A Facile and Scalable Approach to Ultrathin NixMg1−xO Solid Solution Nanoplates and Their Performance for Carbon Dioxide Reforming of Methane
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
2.2. Catalytic Performances of NixMg1−xO
2.3. Durability Tests of NixMg1-xO Catalysts
2.4. Coking Characteristics of NixMg1−xO Catalysts
3. Materials and Methods
3.1. Catalysts’ Synthesis
Synthesis of Ultrathin NixMg1-xO Solid Solution Nanoplates
3.2. Characterization
3.3. Catalytic Reaction
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Goeppert, A.; Czaun, M.; Jones, J.P.; Prakash, G.K.S.; Olah, G.A. Recycling of carbon dioxide to methanol and derived products-closing the loop. Chem. Soc. Rev. 2014, 43, 7995–8048. [Google Scholar] [CrossRef]
- Tang, P.; Zhu, Q.; Wu, Z.; Ma, D. Methane activation: The past and future. Energy Environ. Sci. 2014, 7, 2580–2591. [Google Scholar] [CrossRef]
- Hu, Y.H.; Ruckenstein, E. Binary MgO-based solid solution catalysts for methane conversion to syngas. Cat. Rev. Sci. Eng. 2002, 44, 423–453. [Google Scholar] [CrossRef]
- Bradford, M.C.J.; Vannice, M.A. CO2 reforming of CH4. Cat. Rev. Sci. Eng. 1999, 41, 1–42. [Google Scholar] [CrossRef]
- Bhavani, A.G.; Kim, W.Y.; Lee, J.S. Barium substituted lanthanum manganite perovskite for CO2 reforming of methane. ACS Catal. 2013, 3, 1537–1544. [Google Scholar] [CrossRef]
- Nair, M.M.; Kaliaguine, S.; Kleitz, F. Nanocast LaNiO3 perovskites as precursors for the preparation of coke-resistant dry reforming catalysts. ACS Catal. 2014, 4, 3837–3846. [Google Scholar] [CrossRef]
- Xie, X.; Otremba, T.; Littlewood, P.; Schomäcker, R.; Thomas, A. One-pot synthesis of supported, nanocrystalline nickel manganese oxide for dry deforming of methane. ACS Catal. 2013, 3, 224–229. [Google Scholar] [CrossRef]
- Olah, G.A.; Goeppert, A.; Czaun, M.; Prakash, G.K. Bi-reforming of methane from any source with steam and carbon dioxide exclusively to metgas (CO-2H2) for methanol and hydrocarbon synthesis. J. Am. Chem. Soc. 2013, 135, 648–650. [Google Scholar] [CrossRef]
- Djinović, P.; Batista, J.; Pintar, A. Efficient catalytic abatement of greenhouse gases: Methane reforming with CO2 using a novel and thermally stable Rh–CeO2 catalyst. Int. J. Hydrogen Energy 2012, 37, 2699–2707. [Google Scholar] [CrossRef]
- Qu, Y.Q.; Sutherland, A.M.; Guo, T. Carbon dioxide reforming of methane by Ni/Co nanoparticle catalysts immobilized on single-walled carbon nanotubes. Energy Fuels 2008, 22, 2183–2187. [Google Scholar] [CrossRef]
- Qu, Y.; Sutherland, A.M.; Lien, J.; Suarez, G.D.; Guo, T. Probing site activity of monodisperse Pt nanoparticle catalysts using steam reforming of methane. J. Phys. Chem. Lett. 2010, 1, 254–259. [Google Scholar] [CrossRef]
- Kambolis, A.; Matralis, H.; Trovarelli, A.; Papadopoulou, C. Ni/CeO2-ZrO2 catalysts for the dry reforming of methane. Appl. Catal. A 2010, 377, 16–26. [Google Scholar] [CrossRef]
- Silva, A.M.d.; Souza, K.R.d.; Jacobs, G.; Graham, U.M.; Davis, B.H.; Mattos, L.V.; Noronha, F.B. Steam and CO2 reforming of ethanol over Rh/CeO2 catalyst. Appl. Catal. B 2011, 102, 94–109. [Google Scholar] [CrossRef] [Green Version]
- Sadykov, V.A.; Gubanova, E.L.; Sazonova, N.N.; Pokrovskaya, S.A.; Chumakova, N.A.; Mezentseva, N.V.; Bobin, A.S.; Gulyaev, R.V.; Ishchenko, A.V.; Krieger, T.A.; et al. Dry reforming of methane over Pt/PrCeZrO catalyst: Kinetic and mechanistic features by transient studies and their modeling. Catal. Today 2011, 171, 140–149. [Google Scholar] [CrossRef]
- García-Diéguez, M.; Pieta, I.S.; Herrera, M.C.; Larrubia, M.A.; Malpartida, I.; Alemany, L.J. Transient study of the dry reforming of methane over Pt supported on different γ-Al2O3. Catal. Today 2010, 149, 380–387. [Google Scholar] [CrossRef]
- Wang, N.; Shen, K.; Huang, L.; Yu, X.; Qian, W.; Chu, W. Facile route for synthesizing ordered mesoporous Ni–Ce–Al oxide materials and their catalytic performance for methane dry reforming to hydrogen and syngas. ACS Catal. 2013, 3, 1638–1651. [Google Scholar] [CrossRef]
- Asencios, Y.J.O.; Assaf, E.M. Combination of dry reforming and partial oxidation of methane on NiO–MgO–ZrO2 catalyst: Effect of nickel content. Fuel Process Technol. 2013, 106, 247–252. [Google Scholar] [CrossRef]
- Odedairo, T.; Chen, J.; Zhu, Z. Metal–support interface of a novel Ni–CeO2 catalyst for dry reforming of methane. Catal. Commun. 2013, 31, 25–31. [Google Scholar] [CrossRef]
- Ocsachoque, M.; Pompeo, F.; Gonzalez, G. Rh–Ni/CeO2–Al2O3 catalysts for methane dry reforming. Catal. Today 2011, 172, 226–231. [Google Scholar] [CrossRef]
- de Sousa, H.S.A.; da Silva, A.N.; Castro, A.J.R.; Campos, A.; Filho, J.M.; Oliveira, A.C. Mesoporous catalysts for dry reforming of methane: Correlation between structure and deactivation behaviour of Ni-containing catalysts. Int. J. Hydrogen Energy 2012, 37, 12281–12291. [Google Scholar] [CrossRef]
- Zhang, S.; Muratsugu, S.; Ishiguro, N.; Tada, M. Ceria-doped Ni/SBA-16 catalysts for dry reforming of methane. ACS Catal. 2013, 3, 1855–1864. [Google Scholar] [CrossRef]
- Wang, N.; Chu, W.; Zhang, T.; Zhao, X.S. Synthesis, characterization and catalytic performances of Ce-SBA-15 supported nickel catalysts for methane dry reforming to hydrogen and syngas. Int. J. Hydrogen Energy 2012, 37, 19–30. [Google Scholar] [CrossRef]
- Xu, L.; Song, H.; Chou, L. One-pot synthesis of ordered mesoporous NiO–CaO–Al2O3 composite oxides for catalyzing CO2 reforming of CH4. ACS Catal. 2012, 2, 1331–1342. [Google Scholar] [CrossRef]
- Li, S.; Gong, J. Strategies for improving the performance and stability of Ni-based catalysts for reforming reactions. Chem. Soc. Rev. 2014, 43, 7245–7256. [Google Scholar] [CrossRef] [PubMed]
- Zanganeh, R.; Rezaei, M.; Zamaniyan, A. Preparation of nanocrystalline NiO–MgO solid solution powders as catalyst for methane reforming with carbon dioxide: Effect of preparation conditions. Adv. Powder Technol. 2014, 25, 1111–1117. [Google Scholar] [CrossRef]
- Li, Y.; Lu, G.; Ma, J. Highly active and stable nano NiO–MgO catalyst encapsulated by silica with a core–shell structure for CO2 methanation. RSC Adv. 2014, 4, 17420–17428. [Google Scholar] [CrossRef]
- Nurunnabi, M.; Kado, S.; Suzuki, K.; Fujimoto, K.-I.; Kunimori, K.; Tomishige, K. Synergistic effect of Pd and Ni on resistance to carbon deposition over NiO–MgO solid solution supported Pd catalysts in oxidative steam reforming of methane under pressurized conditions. Catal. Commun. 2006, 7, 488–493. [Google Scholar] [CrossRef]
- Hu, Y.H.; Ruckenstein, E. CH4 TPR-MS of NiO-MgO solid solution catalysts. Langmuir 1997, 13, 2055–2058. [Google Scholar] [CrossRef]
- Chen, Y.-G.; Tomishige, K.; Yokoyama, K.; Fujimoto, K. Catalytic performance and catalyst structure of nickel–magnesia catalysts for CO2 reforming of methane. J. Catal. 1999, 184, 479–490. [Google Scholar] [CrossRef]
- Koo, K.Y.; Roh, H.-S.; Seo, Y.T.; Seo, D.J.; Yoon, W.L.; Park, S.B. Coke study on MgO-promoted Ni/Al2O3 catalyst in combined H2O and CO2 reforming of methane for gas to liquid (GTL) process. Appl. Catal. A 2008, 340, 183–190. [Google Scholar] [CrossRef]
- García, V.; Fernández, J.J.; Ruíz, W.; Mondragón, F.; Moreno, A. Effect of MgO addition on the basicity of Ni/ZrO2 and on its catalytic activity in carbon dioxide reforming of methane. Catal. Commun. 2009, 11, 240–246. [Google Scholar] [CrossRef]
- Liu, D.; Quek, X.Y.; Cheo, W.N.E.; Lau, R.; Borgna, A.; Yang, Y. MCM-41 supported nickel-based bimetallic catalysts with superior stability during carbon dioxide reforming of methane: Effect of strong metal–support interaction. J. Catal. 2009, 266, 380–390. [Google Scholar] [CrossRef]
- Nurunnabi, M.; Mukainakano, Y.; Kado, S.; Li, B.; Kunimori, K.; Suzuki, K.; Fujimoto, K.-I.; Tomishige, K. Additive effect of noble metals on NiO-MgO solid solution in oxidative steam reforming of methane under atmospheric and pressurized conditions. Appl. Catal. A 2006, 299, 145–156. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, Z.; Zhou, X.; Zhu, K. A unique method to fabricate NixMg1−xO (111) nano-platelet solid solution catalyst for CH4-CO2 dry reforming. Catal. Commun. 2013, 34, 11–15. [Google Scholar] [CrossRef]
- Tomishige, K. Syngas production from methane reforming with CO2/H2O and O2 over NiO–MgO solid solution catalyst in fluidized bed reactors. Catal. Today 2004, 89, 405–418. [Google Scholar] [CrossRef]
- Ohira, T.; Yamamoto, O. Effective factor on antibacterial characteristics of Mg1−XNiXO solid solution. Chem. Eng. Res. Des. 2013, 91, 1055–1062. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Liu, H.-M.; Xu, B.-Q. Durable Ni/MgO catalysts for CO2 reforming of methane: Activity and metal–support interaction. J. Mol. Catal. A Chem. 2009, 299, 44–52. [Google Scholar] [CrossRef]
- Ruckenstein, E.; Hu, Y.H. Combination of CO2 reforming and partial oxidation of methane over NiO-MgO solid solution catalysts. Ind. Eng. Chem. Res. 1998, 37, 1744–1747. [Google Scholar] [CrossRef]
- Prieto, G.; Shakeri, M.; Jong, K.P.D.; Jongh, P.E.D. Quantitative relationship between support porosity and the stability of pore-confined metal nanoparticles studied on CuZnO-SiO2 methanol. ACS Nano 2014, 8, 2522–2531. [Google Scholar] [CrossRef]
- Djaidja, A.; Libs, S.; Kiennemann, A.; Barama, A. Characterization and activity in dry reforming of methane on NiMg/Al and Ni/MgO catalysts. Catal. Today 2006, 113, 194–200. [Google Scholar] [CrossRef]
- Guo, J.; Lou, H.; Zhao, H.; Chai, D.; Zheng, X. Dry reforming of methane over nickel catalysts supported on magnesium aluminate spinels. Appl. Catal. A 2004, 273, 75–82. [Google Scholar] [CrossRef]
- Liu, S.; Guan, L.; Li, J.; Zhao, N.; Wei, W.; Sun, Y. CO2 reforming of CH4 over stabilized mesoporous Ni–CaO–ZrO2 composites. Fuel 2008, 87, 2477–2481. [Google Scholar] [CrossRef]
- Juan-Juan, J.; Román-Martínez, M.C.; Illán-Gómez, M.J. Effect of potassium content in the activity of K-promoted Ni/Al2O3 catalysts for the dry reforming of methane. Appl. Catal. A 2006, 301, 9–15. [Google Scholar] [CrossRef]
- Djinović, P.; Črnivec, I.G.O.; Erjavec, B.; Pintar, A. Influence of active metal loading and oxygen mobility on coke-free dry reforming of Ni–Co bimetallic catalysts. Appl. Catal. B 2012, 125, 259–270. [Google Scholar] [CrossRef]
- Frusteria, F.; Spadaroa, L.; Arenab, F.; Chuvilinc, A. TEM evidence for factors affecting the genesis of carbon species on bare and K-promoted Ni-MgO catalysts during the dry reforming of methane. Carbon 2002, 40, 1063–1070. [Google Scholar] [CrossRef]
- Huang, T.; Huang, W.; Huang, J.; Ji, P. Methane reforming reaction with carbon dioxide over SBA-15 supported Ni–Mo bimetallic catalysts. Fuel Process Technol. 2011, 92, 1868–1875. [Google Scholar] [CrossRef]
- Han, Y.K.; Ahn, C.-I.; Bae, J.-W.; Kim, A.R.; Han, G.Y. Effects of carbon formation on catalytic performance for CO2 reforming with methane on Ni/Al2O3 catalyst:Comparison of fixed-bed with fluidized-bed reactors. Ind. Eng. Chem. Res. 2013, 52, 13288–13296. [Google Scholar] [CrossRef]
- Du, X.; Zhang, D.; Shi, L.; Gao, R.; Zhang, J. Morphology dependence of catalytic properties of Ni/CeO2 nanostructures for carbon dioxide reforming of methane. J. Phys. Chem. C 2012, 116, 10009–10016. [Google Scholar] [CrossRef]




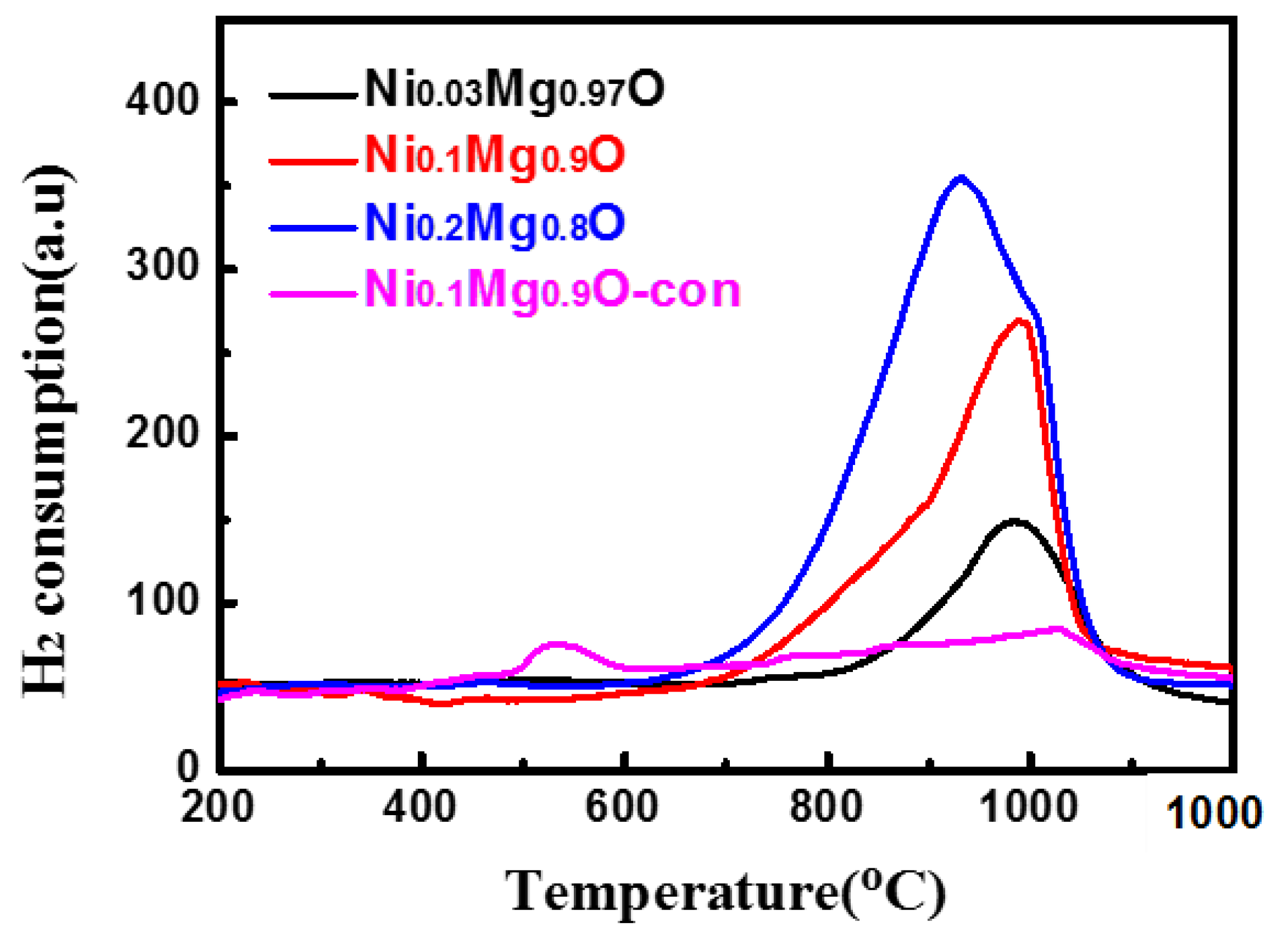



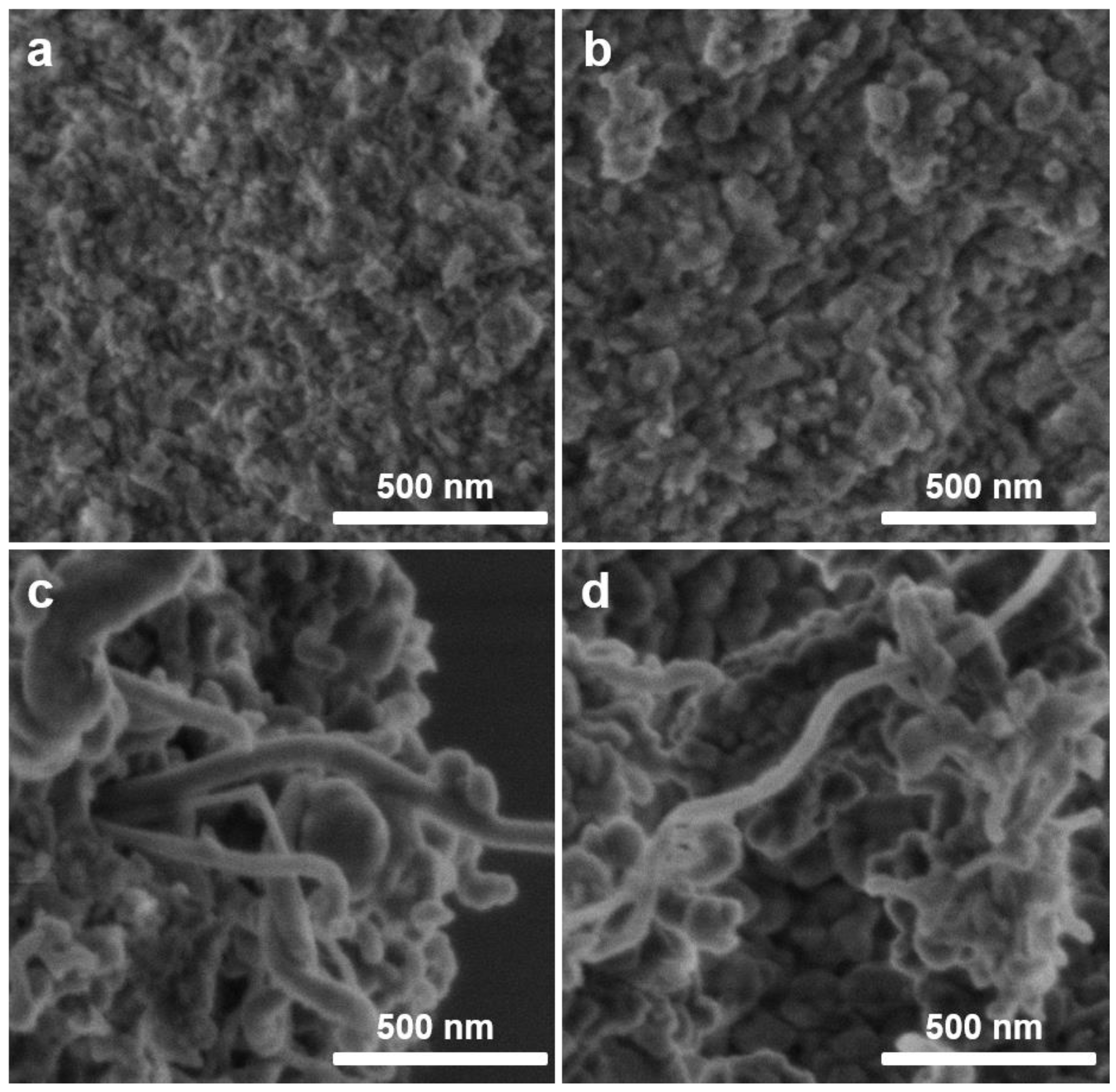
| Catalyst | BET 1 (m2g−1) | Vpore (cm3) | Pore Size (nm) | BET 1spend (m2g−1) | Carbon 2 (wt.%) |
|---|---|---|---|---|---|
| Ni0.03MgO0.97O | 117.8 | 0.35 | 50.62 | 94.5 | 2.0 |
| Ni0.1MgO0.9O | 140.3 | 1.20 | 121.8 | 89.5 | 2.1 |
| Ni0.2MgO0.8O | 164.9 | 1.16 | 95.10 | 76.5 | 31.6 |
| Ni0.1MgO0.9O-con | 12.5 | 0.11 | 33.45 | 10.5 | 8.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Zhang, Z.; Wang, Y.; Liu, Y.; Kang, Q. A Facile and Scalable Approach to Ultrathin NixMg1−xO Solid Solution Nanoplates and Their Performance for Carbon Dioxide Reforming of Methane. Catalysts 2020, 10, 544. https://doi.org/10.3390/catal10050544
Zhang G, Zhang Z, Wang Y, Liu Y, Kang Q. A Facile and Scalable Approach to Ultrathin NixMg1−xO Solid Solution Nanoplates and Their Performance for Carbon Dioxide Reforming of Methane. Catalysts. 2020; 10(5):544. https://doi.org/10.3390/catal10050544
Chicago/Turabian StyleZhang, Guoqiang, Zhiyun Zhang, Yunqiang Wang, Yanqiu Liu, and Qiping Kang. 2020. "A Facile and Scalable Approach to Ultrathin NixMg1−xO Solid Solution Nanoplates and Their Performance for Carbon Dioxide Reforming of Methane" Catalysts 10, no. 5: 544. https://doi.org/10.3390/catal10050544





