibitors. Bioorg. Med. Chem. 2008, 16, 890–901. [Google Scholar] [CrossRef]
- Tischer, J.S.; Possan, H.; Luiz, J.; Malagutti, N.B.; Matello, R.; Valério, A.; Dalmagro, J.; De Oliveira, D.; Oliveira, J.V. Synthesis of eugenyl acetate through heterogeneous catalysis. J. Essent. Oil Res. 2019, 31, 312–318. [Google Scholar] [CrossRef]
- Chiaradia, V.; Paroul, N.; Cansian, R.L.; Júnior, C.V.; Detofol, M.R.; Lerin, L.A.; Oliveira, J.V.; Oliveira, D. Synthesis of eugenol esters by lipase-catalyzed reaction in solvent-free system. Appl. Biochem. Biotechnol. 2012, 168, 742–751. [Google Scholar] [CrossRef]
- Narkhede, N.; Patel, A.; Singh, S. Mono lacunary phosphomolybdate supported on MCM-41: Synthesis, characterization and solvent free aerobic oxidation of alkenes and alcohols. Dalton Trans. 2014, 43, 2512–2520. [Google Scholar] [CrossRef]
- Nascimento, L.A.S.; Angélica, R.S.; Costa, C.E.F.; Zamian, J.R.; Rocha Filho, G.N. Conversion of waste produced by the deodorization of palm oil as feedstock for the production of biodiesel using a catalyst prepared from waste material. Bioresour. Technol. 2011, 102, 8314–8317. [Google Scholar] [CrossRef]
- Nascimento, L.A.S.; Angélica, R.S.; Costa, C.E.F.; Zamian, J.R.; Rocha Filho, G.N. Comparative study between catalysts for esterification prepared from kaolins. Appl. Clay Sci. 2011, 51, 267–273. [Google Scholar] [CrossRef]
- Pires, L.H.O.; Oliveira, A.N.; Monteiro Junior, O.V.; Angélica, R.S.; Costa, C.E.F.; Zamian, J.R.; Nascimento, L.A.S.; Rocha Filho, G.N. Esterification of a waste produced from the palm oil industry over 12-tungstophosforic acid supported on kaolin waste and mesoporous materials. Appl. Catal. B Environ. 2014, 160–161, 122–128. [Google Scholar] [CrossRef]
- Oliveira, A.N.; da Costa, L.R.S.; Pires, L.H.O.; Nascimento, L.A.S.; Angélica, R.S.; Da Costa, C.E.F.; Zamian, J.R.; Da Rocha Filho, G.N. Microwave-assisted preparation of a new esterification catalyst from wasted flint kaolin. Fuel 2013, 103, 626–631. [Google Scholar] [CrossRef]
- Oliveira, A.N.; Lima, E.T.L.; Oliveira, D.T.; Andrade, E.H.A.; Angélica, R.S.; Costa, C.E.F.; Rocha Filho, G.N.; Costa, F.F.; Luque, R.; Nascimento, L.A.S. Acetylation of Eugenol over 12-Molybdophosphoric Acid Anchored in Mesoporous Silicate Support Synthesized from Flint Kaolin. Materials 2019, 12, 2995. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, L.A.S.; Tito, L.M.Z.; Angélica, R.S.; Costa, C.E.F.; Zamian, J.R.; Rocha Filho, G.N. Esterification of oleic acid over solid acid catalysts prepared from Amazon flint kaolin. Appl. Catal. B Environ. 2011, 101, 495–503. [Google Scholar] [CrossRef]
- Carmo, A.C.; de Souza, L.K.C.; da Costa, C.E.F.; Longo, E.; Zamian, J.R.; da Rocha Filho, G.N. Production of biodiesel by esterification of palmitic acid over mesoporous aluminosilicate Al-MCM-41. Fuel 2009, 88, 461–468. [Google Scholar] [CrossRef]
- Oliveira, A.N.; Lima, M.A.B.; Pires, L.H.O.; Silva, M.R.; Luz, P.T.S.; Angélica, R.S.; Rocha Filho, G.N.; Costa, C.E.F.; Luque, R.; Nascimento, L.A.S. Bentonites Modified with Phosphomolybdic Heteropolyacid (HPMo) for Biowaste to Biofuel Production. Materials 2019, 12, 1431. [Google Scholar] [CrossRef]
- Lima, E.T.L.; Queiroz, L.S.; de Pires, L.H.O.; Angélica, R.S.; Costa, C.E.F.; Zamian, J.R.; Rocha Filho, G.N.; Luque, R.; Nascimento, L.A.S. Valorization of Mining Waste in the Synthesis of Organofunctionalized Aluminosilicates for the Esteri fi cation of Waste from Palm Oil Deodorization. ACS Sustain. Chem. Eng. 2019, 7, 7543–7551. [Google Scholar] [CrossRef]
- Queiroz, R.M.; Pires, L.H.O.; de Souza, R.C.P.; Zamian, J.R.; de Souza, A.G.; da Rocha Filho, G.N.; da Costa, C.E.F. Thermal characterization of hydrotalcite used in the transesterification of soybean oil. J. Therm. Anal. Calorim. 2009, 97, 163–166. [Google Scholar] [CrossRef]
- Coral, N.; Rodrigues, E.; Rumjanek, V.; Emmerson, C. Soybean biodiesel methyl esters, free glycerin and acid number quanti fi cation by 1 H nuclear magnetic resonance spectroscopy. Magn. Reson. Chem. 2013, 51, 69–71. [Google Scholar] [CrossRef]
- Laroque, D.A.; Loss, R.A.; Silva, M.J.A.; Pereira, G.N.; Valerio, A.; Hense, H.; de Oliveira, D.; Oliveira, V. Synthesis of Eugenyl Acetate in Solvent-Free Acetylation: Process Optimization and Kinetic Evaluation. J. Chem. Eng. Process Technol. 2015, 6, 4–11. [Google Scholar] [CrossRef]
- Lerin, L.A.; Catani, M.; Oliveira, D.; Massi, A.; Bortolini, O.; Cavazzini, A.; Giovannini, P.P. Continuous ion-exchange resin catalysed esterification of eugenol for the optimized production of eugenyl acetate using a packed bed microreactor. RSC Adv. 2015, 5, 76898–76903. [Google Scholar] [CrossRef]
- De Souza, L.K.C.; Pardauil, J.J.R.; Zamian, J.R.; Geraldo, N.; Filho, R.; Barrado, C.M.; Angélica, R.S.; Carlos, E.F. Rapid synthesis and characterization of CeMCM-41. Powder Technol. 2012, 229, 1–6. [Google Scholar] [CrossRef]
- Pires, L.H.O.; Queiroz, R.M.; Souza, R.P.; Carlos, E.F.; Zamian, J.R.; Weber, I.T.; Geraldo, N.; Filho, R. Synthesis and characterization of spherical Tb-MCM-41. J. Alloys Compd. 2010, 490, 667–671. [Google Scholar] [CrossRef]
- De Souza, L.K.C.; Pardauil, J.J.R.; Zamian, J.R.; da Rocha Filho, G.N.; Costa, C.E.F. Influence of the incorporated metal on template removal from MCM-41 type mesoporous materials. J. Therm. Anal. Calorim. 2011, 106, 355–361. [Google Scholar] [CrossRef]
- Zhou, C.; Sun, T.; Gao, Q.; Alshameri, A.; Zhu, P.; Wang, H.; Qiu, X.; Ma, Y.; Yan, C. Synthesis and characterization of ordered mesoporous aluminosilicate molecular sieve from natural halloysite. J. Taiwan Inst. Chem. Eng. 2014, 45, 1073–1079. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, Y.; Ouyang, J.; Yang, H. Mesoporous material Al-MCM-41 from natural halloysite. Phys. Chem. Miner. 2014, 41, 497–503. [Google Scholar] [CrossRef]
- Santos, E.C.; Costa, L.S.; Oliveira, E.S.; Bessa, R.A.; Freitas, A.D.L.; Oliveira, C.P.; Nascimento, R.F.; Loiola, A.R. Al-MCM-41 synthesized from kaolin via hydrothermal route: Structural characterization and use as an efficient adsorbent of methylene blue. J. Braz. Chem. Soc. 2018, 29, 2378–2386. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Liu, Y.; Liu, Z.; Guo, Y.; Liu, G.; Yang, Z.; Xu, M.; Wang, L. Synthesis of highly regular mesoporous Al-MCM-41 from metakaolin. Appl. Clay Sci. 2009, 44, 185–188. [Google Scholar] [CrossRef]
- Madhusoodana, C.D.; Kameshima, Y.; Nakajima, A.; Okada, K.; Kogure, T.; MacKenzie, K.J.D. Synthesis of high surface area Al-containing mesoporous silica from calcined and acid leached kaolinites as the precursors. J. Colloid Interface Sci. 2006, 297, 724–731. [Google Scholar] [CrossRef]
- Kang, F.; Wang, Q.; Xiang, S. Synthesis of mesoporous Al-MCM-41 materials using metakaolin as aluminum source. Mater. Lett. 2005, 59, 1426–1429. [Google Scholar] [CrossRef]
- Du, C.; Yang, H. Investigation of the physicochemical aspects from natural kaolin to Al-MCM-41 mesoporous materials. J. Colloid Interface Sci. 2012, 369, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Rocha Junior, C.A.F.; Angélica, R.S.; Neves, R.F. Sinthesis of faujasite-type zeolite: Comparison between processed and flint kaolin. Cerâmica 2015, 61, 259–268. [Google Scholar] [CrossRef]
- Carneiro, B.S.; Angélica, R.S.; Scheller, T.; de Castro, E.A.S.; de Neves, R.F. Mineralogical and geochemical characterization of the hard kaolin from the Capim region, Pará, northern Brazil. Cerâmica 2003, 49, 237–244. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, F.; Wang, X.; Cheng, F. Facile Preparation of Ammonium Molybdophosphate/Al-MCM-41 Composite Material from Natural Clay and Its Use in Cesium Ion Adsorption. Eur. J. Inorg. Chem. 2015, 2015, 2125–2131. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, F.; Li, S.; Cheng, F. Synthesis of SBA-15 encapsulated ammonium molybdophosphate using Qaidam natural clay and its use in cesium ion adsorption. RSC Adv. 2015, 5, 35453–35460. [Google Scholar] [CrossRef]
- Díaz, U.; Brunel, D.; Corma, A. Catalysis using multifunctional organosiliceous hybrid materials. Chem. Soc. Rev. 2013, 42, 4083–4097. [Google Scholar] [CrossRef]
- Ng, E.; Norbayu, S.; Subari, M.; Marie, O.; Mukti, R.R.; Juan, J. Sulfonic acid functionalized MCM-41 as solid acid catalyst for tert -butylation of hydroquinone enhanced by microwave heating. Appl. Catal. A Gen. 2013, 450, 34–41. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, Z.; Zhang, F. Esterification of oleic acid to biodiesel catalyzed by a highly acidic carbonaceous catalyst. Catal. Today 2019, 319, 172–181. [Google Scholar] [CrossRef]
- Boveri, M.; Aguilar-Pliego, J.; Pérez-Pariente, J.; Sastre, E. Optimization of the preparation method of HSO3-functionalized MCM-41 solid catalysts. Catal. Today 2005, 107–108, 868–873. [Google Scholar] [CrossRef]
- Guo, K.; Han, F.; Arslan, Z.; McComb, J.; Mao, X.; Zhang, R.; Sudarson, S.; Yu, H. Adsorption of Cs from Water on Surface-Modified MCM-41 Mesosilicate. Water Air Soil Pollut. 2015, 226, 2–9. [Google Scholar] [CrossRef]
- Fontes, M.S.B.; Melo, D.M.A.; Costa, C.C.; Braga, R.M.; Melo, M.A.F.; Alves, J.A.B.L.R.; Silva, M.L.P. Effect of different silica sources on textural parameters of molecular sieve MCM-41. Cerâmica 2016, 62, 85–90. [Google Scholar] [CrossRef]
- Kumar, P.; Mal, N.; Oumi, Y.; Yamana, K.; Sano, T. Mesoporous materials prepared using coal fly ash as the silicon and aluminium source. J. Mater. Chem. 2001, 11, 3285–3290. [Google Scholar] [CrossRef]
- Yang, H.; Deng, Y.; Du, C.; Jin, S. Novel synthesis of ordered mesoporous materials Al-MCM-41 from bentonite. Appl. Clay Sci. 2010, 47, 351–355. [Google Scholar] [CrossRef]
- Sing, K. The use of nitrogen adsorption for the characterisation of porous materials. Colloids Surf. A Physicochem. Eng. Asp. 2001, 187–188, 3–9. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, A.R.; Rouquérol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems including catalysis reporting physisorption data for gas/solid systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Patel, A.; Brahmkhatri, V. Kinetic study of oleic acid esterification over 12-tungstophosphoric acid catalyst anchored to different mesoporous silica supports. Fuel Process. Technol. 2013, 113, 141–149. [Google Scholar] [CrossRef]
- Brahmkhatri, V.; Patel, A. 12-Tungstophosphoric acid anchored to SBA-15: An efficient, environmentally benign reusable catalysts for biodiesel production by esterification of free fatty acids. Appl. Catal. A Gen. 2011, 403, 161–172. [Google Scholar] [CrossRef]
- Ahmed, A.I.; Samra, S.E.; El-Hakam, S.A.; Khder, A.S.; El-Shenawy, H.Z.; El-Yazeed, W.S.A. Characterization of 12-molybdophosphoric acid supported on mesoporous silica MCM-41 and its catalytic performance in the synthesis of hydroquinone diacetate. Appl. Surf. Sci. 2013, 282, 217–225. [Google Scholar] [CrossRef]
- Méndez, F.J.; Llanos, A.; Echeverría, M.; Jáuregui, R.; Villasana, Y.; Díaz, Y.; Liendo-Polanco, G.; Ramos-García, M.A.; Zoltan, T.; Brito, J.L. Mesoporous catalysts based on Keggin-type heteropolyacids supported on MCM-41 and their application in thiophene hydrodesulfurization. Fuel 2013, 110, 249–258. [Google Scholar] [CrossRef]
- Costa, B.O.D.; Legnoverde, M.S.; Lago, C.; Decolatti, H.P.; Querini, C.A. Microporous and Mesoporous Materials Sulfonic functionalized SBA-15 catalysts in the gas phase glycerol dehydration. Thermal stability and catalyst deactivation. Microporous Mesoporous Mater. 2016, 230, 66–75. [Google Scholar] [CrossRef]
- Adam, F.; Kueh, C.W. Phenyl-amino sulfonic solid acid-MCM-41 complex: A highly active and selective catalyst for the synthesis of mono-alkylated products in the solvent free tert-butylation of phenol. J. Taiwan Inst. Chem. Eng. 2014, 45, 713–723. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, H.; Fan, M.; Sun, W.; Jiang, P.; Dong, Y. Direct and postsynthesis of tin-incorporated SBA-15 functionalized with sulfonic acid for efficient biodiesel production. Fuel 2019, 235, 426–432. [Google Scholar] [CrossRef]
- Lima, E.T.L. Síntese de Al-MCM-41 a partir do rejeito do caulim e impregnação com grupo sulfônico para fins catalíticos. 2016, 63f. In Dissertação (Mestrado em Química)–Programa de Pós-Graduação em Química; University Federal do Pará: Belém, Brazil, 2016. [Google Scholar]
- Campelo, J.M.; Lafont, F.; Marinas, J.M.; Ojeda, M. Studies of catalyst deactivation in methanol conversion with high, medium and small pore silicoaluminophosphates. Appl. Catal. A Gen. 2000, 192, 85–96. [Google Scholar] [CrossRef]
- Gang, L.; Xinzong, L.; Eli, W. Solvent-free esterification catalyzed by surfactant-combined catalysts at room temperature. New J. Chem. 2007, 31, 348. [Google Scholar] [CrossRef]
- Santos, P.; Zabot, G.L.; Meireles, M.A.A.; Mazutti, M.A.; Martínez, J. Synthesis of eugenyl acetate by enzymatic reactions in supercritical carbon dioxide. Biochem. Eng. J. 2016, 114, 1–9. [Google Scholar] [CrossRef]
- Narkhede, N.; Singh, S.; Patel, A. Recent progress on supported polyoxometalates for biodiesel synthesis via esterification and transesterification. Green Chem. 2015, 17, 89–107. [Google Scholar] [CrossRef]
- Wang, A.; Wang, J.; Lu, C.; Xu, M.; Lv, J.; Wu, X. Esterification for biofuel synthesis over an eco-friendly and e ffi cient kaolinite-supported SO42−/ZnAl2O4 macroporous solid acid catalyst. Fuel 2018, 234, 430–440. [Google Scholar] [CrossRef]
- Hoo, P.; Abdullah, A.Z. Kinetics Modeling and Mechanism Study for Selective Esterification of Glycerol with Lauric Acid Using 12-Tungstophosphoric Acid Post-Impregnated SBA-15. Ind. Eng. Chem. Res. 2015, 54, 7852–7858. [Google Scholar] [CrossRef]
- Baskaran, Y.; Periyasamy, V.; Carani, A. Investigation of antioxidant, anti-inflammatory and DNA-protective properties of eugenol in thioacetamide-induced liver injury in rats. Toxicology 2010, 268, 204–212. [Google Scholar] [CrossRef]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Santin, J.R.; Lemos, M.; Klein-Júnior, L.C.; Machado, I.D.; Costa, P.; De Oliveira, A.P.; Tilia, C.; De Souza, J.P.; De Sousa, J.P.B.; Bastos, J.K.; et al. Gastroprotective activity of essential oil of the Syzygium aromaticum and its major component eugenol in different animal models. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2011, 383, 149–158. [Google Scholar] [CrossRef]
- Rodrigues, T.; Fernandes, A., Jr.; Sousa, J.; Bastos, J.; Sforcin, J. In vitro and in vivo effects of clove on pro-inflammatory cytokines production by macrophages. Nat. Prod. Res. 2009, 23, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Chaibakhsh, N.; Basri, M.; Anuar, S.H.M.; Rahman, M.B.A.; Rezayee, M. Optimization of enzymatic synthesis of eugenol ester using statistical approaches. Biocatal. Agric. Biotechnol. 2012, 1, 226–231. [Google Scholar] [CrossRef]
- Affonso, R.S.; Lessa, B.; Slana, G.B.C.A.; Barboza, L.L.; de Almeida, F.V.; de Souza, F.R.; França, T.C.C. Quantification and Characterization of the Main Components of the Ethanolic Extract of Indian Cloves, Syzygium aromaticum [l] Mer. et Perry. Rev. Virtual Quim. 2014, 6, 1316–1331. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Reddy, C.R.; Nagendrappa, G.; Prakash, B.S.J. Surface acidity study of Mn+-montmorillonite clay catalysts by FT-IR spectroscopy: Correlation with esterification activity. Catal. Commun. 2007, 8, 241–246. [Google Scholar] [CrossRef]
- Reddy, C.R.; Bhat, Y.S.; Nagendrappa, G.; Prakash, B.S.J. Brønsted and Lewis acidity of modified montmorillonite clay catalysts determined by FT-IR spectroscopy. Catal. Today 2009, 141, 157–160. [Google Scholar] [CrossRef]
- NIST NIST (2011) National Institute of Standard and Technology (2011) NIST Standard Reference Database Number 69. Available online: http://webbook.nist.gov/ (accessed on 25 November 2018).














| Samples | SiO2 | Al2O3 | TiO2 | Fe2O3 | SO3 | LF a | Si/Al |
|---|---|---|---|---|---|---|---|
| K b | 46.54 | 39.50 | 13.96 | 1 | |||
| KF | 42.30 | 38.29 | 3.20 | 2.92 | 13.29 | 1 | |
| MF | 49.64 | 37.92 | 2.50 | 2.78 | 1.25 | 1 | |
| MFL | 73.98 | 4.74 | 3.71 | 1.62 | 15.89 | 13 | |
| AlSiM | 86.72 | 3.21 | 3.52 | 1.10 | 5.23 | 23 | |
| (3)SO3H/AlSiM | 69.09 | 1.71 | 2.16 | 0.26 | 8.70 | 18.08 | 34 |
| (5)SO3H/AlSiM | 61.39 | 1.55 | 1.83 | 0.10 | 11.06 | 24.07 | 34 |
| Samples | Textural Property | Structural Property | ||||
|---|---|---|---|---|---|---|
| - | SSA (m2 g−1) a | Vp (cm3 g−1) b | Dp (nm) c | d100 (nm) d | a0 (nm) e | Wt (nm) f |
| KF | 8.9 | 0.05 | 32.20 | - | - | - |
| MF | 9.78 | 0.05 | 31.11 | - | - | - |
| MFL | 433 | 0.56 | 1.20 | - | - | - |
| AlSiM | 1071 | 1.05 | 3.85 | 4.35 | 5.06 | 1.21 |
| (3)SO3H/AlSiM | 998 | 0.78 | 3.25 | 4.07 | 4.70 | 1.45 |
| (5)SO3H/AlSiM | 869 | 0.65 | 3.01 | 4.04 | 4.67 | 1.66 |
| Samples | Mass Losses (%) | Total | ||
|---|---|---|---|---|
| I (T < 200 °C) | II (200–430 °C) | III (T > 430 °C) | ||
| AlSiM | 3.96 | 0.99 | 0.80 | 5.23 |
| (3)SO3H/AlSiM | 8.23 | 6.71 | 5.75 | 20.69 |
| (5)SO3H/AlSiM | 12.65 | 8.09 | 6.55 | 27.29 |
| Amostras | (mmol H+ g−1) a | (μmol g−1) b | Conv. (%) c |
|---|---|---|---|
| AlSiM | 1.31 | 27 | |
| (3)SO3H/AlSiM | 5.93 | 295 | 97 |
| (5)SO3H/AlSiM | 4.89 | 236 | 89 |
| Catalyst | Solvent | T (°C) | R: M | T (min) | Conv. (%) | Reference |
|---|---|---|---|---|---|---|
| UDCaT-5 | Toluene | 110 | 1:5 | 240 | 90 | [10] |
| Lipase (RML) | Chloroform | 50 | 1:3 | 300 | 66 | [11] |
| Lipozyme TL | Acetic anhydride | 70 | 1:5 | 120 | 92.9 | [5] |
| Molecular sieve 4Å | Acetic anhydride | 60 | 1:3 | 120 | 90 | [31] |
| Amberlyst A-21 | Acetic anhydride | 95 | 1:3 | 120 | 95 | [32] |
| Lewatit® GF 101 | Acetic anhydride | 70 | 1:1 | 45 | 100 | [17] |
| 10HPMo/AlSiM | Acetic anhydride | 80 | 1:5 | 40 | 99.9 | [24] |
| (3)SO3H/AlSiM | Acetic anhydride | 80 | 1:5 | 40 | 99.9 | Present work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Nazaré de Oliveira, A.; Tallyta Leite Lima, E.; de Aguiar Andrade, E.H.; Zamian, J.R.; Filho, G.N.d.R.; Costa, C.E.F.d.; Helena de Oliveira Pires, L.; Luque, R.; Nascimento, L.A.S.d. Acetylation of Eugenol on Functionalized Mesoporous Aluminosilicates Synthesized from Amazonian Flint Kaolin. Catalysts 2020, 10, 478. https://doi.org/10.3390/catal10050478
de Nazaré de Oliveira A, Tallyta Leite Lima E, de Aguiar Andrade EH, Zamian JR, Filho GNdR, Costa CEFd, Helena de Oliveira Pires L, Luque R, Nascimento LASd. Acetylation of Eugenol on Functionalized Mesoporous Aluminosilicates Synthesized from Amazonian Flint Kaolin. Catalysts. 2020; 10(5):478. https://doi.org/10.3390/catal10050478
Chicago/Turabian Stylede Nazaré de Oliveira, Alex, Erika Tallyta Leite Lima, Eloisa Helena de Aguiar Andrade, José Roberto Zamian, Geraldo Narciso da Rocha Filho, Carlos Emmerson Ferreira da Costa, Luíza Helena de Oliveira Pires, Rafael Luque, and Luís Adriano Santos do Nascimento. 2020. "Acetylation of Eugenol on Functionalized Mesoporous Aluminosilicates Synthesized from Amazonian Flint Kaolin" Catalysts 10, no. 5: 478. https://doi.org/10.3390/catal10050478
APA Stylede Nazaré de Oliveira, A., Tallyta Leite Lima, E., de Aguiar Andrade, E. H., Zamian, J. R., Filho, G. N. d. R., Costa, C. E. F. d., Helena de Oliveira Pires, L., Luque, R., & Nascimento, L. A. S. d. (2020). Acetylation of Eugenol on Functionalized Mesoporous Aluminosilicates Synthesized from Amazonian Flint Kaolin. Catalysts, 10(5), 478. https://doi.org/10.3390/catal10050478









