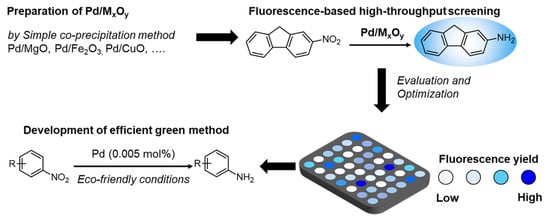
Preparation of Metal Oxides Containing ppm Levels of Pd as Catalysts for the Reduction of Nitroarene and Evaluation of Their Catalytic Activity by the Fluorescence-Based High-Throughput Screening Method
Abstract
1. Introduction
2. Results and Discussion
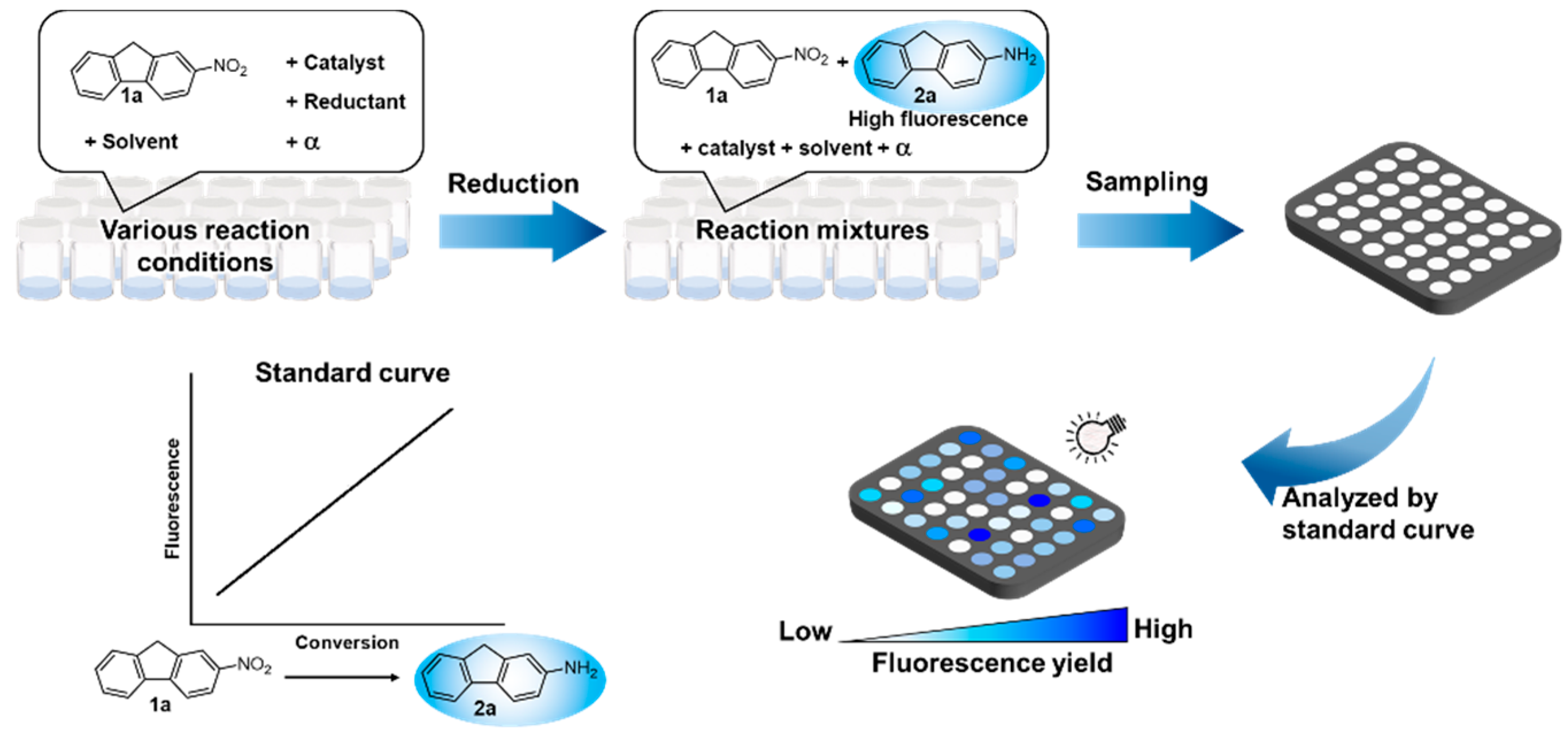
2.1. Development of Fluorescence-Based HTS Method for the Reduction of Nitroarene
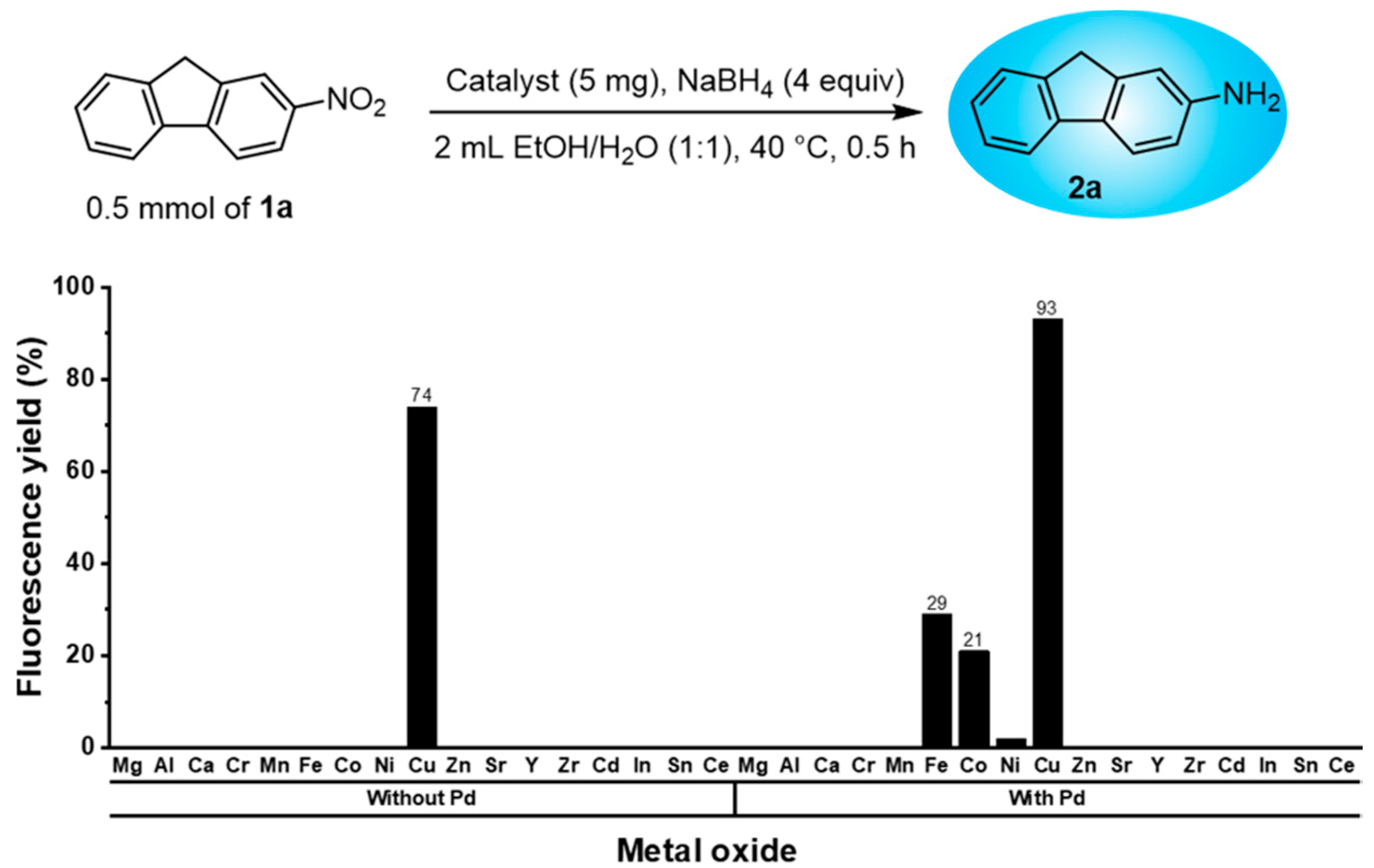
2.2. Preparation of Metal Oxides
2.3. HTS of Metal Oxides Catalysts
2.4. Optimization of Reaction Conditions
2.5. Substrate Scope
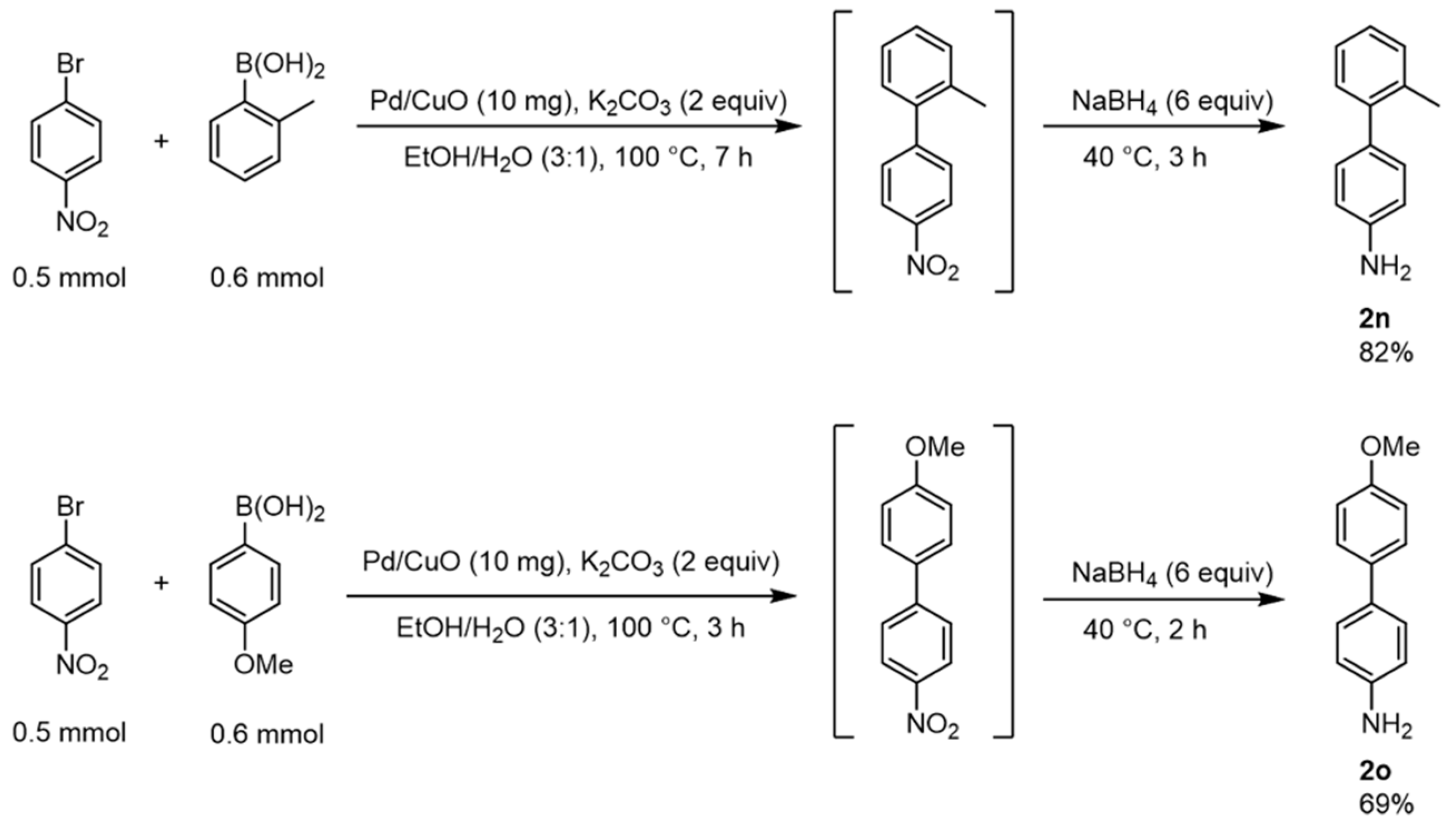
2.6. Synthetic Applications
3. Materials and Methods
3.1. Materials and Instruments
3.2. General Procedure for the Preparation of Pd/MxOy
3.3. General Procedure for High-Throughput Screening Method for the Reduction of Nitroarene
3.4. General Procedure for the Reduction of Nitroarene
3.5. One-Pot Procedure for Suzuki-Miyaura Cross Coupling and Nitro Reduction
3.6. Gram-Scale Reaction of Reduction of Nitroarene
3.7. Procedure for the Reuse of Catalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rappoport, Z. The Chemistry of Anilines, Part 1; John Wiley & Sons Ltd: Chichester, UK, 2007. [Google Scholar]
- Blaser, H.-U.; Steiner, H.; Studer, M. Selective catalytic hydrogenation of functionalized nitroarenes: An update. ChemCatChem 2009, 1, 210–221. [Google Scholar] [CrossRef]
- Orlandi, M.; Brenna, D.; Harms, R.; Jost, S.; Benaglia, M. Recent developments in the reduction of aromatic and aliphatic nitro compounds to amines. Org. Process Res. Dev. 2018, 22, 430–445. [Google Scholar] [CrossRef]
- Formenti, D.; Ferretti, F.; Scharnagl, F.K.; Beller, M. Reduction of nitro compounds using 3d-non-noble metal catalysts. Chem. Rev. 2019, 119, 2611–2680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, M.; Wang, A.; Zhang, T. Selective hydrogenation over supported metal catalysts: From nanoparticles to single atoms. Chem. Rev. 2020, 120, 683–733. [Google Scholar] [CrossRef] [PubMed]
- Garrett, C.E.; Prasad, K. The art of meeting palladium specifications in active pharmaceutical ingredients produced by Pd-catalyzed reactions. Adv. Synth. Catal. 2004, 346, 889–900. [Google Scholar] [CrossRef]
- Feng, J.; Handa, S.; Gallou, F.; Lipshutz, B.H. Safe and selective nitro group reductions catalyzed by sustainable and recyclable Fe/ppm Pd nanoparticles in water at room temperature. Angew. Chem. Int. Ed. 2016, 55, 8979–8983. [Google Scholar] [CrossRef]
- Pang, H.; Gallou, F.; Sohn, H.; Camacho-Bunquin, J.; Delferro, M.; Lipshutz, B.H. Synergistic effects in Fe nanoparticles doped with ppm levels of (Pd + Ni). A new catalyst for sustainable nitro group reductions. Green Chem. 2018, 20, 130–135. [Google Scholar] [CrossRef]
- Uberman, P.M.; García, C.S.; Rodríguez, J.R.; Martín, S.E. PVP-Pd nanoparticles as efficient catalyst for nitroarene reduction under mild conditions in aqueous media. Green Chem. 2017, 19, 739–748. [Google Scholar] [CrossRef]
- Fihri, A.; Bouhrara, M.; Nekoueishahraki, B.; Basset, J.-M.; Polshettiwar, V. Nanocatalysts for Suzuki cross-coupling reactions. Chem. Soc. Rev. 2011, 40, 5181–5203. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, Z.; Wang, B.; Tang, Y.; Nguyen, L.; Li, Y.; Tao, F.F. C−C coupling on single-atom-based heterogeneous catalyst. J. Am. Chem. Soc. 2018, 140, 954–962. [Google Scholar] [CrossRef]
- Holz, J.; Pfeffer, C.; Zuo, H.; Beierlein, D.; Richter, G.; Klemm, E.; Peters, R. In situ generated gold nanoparticles on active carbon as reusable highly efficient catalysts for a Csp3–Csp3 Stille Coupling. Angew. Chem. Int. Ed. 2019, 58, 10330–10334. [Google Scholar] [CrossRef] [PubMed]
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, J.; Zeng, J.; Wang, Q.; Li, Z.; Qin, R.; Wu, C.; Xie, Z.; Zheng, L. The synergy between atomically dispersed Pd and cerium oxide for enhanced catalytic properties. Nanoscale 2017, 9, 6643–6648. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Cao, Y.; Duan, X.; Li, N.; Xu, Q.; Li, H.; He, J.; Chen, D.; Lu, J. Eye-readable detection and oxidation of CO with a platinum-based catalyst and a binuclear rhodium complex. Angew. Chem. Int. Ed. 2019, 58, 12258–12263. [Google Scholar] [CrossRef]
- Min, H.; Lee, S.; Park, M.; Hwang, J.; Jung, H.M.; Lee, S. Preparation of polymer-bound palladium catalyst and its application to the reduction of nitro arenes and the hydrodehalogenation of aryl halides. J. Organomet. Chem. 2014, 755, 7–11. [Google Scholar] [CrossRef]
- Byun, S.; Song, Y.; Kim, B.M. Heterogenized bimetallic Pd−Pt−Fe3O4 nanoflakes as extremely robust, magnetically recyclable catalysts for chemoselective nitroarene reduction. ACS Appl. Mater. Interfaces 2016, 8, 14637–14647. [Google Scholar] [CrossRef]
- Cho, H.R.; Kwon, Y.M.; Lee, Y.J.; Park, Y.A.; Ji, H.G.; Lee, J.H. Morphological control of gold nanoparticles on exfoliated layers of layered double hydroxide: A reusable hybrid catalyst for the reduction of p-nitrophenol. Appl. Clay Sci. 2018, 156, 187–194. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Hong, K.; Lee, T.H.; Moon, C.W.; Hong, S.P.; Zhang, K.; Suh, J.M.; Choi, K.S.; Varma, R.S.; Jang, H.W. Magnetically retrievable nanocomposite adorned with Pd nanocatalysts: Efficient reduction of nitroaromatics in aqueous media. Green Chem. 2018, 20, 3809–3817. [Google Scholar] [CrossRef]
- Monyoncho, E.A.; Ntais, S.; Brazeau, N.; Wu, J.-J.; Sun, C.-L.; Baranova, E.A. Role of the metal-oxide support in the catalytic activity of Pd nanoparticles for ethanol electrooxidation in alkaline media. ChemElectroChem 2016, 3, 218–227. [Google Scholar] [CrossRef]
- Zhao, G.; Yang, F.; Chen, Z.; Liu, Q.; Ji, Y.; Zhang, Y.; Niu, Z.; Mao, J.; Bao, X.; Hu, P.; et al. Metal/oxide interfacial effects on the selective oxidation of primary alcohols. Nat. Commun. 2017, 8, 14039. [Google Scholar] [CrossRef]
- Cai, C.; Chung, J.Y.L.; McWilliams, J.C.; Sun, Y.; Shultz, C.S.; Palucki, M. From high-throughput catalyst screening to reaction optimization: Detailed investigation of regioselective Suzuki coupling of 1,6-naphthyridone dichloride. Org. Process Res. Dev. 2007, 11, 328–335. [Google Scholar] [CrossRef]
- Fang, H.; Xiao, Q.; Wu, F.; Floreancig, P.E.; Weber, S.G. Rapid catalyst screening by a continuous-flow microreactor interfaced with ultra-high-pressure liquid chromatography. J. Org. Chem. 2010, 75, 5619–5626. [Google Scholar] [CrossRef] [PubMed]
- Trapp, O. Gas chromatographic high-throughput screening techniques in catalysis. J. Chromatogr. A 2008, 1184, 160–190. [Google Scholar] [CrossRef] [PubMed]
- Uozumi, Y.; Nakai, Y. An amphiphilic resin-supported palladium catalyst for high-throughput cross-coupling in water. Org. Lett. 2002, 4, 2997–3000. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.-B.; van Leeuwen, P.W.N.M.; Reek, J.N.H. SUPRAphos-based palladium catalysts for the kinetic resolution of racemic cyclohexenyl acetate. Chem. Commun. 2007, 22, 2287–2289. [Google Scholar] [CrossRef] [PubMed]
- Reetz, M.T.; Becker, M.H.; Klein, H.-W.; Stöckigt, D. A method for high-throughput screening of enantioselective catalysts. Angew. Chem. Int. Ed. 1999, 38, 1758–1761. [Google Scholar] [CrossRef]
- Szewczyk, J.W.; Zuckerman, R.L.; Bergman, R.G.; Ellman, J.A. A mass spectrometric labeling strategy for high-throughput reaction evaluation and optimization: Exploring C-H activation. Angew. Chem. Int. Ed. 2001, 40, 216–219. [Google Scholar] [CrossRef]
- Markert, C.; Pfaltz, A. Screening of chiral catalysts and catalyst mixtures by mass spectrometric monitoring of catalytic intermediates. Angew. Chem. Int. Ed. 2004, 43, 2498–2500. [Google Scholar] [CrossRef]
- Reetz, M.T.; Eipper, A.; Tielmann, P.; Mynott, R. A practical NMR-based high-throughput assay for screening enantioselective catalysts and biocatalysts. Adv. Synth. Catal. 2002, 344, 1008–1016. [Google Scholar] [CrossRef]
- Mori, K.; Ichikawa, Y.; Kobayashi, M.; Shibata, Y.; Yamanaka, M.; Akiyama, T. Prediction of suitable catalyst by 1H NMR: Asymmetric synthesis of multisubstituted biaryls by chiral phosphoric acid catalyzed asymmetric bromination. Chem. Sci. 2013, 4, 4235–4239. [Google Scholar] [CrossRef]
- Lavastre, O.; Morken, J.P. Discovery of novel catalysts for allylic alkylation with a visual colorimetric assay. Angew. Chem. Int. Ed. 1999, 38, 3163–3165. [Google Scholar] [CrossRef]
- Kawatsura, M.; Hartwig, J.F. Transition metal-catalyzed addition of amines to acrylic acid derivatives. A high-throughput method for evaluating hydroamination of primary and secondary alkylamines. Organometallics 2001, 20, 1960–1964. [Google Scholar] [CrossRef]
- Löber, O.; Kawatsura, M.; Hartwig, J.F. Palladium-catalyzed hydroamination of 1,3-Dienes: A colorimetric assay and enantioselective additions. J. Am. Chem. Soc. 2001, 123, 4366–4367. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.F.; Messerle, B.A.; Rumble, S.L. Application of UV-Vis spectroscopy to high throughput screening of hydroamination catalysts. New J. Chem. 2009, 33, 818–824. [Google Scholar] [CrossRef]
- Shaughnessy, K.H.; Kim, P.; Hartwig, J.F. A fluorescence-based assay for high-throughput screening of coupling reactions. Application to Heck chemistry. J. Am. Chem. Soc. 1999, 121, 2123–2132. [Google Scholar] [CrossRef]
- Stambuli, J.P.; Stauffer, S.R.; Shaughnessy, K.H.; Hartwig, J.F. Screening of homogeneous catalysts by fluorescence resonance energy transfer. Identification of catalysts for room-temperature Heck reactions. J. Am. Chem. Soc. 2001, 123, 2677–2678. [Google Scholar] [CrossRef]
- Stauffer, S.R.; Beare, N.A.; Stambuli, J.P.; Hartwig, J.F. Palladium-catalyzed arylation of ethyl cyanoacetate. fluorescence resonance energy transfer as a tool for reaction discovery. J. Am. Chem. Soc. 2001, 123, 4641–4642. [Google Scholar] [CrossRef]
- Stauffer, S.R.; Hartwig, J.F. Fluorescence resonance energy transfer (FRET) as a high-throughput assay for coupling reactions. Arylation of amines as a case study. J. Am. Chem. Soc. 2003, 125, 6977–6985. [Google Scholar] [CrossRef]
- Angelovski, G.; Keränen, M.D.; Linnepe, P.; Grudzielanek, S.; Eilbracht, P. A rapid and reliable assay for regioselectivity using fluorescence spectroscopy. Adv. Synth. Catal. 2006, 348, 1193–1199. [Google Scholar] [CrossRef]
- Guo, H.-M.; Tanaka, F. A fluorogenic aldehyde bearing a 1,2,3-triazole moiety for monitoring the progress of aldol reactions. J. Org. Chem. 2009, 74, 2417–2424. [Google Scholar] [CrossRef]
- Xia, B.; Gerard, B.; Solano, D.M.; Wan, J.; Jones II, G.; Porco, J.A., Jr. ESIPT-mediated photocycloadditions of 3-hydroxyquinolinones: Development of a fluorescence quenching assay for reaction screening. Org. Lett. 2011, 13, 1346–1349. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Kim, S.; Kim, Y.; Seo, S.H.; Lee, S.S.; Han, M.S.; Lee, S. A colorimetric high-throughput screening method for palladium-catalyzed coupling reactions of aryl iodides using a gold nanoparticle-based iodide-selective probe. Angew. Chem. Int. Ed. 2011, 50, 4386–4389. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jung, E.; Kim, M.J.; Pyo, A.; Palani, T.; Eom, M.S.; Han, M.S.; Lee, S. A simple, fast, and easy assay for transition metal-catalyzed coupling reactions using a paper-based colorimetric iodide sensor. Chem. Commun. 2012, 48, 8751–8753. [Google Scholar] [CrossRef] [PubMed]
- Pyo, A.; Kim, S.; Kumar, M.R.; Byeun, A.; Eom, M.S.; Han, M.S.; Lee, S. Palladium-catalyzed hydrodehalogenation of aryl halides using paraformaldehyde as the hydride source: High-throughput screening by paper-based colorimetric iodide sensor. Tetrahedron Lett. 2013, 54, 5207–5210. [Google Scholar] [CrossRef]
- Byeun, A.; Baek, K.; Han, M.S.; Lee, S. Palladium-catalyzed C–S bond formation by using N-amido imidazolium salts as ligands. Tetrahedron Lett. 2013, 54, 6712–6715. [Google Scholar] [CrossRef]
- Eom, M.S.; Noh, J.; Kim, H.-S.; Yoo, S.; Han, M.S.; Lee, S. High-throughput screening protocol for the coupling reactions of aryl halides using a colorimetric chemosensor for halide ions. Org. Lett. 2016, 18, 1720–1723. [Google Scholar] [CrossRef]
- Kim, H.-S.; Eom, M.S.; Han, M.S.; Lee, S. Paper-based colorimetric sensor system for high-throughput screening of C-H borylation. Chem. Eur. J. 2017, 23, 6282–6285. [Google Scholar] [CrossRef]
- Son, Y.; Lee, S.; Kim, H.-S.; Eom, M.S.; Han, M.S.; Lee, S. Organosilane-patterned paper-based colorimetric sensors for high-throughput screening of cross-coupling reactions with aryl bromides. Adv. Synth. Catal. 2018, 360, 3916–3923. [Google Scholar] [CrossRef]
- Noh, H.; Lim, T.; Park, B.Y.; Han, M.S. A fluorescence-based high-throughput screening method for olefin metathesis using a ratiometric fluorescent probe. Org. Lett. 2020, 22, 1703–1708. [Google Scholar] [CrossRef]
- Yu, F.; Han, X.; Chen, L. Fluorescent probes for hydrogen sulfide detection and bioimaging. Chem. Commun. 2014, 50, 12234–12249. [Google Scholar] [CrossRef]
- Yuan, J.; Xu, Y.-Q.; Zhou, N.-N.; Wang, R.; Qian, X.-H.; Xu, Y.-F. A highly selective turn-on fluorescent probe based on semi-cyanine for the detection of nitroreductase and hypoxic tumor cell imaging. RSC Adv. 2014, 4, 56207–56210. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Li, J.; Su, Q.; Yuan, W.; Dai, Y.; Han, C.; Wang, Q.; Feng, W.; Li, F. Ultrasensitive near-infrared fluorescence-enhanced probe for in vivo nitroreductase imaging. J. Am. Chem. Soc. 2015, 137, 6407–6416. [Google Scholar] [CrossRef] [PubMed]
- Suman Bagui, A.; Datt, R.; Gupta, V.; Singh, S.P. A simple fluorene core-based non-fullerene acceptor for high performance organic solar cells. Chem. Commun. 2017, 53, 12790–12793. [Google Scholar] [CrossRef] [PubMed]
- Catalfo, A.; Serrentino, M.E.; Librando, V.; Perrini, G.; De Guidi, G. Spectroscopic properties of some drivatives of polycyclic aromatic hydrocarbons. Appl. Spectrosc. 2008, 62, 1233–1237. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.; Xi, W.; Liu, J.-C.; Cui, Y.-T.; Li, T.; Lee, A.F.; Chen, F.; Chen, Y.; Li, L.; Li, L.; et al. Non defect-stabilized thermally stable single-atom catalyst. Nat. Commun. 2019, 10, 234. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-I.; Chang, H.-Y. Homogeneous precipitation of cerium dioxide nanoparticles in alcohol/water mixed solvents. Colloids Surf. A Physicochem. Eng. Asp. 2004, 242, 61–69. [Google Scholar] [CrossRef]
- Ma, G.; Li, S.; Zhang, W.; Yang, Z.; Liu, S.; Fan, X.; Chen, F.; Tian, Y.; Zhang, W.; Yang, S.; et al. A general and mild approach to controllable preparation of manganese-based micro- and manostructured bars for high performance lithium-Ion batteries. Angew. Chem. Int. Ed. 2016, 55, 3667–3671. [Google Scholar] [CrossRef]
- Laguna, M.; Nuñez, N.O.; Becerro, A.I.; Lozano, G.; Moros, M.; de la Fuente, J.M.; Corral, A.; Balcerzyk, M.; Ocaña, M. Synthesis, functionalization and properties of uniform europium-doped sodium lanthanum tungstate and molybdate (NaLa(XO4)2, X = Mo,W) probes for luminescent and X-ray computed tomography bioimaging. J. Colloid Interface Sci. 2019, 554, 520–530. [Google Scholar] [CrossRef]
- Kadam, H.K.; Tilve, S.G. Advancement in methodologies for reduction of nitroarenes. RSC Adv. 2015, 5, 83391–83407. [Google Scholar] [CrossRef]
- Netskina, O.V.; Ozerova, A.M.; Komova, O.V.; Odegova, G.V.; Simagina, V.I. Hydrogen storage systems based on solid-state NaBH4/CoxB composite: Influence of catalyst properties on hydrogen generation rate. Catal. Today 2015, 245, 86–92. [Google Scholar] [CrossRef]
- Farmer, J.A.; Campbell, C.T. Ceria maintains smaller metal catalyst particles by strong metal-support bonding. Science 2010, 329, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Bruix, A.; Rodriguez, J.A.; Ramírez, P.J.; Senanayake, S.D.; Evans, J.; Park, J.B.; Stacchiola, D.; Liu, P.; Hrbek, J.; Illas, F. A new type of strong metal−support interaction and the production of H2 through the transformation of water on Pt/CeO2(111) and Pt/CeOx/TiO2(110) Catalysts. J. Am. Chem. Soc. 2012, 134, 8968–8974. [Google Scholar] [CrossRef] [PubMed]
- Lykhach, Y.; Kozlov, S.M.; Skála, T.; Tovt, A.; Stetsovych, V.; Tsud, N.; Dvořák, F.; Johánek, V.; Neitzel, A.; Mysliveček, J.; et al. Counting electrons on supported nanoparticles. Nat. Mater. 2016, 15, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Valden, M.; Lai, X.; Goodman, D.W. Onset of catalytic activity of gold clusters on titania with the appearance of nonmetallic properties. Science 1998, 281, 1647–1650. [Google Scholar] [CrossRef] [PubMed]
- McClure, S.M.; Lundwall, M.J.; Goodman, D.W. Planar oxide supported rhodium nanoparticles as model catalysts. Proc. Natl. Acad. Sci. USA 2011, 108, 931–936. [Google Scholar] [CrossRef]
- Graciani, J.; Mudiyanselage, K.; Xu, F.; Baber, A.E.; Evans, J.; Senanayake, S.D.; Stacchiola, D.J.; Liu, P.; Hrbek, J.; Sanz, J.F.; et al. Highly active copper-ceria and copper-ceria-titania catalysts for methanol synthesis from CO2. Science 2014, 345, 546–550. [Google Scholar] [CrossRef]
- Fountoulaki, S.; Daikopoulou, V.; Gkizis, P.L.; Tamiolakis, I.; Armatas, G.S.; Lykakis, I.N. Mechanistic Studies of the Reduction of Nitroarenes by NaBH4 or Hydrosilanes Catalyzed by Supported Gold Nanoparticles. ACS Catal. 2014, 4, 3504–3511. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, R.; Jiang, R.; Zhang, K.; Dong, Y.; Xie, W. Mechanistic Study of Catalytic Hydride Reduction of −NO2 to −NH2 Using Isotopic Solvent and Reducer: The Real Hydrogen Source. J. Phys. Chem. C 2019, 123, 15582–15588. [Google Scholar] [CrossRef]

| Entry | Catalyst | Reducing Agent | Solvent | Yield c |
|---|---|---|---|---|
| 1 | Pd/CuO 2 mg | NaBH4 4 equiv | EtOH/H2O (1:1) | 90 |
| 2 | Pd/CuO 1 mg | NaBH4 4 equiv | EtOH/H2O (1:1) | 75 |
| 3 | Pd/CuO 2 mg | NaBH4 3 equiv | EtOH/H2O (1:1) | 89 |
| 4 | Pd/CuO 2 mg | NaBH4 2 equiv | EtOH/H2O (1:1) | 86 |
| 5 | Pd/CuO 2 mg | NaBH4 1 equiv | EtOH/H2O (1:1) | 46 |
| 6 | Pd/CuO 2 mg | NH3BH3 4 equiv | EtOH/H2O (1:1) | 74 |
| 7 | Pd/CuO 2 mg | NaBH3CN 4 equiv | EtOH/H2O (1:1) | 0 |
| 8 b | Pd/CuO 2 mg | H2 (1 atm) | EtOH/H2O (1:1) | 0 |
| 9 | Pd/CuO 2 mg | NH2NH2 4 equiv | EtOH/H2O (1:1) | 0 |
| 10 | Pd/CuO 2 mg | NaBH4 3 equiv | EtOH | >99 |
| 11 | Pd/CuO 2 mg | NaBH4 3 equiv | H2O | 0 |
| 12 | Pd/CuO 2 mg | NaBH4 3 equiv | Acetonitrile | 9 |
| 13 | Pd/CuO 2 mg | NaBH4 3 equiv | DMF | 0 |
| 14 | Pd/CuO 2 mg | NaBH4 3 equiv | Toluene | 0 |
| 15 | Pd/CuO 2 mg | NaBH4 3 equiv | Dioxane | 0 |
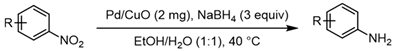
| Entry | Substrate | Product | Time | Yield (%) b |
|---|---|---|---|---|
| 1 |  1a | 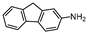 2a | 0.5 h | 94 |
| 2 | 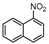 1b | 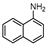 2b | 4 h | 93 |
| 3 | 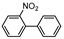 1c | 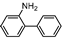 2c | 17.5 h | 73 |
| 4 c | 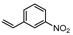 1d | 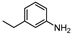 2d | 3 h | 62 |
| 5 |  1e |  2e | 12 h | 86 |
| 6 c | 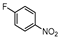 1f | 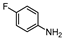 2f | 1 h | 68 |
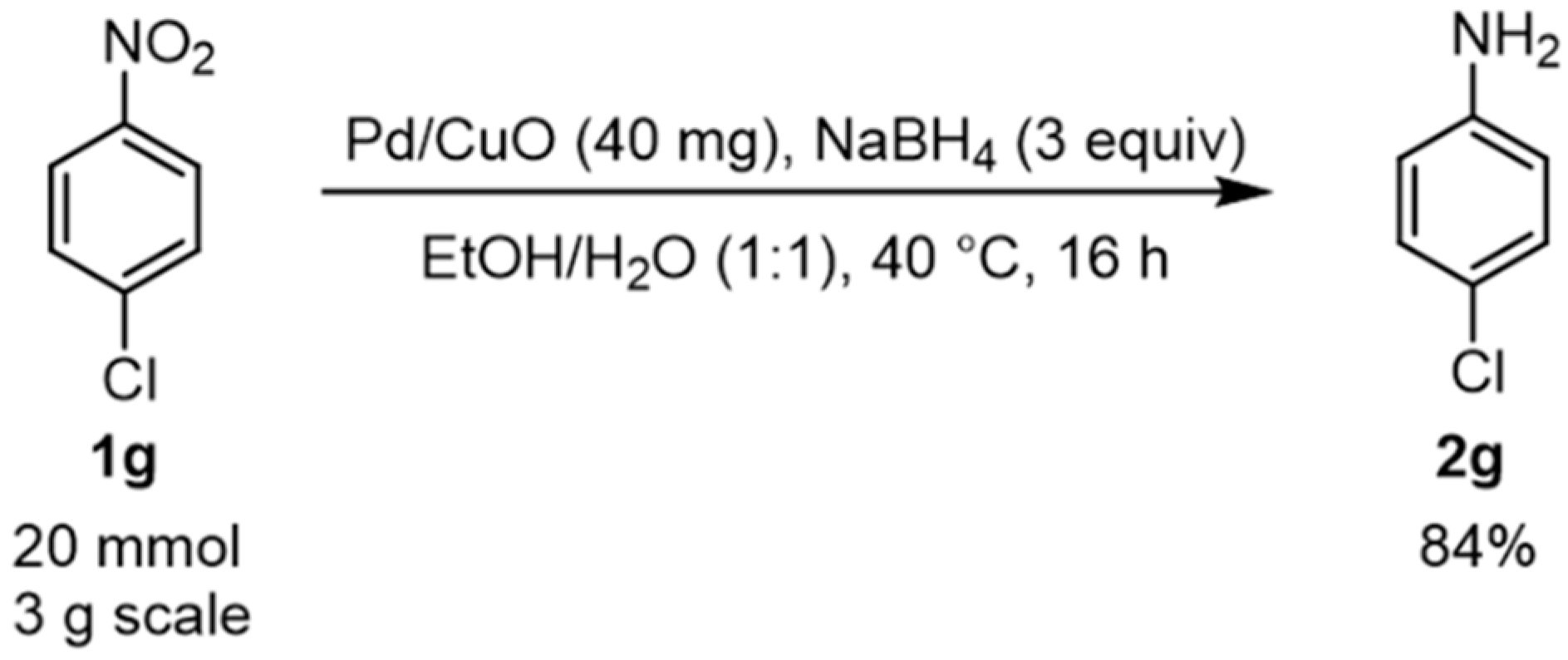
| 7 | 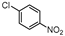 1g | 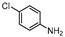 2g | 2 h | 71 |
| 8 | 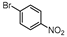 1h | 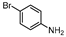 2h | 1.5 h | 61 |
| 9 |  1i |  2i | 11 h | 80 |
| 10 | 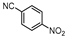 1j |  2j | 6 h | 53 |
| 11 | 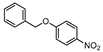 1k | 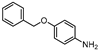 2k | 1.5 h | 98 |
| 12 c | 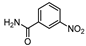 1l | 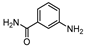 2l | 3 h | 88 |
| 13 c,d | 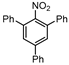 1m | 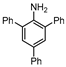 2m | 40 h | 80 |

| Cycle | Time | Yield (%) b |
|---|---|---|
| 1st | 1.0 h | 96 |
| 2nd | 1.5 h | 95 |
| 3rd | 1.5 h | 98 |
| 4th | 5.0 h | 96 |
| 5th | 5.5 h | 95 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, T.; Han, M.S. Preparation of Metal Oxides Containing ppm Levels of Pd as Catalysts for the Reduction of Nitroarene and Evaluation of Their Catalytic Activity by the Fluorescence-Based High-Throughput Screening Method. Catalysts 2020, 10, 542. https://doi.org/10.3390/catal10050542
Lim T, Han MS. Preparation of Metal Oxides Containing ppm Levels of Pd as Catalysts for the Reduction of Nitroarene and Evaluation of Their Catalytic Activity by the Fluorescence-Based High-Throughput Screening Method. Catalysts. 2020; 10(5):542. https://doi.org/10.3390/catal10050542
Chicago/Turabian StyleLim, Taeho, and Min Su Han. 2020. "Preparation of Metal Oxides Containing ppm Levels of Pd as Catalysts for the Reduction of Nitroarene and Evaluation of Their Catalytic Activity by the Fluorescence-Based High-Throughput Screening Method" Catalysts 10, no. 5: 542. https://doi.org/10.3390/catal10050542
APA StyleLim, T., & Han, M. S. (2020). Preparation of Metal Oxides Containing ppm Levels of Pd as Catalysts for the Reduction of Nitroarene and Evaluation of Their Catalytic Activity by the Fluorescence-Based High-Throughput Screening Method. Catalysts, 10(5), 542. https://doi.org/10.3390/catal10050542